Effect of Probiotics Supplementations on Health Status of Athletes
Abstract
1. Introduction
2. Effect of Probiotic Supplementation on the Health Status of Athletes
2.1. Single-Strain Probiotic Intervention
2.2. Multispecies Probiotic Intervention
2.3. Multigenus Probiotic Intervention
3. Impact of Probiotic Supplementation on Athlete Health: Non-significant Outcomes
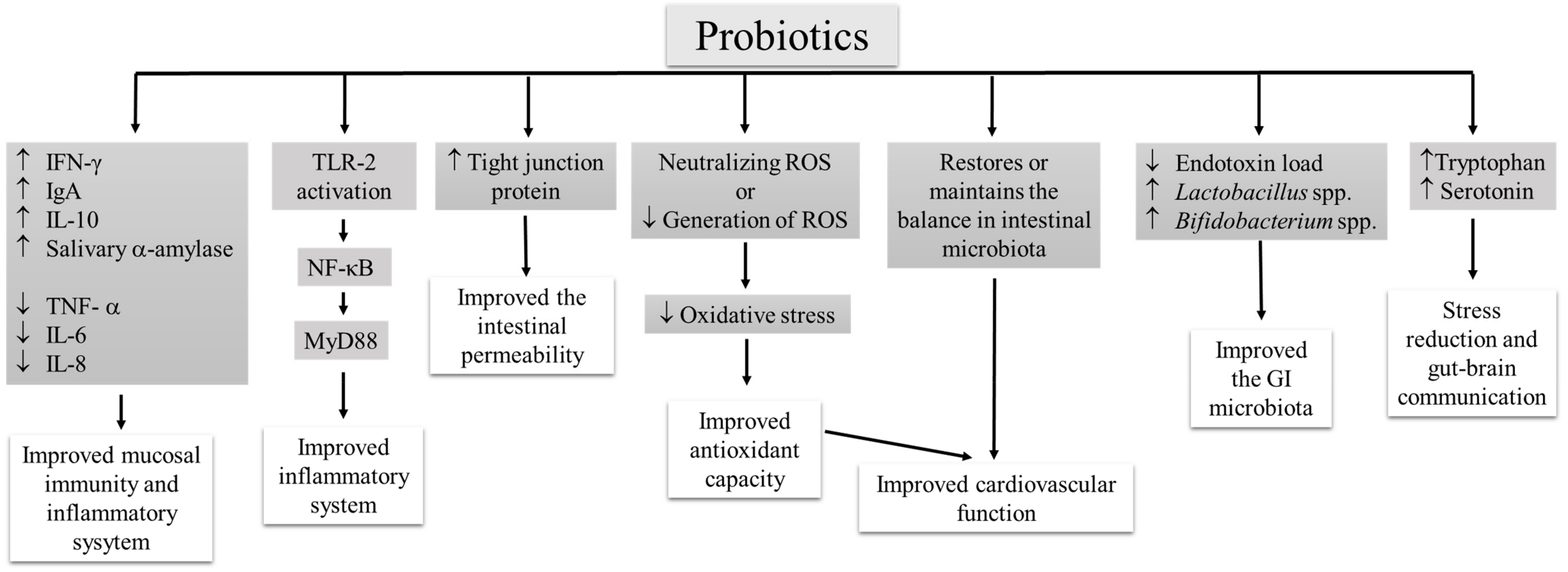
4. Mechanism of Probiotic Action
5. Factors Influencing the Effect of Probiotic Supplementation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bermon, S.; Petriz, B.; Kajeniene, A.; Prestes, J.; Castell, L.; Franco, O.L. The microbiota: An exercise immunology perspective. Exerc. Immunol. Rev. 2015, 21, 70–79. [Google Scholar] [PubMed]
- Pyne, D.B.; West, N.P.; Cox, A.J.; Cripps, A.W. Probiotics supplementation for athletes: Clinical and physiological effects. Eur. J. Sport Sci. 2015, 15, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 24, 43. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Teixeira, A.; Rama, L.; Prestes, J.; Rosado, F.; Hankey, J.; Scheer, V.; Hemmings, K.; Ansley-Robson, P.; Costa, R.J. Circulatory endotoxin concentration and cytokine profile in response to exertional-heat stress during a multi-stage ultra-marathon competition. Exerc. Immunol. Rev. 2015, 21, 114–128. [Google Scholar] [PubMed]
- Pugh, J.N.; Fearn, R.; Morton, J.P.; Close, G.L. Gastrointestinal symptoms in elite athletes: Time to recognise the problem? Br. J. Sports Med. 2018, 52, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC Consensus Statement: Dietary supplements and the high-performance athlete. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S. A comprehensive review on clinical outcome of probiotic and synbiotic therapy for inflammatory bowel diseases. Asian Pac. J. Trop. Biomed. 2018, 8, 179–186. [Google Scholar] [CrossRef]
- Blaabjerg, S.; Artzi, D.M.; Aabenhus, R. Probiotics for the prevention of antibiotic-associated diarrhea in outpatients-a systematic review and meta-analysis. Antibiotics 2017, 6, 21. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Influence of probiotic supplementation on climacteric symptoms in menopausal women-A mini review. Int. J. App. Pharm. 2018, 10, 43–46. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Probiotic based therapy for atopic dermatitis: Outcomes of clinical studies. Asian Pac. J. Trop. Biomed. 2018, 8, 328–332. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A review on anti-aging properties of probiotics. Int. J. App. Pharm. 2018, 10, 23–27. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Prasanth, M.I.; Kesika, P.; Chaiyasut, C. Probiotics in human mental health and diseases-A mini review. Trop. J. Pharm. Res. 2019, 18, 889–895. [Google Scholar]
