The Effect of Treatment during A Haze/Post-Haze Year on Subsequent Respiratory Morbidity Status among Successful Treatment Tuberculosis Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Study Procedure
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
3.1. Respondents’ Characteristics
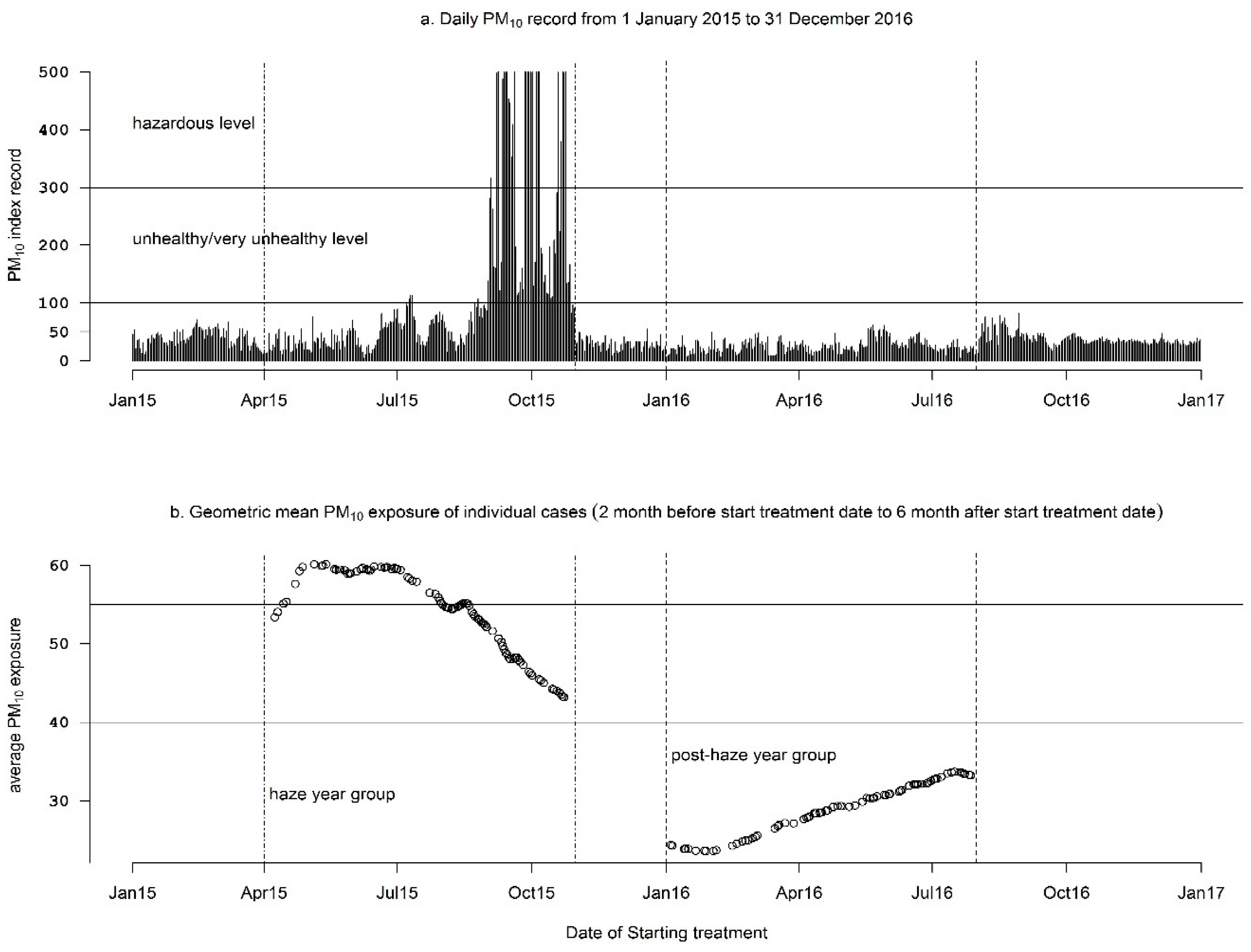
3.2. Air Pollutant Exposure
3.3. Comparison of Respiratory Morbidity Status between Respondents Treated in 2015 and 2016
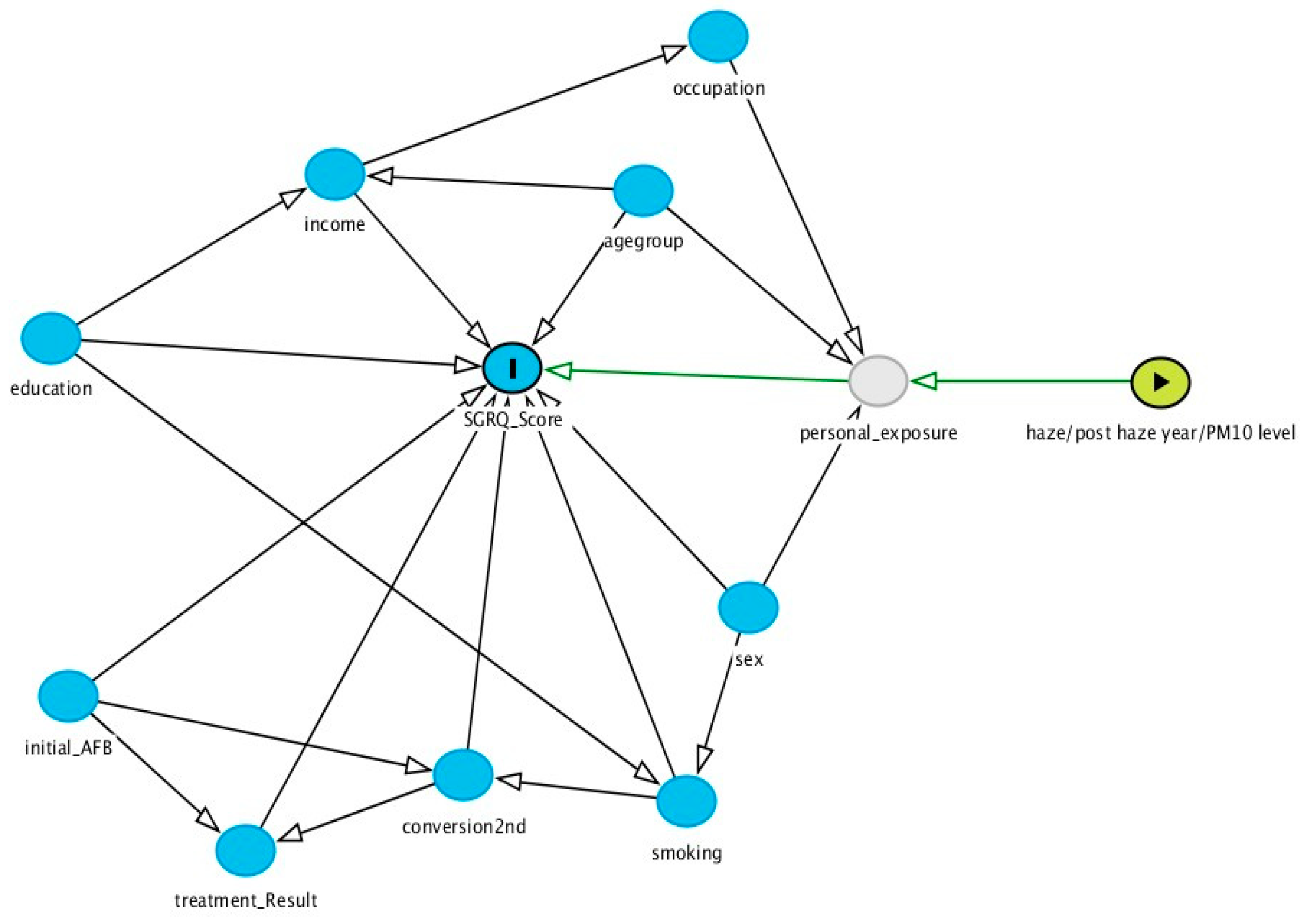
3.4. Factors Associated with Score of SGRQ Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2017; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-156551-6. [Google Scholar]
- Aggarwal, A. Health-related quality of life: A neglected aspect of pulmonary tuberculosis. Lung India 2010, 27, 1–3. [Google Scholar] [CrossRef]
- Guo, N.; Marra, F.; Marra, C.A. Measuring health-related quality of life in tuberculosis: A systematic review. Health Qual. Life Outcomes 2009, 7, 14. [Google Scholar] [CrossRef]
- Pratiwi, P.D.; Perwitasari, D.A. Validation of St. George’s Respiratory Questionnaire (SGRQ) in Chronic Obstructive Pulmonary Disease (COPD) at Respira Lung Hospital Yogyakarta. J. Manag. Pharm. Pract. 2017, 7, 75–82. [Google Scholar] [CrossRef]