- Sivamaruthi, B.S.; Kesika, P.; Suganthy, N.; Chaiyasut, C. A review on role of microbiome in obesity and antiobesity properties of probiotic supplements. BioMed Res. Int. 2019, 2019, 3291367. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A mini review of human studies on cholesterol-lowering properties of probiotics. Sci. Pharm. 2019, 87, 26. [Google Scholar] [CrossRef]
- Vasquez, E.C.; Pereira, T.M.C.; Peotta, V.A.; Baldo, M.P.; Campos-Toimil, M. Probiotics as beneficial dietary supplements to prevent and treat cardiovascular diseases: Uncovering their impact on oxidative stress. Oxid. Med. Cell. Longev. 2019, 2019, 3086270. [Google Scholar] [CrossRef]
- Williams, N.C.; Killer, S.C.; Svendsen, I.S.; Jones, A.W. Immune nutrition and exercise: Narrative review and practical recommendations. Eur. J. Sport Sci. 2019, 19, 49–61. [Google Scholar] [CrossRef]
- Vitale, K.; Getzin, A. Nutrition and supplement update for the endurance athlete: Review and recommendations. Nutrients 2019, 11, 1289. [Google Scholar] [CrossRef]
- Pane, M.; Amoruso, A.; Deidda, F.; Graziano, T.; Allesina, S.; Mogna, L. Gut microbiota, probiotics, and sport from clinical evidence to agonistic performance. J. Clin. Gastroenterol. 2018, 52, S46–S49. [Google Scholar] [CrossRef]
- Kekkonen, R.A.; Vasankari, T.J.; Vuorimaa, T.; Haahtela, T.; Julkunen, I.; Korpela, R. The effect of probiotics on respiratory infections and gastrointestinal symptoms during training in marathon runners. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 352–363. [Google Scholar] [CrossRef]
- Clancy, R.L.; Gleeson, M.; Cox, A.; Callister, R.; Dorrington, M.; D’Este, C.; Pang, G.; Pyne, D.; Fricker, P.; Henriksson, A. Reversal in fatigued athletes of a defect in interferon γ secretion after administration of Lactobacillus acidophilus. Br. J. Sports Med. 2006, 40, 351–354. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Oliveira, M.; Tauler, P. Daily probiotic’s (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Struszczak, L. Effects of Lactobacillus casei Shirota ingestion on common cold infection and herpes virus antibodies in endurance athletes: A placebo-controlled, randomized trial. Eur. J. Appl. Physiol. 2016, 116, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Michalickova, D.; Minic, R.; Dikic, N.; Andjelkovic, M.; Kostic-Vucicevic, M.; Stojmenovic, T.; Nikolic, I.; Djordjevic, B. Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: A randomized, double-blind, placebo-controlled trial. Appl. Physiol. Nutr. Metab. 2016, 41, 782–789. [Google Scholar] [CrossRef] [PubMed]
- West, N.P.; Pyne, D.B.; Cripps, A.W.; Hopkins, W.G.; Eskesen, D.C.; Jairath, A.; Christophersen, C.T.; Conlon, M.A.; Fricker, P.A. Lactobacillus fermentum (PCC®) supplementation and gastrointestinal and respiratory tract illness symptoms: A randomized control trial in athletes. Nutr. J. 2011, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; Pyne, D.B.; Saunders, P.U.; Fricker, P.A. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br. J. Sports Med. 2010, 44, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Komano, Y.; Shimada, K.; Naito, H.; Fukao, K.; Ishihara, Y.; Fujii, T.; Kokubo, T.; Daida, H. Efficacy of heat-killed Lactococcus lactis JCM 5805 on immunity and fatigue during consecutive high intensity exercise in male athletes: A randomized, placebo-controlled, double-blinded trial. J. Int. Soc. Sports Nutr. 2018, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Wei, C.C.; Huang, C.C.; Chen, W.L.; Huang, H.Y. The beneficial effects of Lactobacillus plantarum PS128 on high-intensity, exercise-induced oxidative stress, inflammation, and performance in triathletes. Nutrients 2019, 11, 353. [Google Scholar] [CrossRef]
- Martarelli, D.; Verdenelli, M.C.; Scuri, S.; Cocchioni, M.; Silvi, S.; Cecchini, C.; Pompei, P. Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr. Microbiol. 2011, 62, 1689–1696. [Google Scholar] [CrossRef]
- Coman, M.M.; Verdenelli, M.C.; Silvi, S.; Cecchini, C.; Gabbianelli, R.; Amadio, E.; Orpianesi, C.; Cresci, A. Knowledge and acceptance of functional foods: A preliminary study on influence of a synbiotic-fermented milk on athlete health. Int. J. Probiotics Prebiotics 2017, 12, 33–41. [Google Scholar]
- Pumpa, K.L.; McKune, A.J.; Harnett, J. A novel role of probiotics in improving host defence of elite rugby union athlete: A double blind randomised controlled trial. J. Sci. Med. Sport 2019, 22, 876–881. [Google Scholar] [CrossRef]
- Salarkia, N.; Ghadamli, L.; Zaeri, F.; Rad, L.S. Effects of probiotic yogurt on performance, respiratory and digestive systems of young adult female endurance swimmers: A randomized controlled trial. Med. J. Islam. Repub. Iran 2013, 27, 141–146. [Google Scholar] [PubMed]
- Haywood, B.A.; Black, K.E.; Baker, D.; McGarvey, J.; Healey, P.; Brown, R.C. Probiotic supplementation reduces the duration and incidence of infections but not severity in elite rugby union players. J. Sci. Med. Sport 2014, 17, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Suckling, C.A.; Peedle, G.Y.; Murphy, J.A.; Dawkins, T.G.; Roberts, M.G. An exploratory investigation of endotoxin levels in novice long distance triathletes, and the effects of a multi-strain probiotic/prebiotic, antioxidant intervention. Nutrients 2016, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, M.; Bogner, S.; Schippinger, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J.F. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Geiger, D.; Schauer, M.; Gostner, J.M.; Gatterer, H.; Burtscher, M.; Fuchs, D. Probiotic supplements beneficially affect tryptophan–kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: A randomized, double-blinded, placebo-controlled trial. Nutrients 2016, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Oliveira, M.; McCauley, T.; Tauler, P.; Lawrence, C. Effects of a Lactobacillus salivarius probiotic intervention on infection, cold symptom duration and severity, and mucosal immunity in endurance athletes. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Kekkonen, R.; Korpela, R.; Delgado, L.; Haahtela, T. Allergy in marathon runners and effect of Lactobacillus GG supplementation on allergic inflammatory markers. Respir. Med. 2007, 101, 1123–1131. [Google Scholar] [CrossRef]