- Wahyuni, A.S.; Soeroso, N.; Harahap, J.; Amelia, R.; Alona, I. Quality of life of pulmonary TB patients after intensive phase treatmentin the health centers of Medan city, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018, 125, 012142. [Google Scholar] [CrossRef]
- Mamani, M.; Majzoobi, M.M.; Ghahfarokhi, S.M.; Esna-Ashari, F.; Keramat, F. Assessment of health-related quality of life among patients with Tuberculosis in Hamadan, Western Iran. Oman Med. J. 2014, 29, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Hama, M.; Ushiki, A.; Kosaka, M.; Yamazaki, Y.; Yasuo, M.; Yamamoto, H.; Hanaoka, M. Health-related quality of life in patients with pulmonary non-tuberculous mycobacteria infection. Int. J. Tuberc. Lung Dis. 2016, 20, 747–752. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muniyandi, M.; Rajeswari, R.; Balasubramanian, R.; Nirupa, C.; Gopi, P.G.; Jaggarajamma, K.; Sheela, F.; Narayanan, P.R. Evaluation of post-treatment health-related quality of life (HRQoL) among tuberculosis patients. Int. J. Tuberc. Lung Dis. 2007, 11, 887–892. [Google Scholar] [PubMed]
- Ferrer, M.; Villasante, C.; Alonso, J.; Sobradillo, V.; Gabriel, R.; Vilagut, G.; Masa, J.F.; Viejo, J.L.; Jiménez-Ruiz, C.A.; Miravitlles, M. Interpretation of quality of life scores from the St George’s Respiratory Questionnaire. Eur. Respir. J. 2002, 19, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Sherpa, C.T.; LeClerq, S.L.; Singh, S.; Naithani, N.; Pangeni, R.; Karki, A.; Chokhani, R.; Han, M.; Gyetko, M.; Tielsch, J.M.; et al. Validation of the St. George’s Respiratory Questionnaire in Nepal. Chronic Obstr. Pulm. Dis. J. COPD Found. 2015, 2, 281–289. [Google Scholar] [CrossRef][Green Version]
- Guillerm, N.; Cesari, G. Fighting ambient air pollution and its impact on health: From human rights to the right to a clean environment. Int. J. Tuberc. Lung Dis. 2015, 19, 887–897. [Google Scholar] [CrossRef]
- Pinichka, C.; Makka, N.; Sukkumnoed, D.; Chariyalertsak, S.; Inchai, P.; Bundhamcharoen, K. Burden of disease attributed to ambient air pollution in Thailand: A GIS-based approach. PLoS ONE 2017, 12, e0189909. [Google Scholar] [CrossRef] [PubMed]
- WHO. Ambient (Outdoor) Air Quality and Health. Available online: http://www.who.int/en/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 28 April 2018).
- Kusumaningtyas, S.D.A.; Aldrian, E. Impact of the June 2013 Riau province Sumatera smoke haze event on regional air pollution. Environ. Res. Lett. 2016, 11, 075007. [Google Scholar] [CrossRef]
- Koplitz, S.N.; Mickley, L.J.; Marlier, M.E.; Buonocore, J.J.; Kim, P.S.; Liu, T.; Sulprizio, M.P.; DeFries, R.S.; Jacob, D.J.; Schwartz, J.; et al. Public health impacts of the severe haze in Equatorial Asia in September–October 2015: Demonstration of a new framework for informing fire management strategies to reduce downwind smoke exposure. Environ. Res. Lett. 2016, 11, 094023. [Google Scholar] [CrossRef]
- Yang, W.; Yuan, G.; Han, J. Is China’s air pollution control policy effective? Evidence from Yangtze River Delta cities. J. Clean. Prod. 2019, 220, 110–133. [Google Scholar] [CrossRef]
- Indonesia Decree of the Head of the Environmental Impact Control No. 107/1997 on Calculation, Reporting and Information of Air Quality Index. Available online: http://www.cets-uii.org/BML/Udara/ISPU/ISPU%20(Indeks%20Standar%20Pencemar%20Udara).htm (accessed on 2 October 2019).
- Greenstone, M. Indonesia’s Worsening Air Quality and Its Impact on Life Expectancy. Available online: https://aqli.epic.uchicago.edu/wp-content/uploads/2019/03/Indonesia-Report.pdf (accessed on 11 November 2019).
- Popovic, I.; Soares Magalhaes, R.J.; Ge, E.; Marks, G.B.; Dong, G.H.; Wei, X.; Knibbs, L.D. A systematic literature review and critical appraisal of epidemiological studies on outdoor air pollution and tuberculosis outcomes. Environ. Res. 2019, 170, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.S.; Schoenbach, V.J.; Richardson, D.B.; Gammon, M.D. Particulate air pollution and susceptibility to the development of pulmonary tuberculosis disease in North Carolina: An ecological study. Int. J. Environ. Health Res. 2014, 24, 103–112. [Google Scholar] [CrossRef]
- Zhu, S.; Xia, L.; Wu, J.; Chen, S.; Chen, F.; Zeng, F.; Chen, X.; Chen, C.; Xia, Y.; Zhao, X.; et al. Ambient air pollutants are associated with newly diagnosed tuberculosis: A time-series study in Chengdu, China. Sci. Total Environ. 2018, 631–632, 47–55. [Google Scholar] [CrossRef]
- Chuang, H.C.; Chen, K.Y.; Chuang, K.J.; Liu, H.C.; Lee, K.Y.; Feng, P.H.; Su, C.L.; Lin, C.L.; Lee, C.N. Particulate matter is associated with sputum culture conversion in patients with culture-positive tuberculosis. Ther. Clin. Risk Manag. 2016, 12, 41–46. [Google Scholar] [CrossRef]
- Jung, J.H.; Kang, I.G.; Cha, H.E.; Choe, S.H.; Kim, S.T. Effect of Asian Sand Dust on Mucin Production in NCI-H292 Cells and Allergic Murine Model. Otolaryngol. Head Neck Surg. 2012, 146, 887–894. [Google Scholar] [CrossRef]
- Maestrelli, P.; Canova, C.; Scapellato, M.; Visentin, A.; Tessari, R.; Bartolucci, G.; Simonato, L.; Lotti, M. Personal exposure to particulate matter is associated with worse health perception in Adult Asthma. J. Investig. Allergol. Clin. Immunol. 2011, 21, 120. [Google Scholar]
- Indonesian Haze: Why It’s Everyone’s Problem—CNN.com. Available online: http://edition.cnn.com/2015/09/17/asia/indonesian-haze-southeast-asia-pollution/index.html (accessed on 7 July 2017).