- Leite, G.S.F.; Ayane, S.; West, N.P.; Lancha, A.H. Probiotics and sports: A new magic bullet? Nutrition 2019, 60, 152–160. [Google Scholar] [CrossRef]
- Gluck, U.; Gebbers, J.O. Ingested probiotics reduce nasal colonization with pathogenic bacteria. Am. J. Clin. Nutr. 2003, 77, 517–520. [Google Scholar] [CrossRef]
- Cario, E.; Gerken, G.; Podolsky, D.K. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 2007, 132, 1359–1374. [Google Scholar] [CrossRef]
- Wells, J.M.; Rossi, O.; Meijerink, M.; van Baarlen, P. Epithelial crosstalk at the microbiota-mucosal interface. Proc. Natl. Acad. Sci. USA 2011, 108, 4607–4614. [Google Scholar] [CrossRef] [PubMed]
- Vecchia, L.D.; Traversi, E.; Porta, A.; Lucini, D.; Pagani, M. On site assessment of cardiac function and neural regulation in amateur half marathon runners. Open Heart 2014, 1, e000005. [Google Scholar] [CrossRef] [PubMed]
- Vecchia, L.A.D.; Barbic, F.; De Maria, B.; Cozzolino, D.; Gatti, R.; Dipaola, F.; Brunetta, E.; Zamuner, A.R.; Porta, A.; Furlan, R. Can strenuous exercise harm the heart? Insights from a study of cardiovascular neural regulation in amateur triathletes. PLoS ONE 2019, 14, e0216567. [Google Scholar]
- West, N.P.; Horn, P.L.; Pyne, D.B.; Gebski, V.J.; Lahtinen, S.J.; Fricker, P.A.; Cripps, A.W. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin. Nutr. 2014, 33, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Shing, C.M.; Peake, J.M.; Lim, C.L.; Briskey, D.; Walsh, N.P.; Fortes, M.B.; Ahuja, K.D.; Vitetta, L. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur. J. Appl. Physiol. 2014, 114, 93–103. [Google Scholar] [CrossRef]
- Gill, S.K.; Allerton, D.M.; Ansley-Robson, P.; Hemmings, K.; Cox, M.; Costa, R.J. Does short-term high dose probiotic supplementation containing lactobacillus casei attenuate exertional-heat stress induced endotoxaemia and cytokinaemia? Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 268–275. [Google Scholar] [CrossRef]
| Subjects | Probiotics | Dose and Duration | Benefits/Impacts | References |
|---|---|---|---|---|
| Healthy marathon runners; n = 141 (16 females, 125 males); Age = 22 to 69 years | Lactobacillus rhamnosus GG | 4 × 1010 CFU or 1010 CFU per day; 3 months | No changes in the incidence of GI-symptoms episodes, URTIs, and hematological parameters ↓ Duration of GI-symptoms episodes | Kekkonen et al. [19] |
| Healthy and fatigued athletes; n = 27 (10 females, 17 males); Age = 16 to 41 years | L. acidophilus LAFTI®L10 | 2 × 1010 CFU per day; 4 weeks | ↑ Salivary IFN-γ ↑ IFN-γ secretion from WBC | Clancy et al. [20] |