- Welling, J.B.A.; Hartman, J.E.; Ten Hacken, N.H.T.; Klooster, K.; Slebos, D.J. The minimal important difference for the St George’s Respiratory Questionnaire in patients with severe COPD. Eur. Respir. J. 2015, 46, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.W.; Forde, Y. St George’s Respiratory Questionare Manual. Available online: http://www.healthstatus.sgul.ac.uk/SGRQ_download/SGRQ%20Manual%20June%202009.pdf (accessed on 15 March 2017).
- Agustine, I.; Yulinawati, H.; Gunawan, D.; Suswantoro, E. Potential impact of particulate matter less than 10 micron (PM10) to ambient air quality of Jakarta and Palembang. IOP Conf. Ser. Earth Environ. Sci. 2018, 106, 012057. [Google Scholar] [CrossRef]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liśkiewicz, M.; Ellison, G.T.H. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Pasipanodya, J.G.; Miller, T.L.; Vecino, M.; Munguia, G.; Bae, S.; Drewyer, G.; Weis, S.E. Using the St. George Respiratory Questionnaire to ascertain health quality in Persons with treated pulmonary tuberculosis. Chest 2007, 132, 1591–1598. [Google Scholar] [CrossRef]
- Perwitasari, D.A.; Mulyani, U.A.; Thobari, J.A. Pengukuran Kualitas Hidup Pasien Tuberculosis Menggunakan Instrumen St Geotge Respiratory Questionare (SGRQ) di Yogyakarta. Available online: http://eprints.uad.ac.id/6621/ (accessed on 10 May 2018).
- Rekha, V.V.B.; Ramachandran, R.; Rao, K.V.K.; Rahman, F.; Adhilakshmi, A.R.; Murugesan, P.; Sundaram, V.; Narayanan, P.R. Assesment of long term status of sputum positive pulmonary TB patients succesfully treated with short course chemotherapy. Indian J. Tuberc. 2009, 56, 132–140. [Google Scholar]
- Obaseki, D.; Erhabor, G.; Awopeju, O.; Obaseki, J.; Adewole, O. Determinants of health related quality of life in a sample of patients with chronic obstructive pulmonary disease in Nigeria using the St. George’s respiratory questionnaire. Afr. Health Sci. 2013, 13, 694–702. [Google Scholar] [CrossRef]
- de Farias, S.N.P.; da Silva Medeiros, C.R.; Paz, E.P.A.; de Lobo, A.J.S.; Ghelman, L.G. Completeness in caring: Study of quality of life in clients with tuberculosis. Esc. Anna Nery 2013, 17, 749–754. [Google Scholar] [CrossRef]
- Li, C.T.; Chu, K.H.; Reiher, B.; Kienene, T.; Chien, L.Y. Evaluation of health-related quality of life in patients with tuberculosis who completed treatment in Kiribati. J. Int. Med. Res. 2017, 45, 610–620. [Google Scholar] [CrossRef]
- Brown, J.; Capocci, S.; Smith, C.; Morris, S.; Abubakar, I.; Lipman, M. Health status and quality of life in tuberculosis. Int. J. Infect. Dis. 2015, 32, 68–75. [Google Scholar] [CrossRef]
- Campos, A.C.V.; Ferreira, E.F.E.; Vargas, A.M.D.; Albala, C. Aging, Gender and Quality of Life (AGEQOL) study: Factors associated with good quality of life in older Brazilian community-dwelling adults. Health Qual. Life Outcomes 2014, 12, 166. [Google Scholar] [CrossRef]
- Smoking Rates by Country Population 2019. Available online: http://worldpopulationreview.com/countries/smoking-rates-by-country/ (accessed on 6 November 2019).
- Bridevaux, P.O. Secondhand smoke and health-related quality of life in never smokers: Results from the SAPALDIA Cohort Study 2. Arch. Intern. Med. 2007, 167, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Awaisu, A.; Haniki Nik Mohamed, M.; Noordin, N.; Muttalif, A.; Aziz, N.; Syed Sulaiman, S.; Mahayiddin, A. Impact of connecting tuberculosis directly observed therapy short-course with smoking cessation on health-related quality of life. Tob. Induc. Dis. 2012, 10, 2. [Google Scholar] [CrossRef]
| Characteristics | Treated on 2015 N = 133 (%) | Treated on 2016 N = 103 (%) | p-Value |
|---|---|---|---|
| Sex group | |||
| Female | 31 (23.3) | 31 (30.1) | 0.30 |
| Male | 102 (76.7) | 72 (69.9) | |
| Age group | 0.11 | ||
| <33 year | 46 (34.6) | 34 (33.0) | |
| 33–49 year | 37 (27.8) | 41 (39.8) | |
| >49 year | 50 (37.6) | 28 (27.2) | |
| Education group | 0.98 | ||
| Low | 71 (53.4) | 56 (54.4) | |
| High | 62 (46.6) | 47 (45.6) | |
| Occupation group | |||
| Outdoor job | 93 (69.9) | 77 (74.7) | 0.50 |
| Indoor job | 40 (30.1) | 26 (25.2) | |
| Income group | 1 | ||
| <median | 74 (55.6) | 57 (55.3) | |
| ≥median | 59 (44.4) | 46 (44.7) | |
| Smoking group | 0.94 | ||
| Non-smoker | 78 (58.6) | 59 (57.3) | |
| Smoker | 55 (41,4) | 44 (42.7) | |
| Initial AFB group | |||
| Negative | 39 (29.3) | 34 (33.0) | 0.64 |
| Positive | 94 (70.7) | 69 (67.0) | |
| Conversion Month group | |||
| Month 2nd | 130 (97.7) | 98 (95.1) | 0.46 |
| Month 3rd | 3 (2.3) | 5 (4.9) | |
| Treatment Result group | 0.51 | ||
| Completed | 74 (55.6) | 52 (50.5) | |
| Cured | 59 (44.4) | 51 (49.5) | |
| PM10 exposure group | < 0.01 | ||
| <40 | 0 | 103 (100) | |
| 40–55 | 67 (51.0) | ||
| ≥55 | 66 (49.0) |
| Group | Symptom Score | Activity Score | Impact Score | Total Score |
|---|---|---|---|---|
| Median haze year (2015) group (IQR) | 17.9 (12.3, 30.7) | 23.7 * (17.2, 30.9) | 15.4 (9.5, 22.4) | 19.2 (13.0, 25.4) |
| Median post-haze year (2016) group (IQR) | 17.9 (11.1, 26.6) | 18.4 * (11.9, 24.8) | 14.6 (9.5, 21.4) | 17.7 (12.9, 23.9) |
| Variables | Level | Symptom Domain | Activity Domain | Impact Domain | Total Domain |
|---|---|---|---|---|---|
| Sex group | Female | 0 | 0 | 0 | 0 |
| Male | 2.72 (−0.56, 8,35) | 1.07 (0.25, 10.58) * | 2.00 (−0.07, 5.11) | 3.28 (0.75, 6.02) * | |
| Age group | <33 year | 0 | 0 | 0 | 0 |
| 33–49 year | 3.99 (2.68, 7.24) * | 1.23 (−0.22, 7.19) | 5.41 (3.52, 7.92) * | 5.01 (2.04, 6.52) * | |
| >49 year | 14.82 (11.67, 19.16) * | 7.52 (6.41, 16.88) * | 7.71 (5.51, 9.47) * | 9.49 (6.86, 11.08) * | |
| Education group | Low | 0 | 0 | 0 | 0 |
| High | 0.00 (−2.96, 4.89) | 0.10 (−1.83, 4.81) | −1.57 (−3.91, 1.12) | −0.15 (−2.36, 2.41) | |
| Occupation group | Outdoor job | 0 | 0 | 0 | 0 |
| Indoor job | −1.14 (−4.73, 6.51) | 0.02 (−5.8, 2.81) | 0.53 (−1.66, 2.87) | 0.54 (−3.38,3.54) | |
| Income group | <median | 0 | 0 | 0 | 0 |
| ≥median | 0.00 (−4.88, 2.95) | −0.26 (−5.04, 3.98) | −0.15 (−2.52, 2.53) | −0.77 (−3.26, 1.64) | |
| Smoking group | Non-smoker | 0 | 0 | 0 | 0 |
| Smoker | 7.84 (3.96, 12.35) * | 6.04 (5.66, 6.75) * | 5.17 (1.66, 7.85) * | 5.43 (1.19, 8.17) * | |
| Initial AFB group | Negative | 0 | 0 | 0 | 0 |
| Positive | −1.07 (−5.77, 2.91) | 0.10 (−5.48, 1.79) | 0.42 (−2.90, 3.89) | −0.25 (−3.08, 3.08) | |
| Conversion month group | Month 2nd | 0 | 0 | 0 | 0 |
| Month 3rd | −4.88 (−9.88, 17.59) | 0.42 (−2.91, 12.53) | 0.62 (−2.23, 17.95) | 0.58 (−3.81, 17.81) | |
| Treatment result group | Completed | 0 | 0 | 0 | 0 |
| Cured | −2.06 (−6.03, 0.33) | −0.77 (−5.42, 0.51) | 0.97 (−1.90, 3.05) | 0.31 (−1.96, 2.75) | |
| PM10 exposure group | <40 | 0 | 0 | 0 | 0 |
| 40–55 | 3.65 (−2.37, 7.87) | 0.26 (−0..44, 6.38) | 0.51 (−2.18, 3.97) | 1.72 (−0.25, 4.57) | |
| ≥55 | −0.45 (−2.66, 3.48) | 5.67 (0.99, 6.18) * | 1.18 (−1.92, 3.64) | −0.13 (−2.69, 4.37) | |
| Year of Treatment | 2016 | 0 | 0 | 0 | 0 |
| 2015 | 0.00 (−2.33, 6.01) | 5.32 (0.12, 6.16) * | 0.80 (−2.04, 2.85) | 1.49 (−1.01, 4.19) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suyanto, S.; Geater, A.; Chongsuvivatwong, V. The Effect of Treatment during A Haze/Post-Haze Year on Subsequent Respiratory Morbidity Status among Successful Treatment Tuberculosis Cases. Int. J. Environ. Res. Public Health 2019, 16, 4669. https://doi.org/10.3390/ijerph16234669
Suyanto S, Geater A, Chongsuvivatwong V. The Effect of Treatment during A Haze/Post-Haze Year on Subsequent Respiratory Morbidity Status among Successful Treatment Tuberculosis Cases. International Journal of Environmental Research and Public Health. 2019; 16(23):4669. https://doi.org/10.3390/ijerph16234669
Chicago/Turabian StyleSuyanto, Suyanto, Alan Geater, and Virasakdi Chongsuvivatwong. 2019. "The Effect of Treatment during A Haze/Post-Haze Year on Subsequent Respiratory Morbidity Status among Successful Treatment Tuberculosis Cases" International Journal of Environmental Research and Public Health 16, no. 23: 4669. https://doi.org/10.3390/ijerph16234669
APA StyleSuyanto, S., Geater, A., & Chongsuvivatwong, V. (2019). The Effect of Treatment during A Haze/Post-Haze Year on Subsequent Respiratory Morbidity Status among Successful Treatment Tuberculosis Cases. International Journal of Environmental Research and Public Health, 16(23), 4669. https://doi.org/10.3390/ijerph16234669