| Endurance athletes; n = 84 (30 females, 54 males); Age = 18 to 55 years | L. casei Shirota | 1.3 × 1010 CFU per day; 16 weeks | No change in severity of URTI ↓ Incidence of URTI ↑ Saliva IgA level | Gleeson et al. [21] |
| Endurance athletes; n = 268 (113 females, 155 males); Age = 18 to 32 years | L. casei Shirota | 1.3 × 1010 CFU per day; 20 weeks | No influence on URTI, but improved the immune status | Gleeson et al. [22] |
| Elite athletes; n = 30 (6 females, 24 males); Age = 18 to 28 years | L. helveticus LaftiL10 | 2 × 1010 CFU per day; 14 weeks | Improved the immunity of athletes | Michalickova et al. [23] |
| Competitive cyclists; n = 62 (35 females, 64 males); Age = 25 to 45 years | L. fermentum VRI-003 (PCC®) | 1 × 109 CFU per day; 11 weeks | ↑ Lactobacillus spp. count ↓ Severity of GI-symptoms in male ↓ Symptoms and illness load of URTIs in male No significant change in mucosal immunity | West et al. [24] |
| Elite distance runners; n = 20 males; Age = 20 to 35 years | L. fermentum VRI-003 | 1.2 × 1010 CFU per day; 28 days (1st treatment month) | ↓ Severity and incidence of URTI ↑ Whole blood culture IFN-γ | Cox et al. [25] |
| Athletes; n = 51 males; Age = 20 years | Lactococcus lactis JCM 5805 | 10 × 1010 CFU per day; 13 days | ↑ pDC maturation ↓ Incidence of URTI ↓ Fatigue | Komano et al. [26] |
| Triathletes; n = 18 (study 1); n= 16 (study 2); Age = 19 to 26 years | L. plantarum PS128 | 3 × 1010 CFU per day; 3 or 8 weeks | ↓ Oxidative stress ↓ Pro-inflammation ↑ Anti-inflammation ↑ Plasma-branched amino acids | Huang et al. [27] |
| Amateur athletes; n = 24 males; Age = 25 to 39 years | L. rhamnosus IMC 501®, and L. paracasei IMC 502® (1:1 ratio) | ~109 cells per day; 4 weeks | Neutralize the ROS ↑ Antioxidant potential | Martarelli et al. [28] |
| Athletes; n = 10 (7 females, 3 males); Age = 20 to 45 years | * L. rhamnosus IMC 501® and L. paracasei IMC 502® | 109 CFU per strain; 2 × 109 CFU per day; 4 weeks | Improve gut health, oxidative status, and mucosal immunity | Coman et al. [29] |
| Elite rugby union athletes; n = 19; Age = 20 to 35 years | L. rhamnosus, L. casei, L. acidophilus, L. plantarum, L. fermentum, Bifidobacterium lactis, B. bifidum, Streptococcus thermophilus, Saccharomyces boulardi | 12 × 1010 CFU per day; 27 weeks | ↓ Incidence of GI and URTIs ↑ Salivary α-amylase | Pumpa et al. [30] |
| Female endurance swimmers; n = 46; Age = 11 to 17 years | L. acidophilus, L. delbrueckii subsp. bulgaricus, B. bifidum, and Streptococcus salivarius subsp. thermophilus | 400 mL per day (4 × 1010 per ml); 8 weeks | ↓ Number of episodes of URTIs ↓ Dyspnea and ear pain | Salarkia et al. [31] |
| Rugby players; n = 30 males; Age = 19 to 29 years | L. gasseri, B. bifidum, B. longum | 3 × 109 CFU per day; 4 weeks | ↓ Incidence of URTI ↓ GI episodes | Haywood et al. [32] |
| Long-distance triathletes; n = 30 (5 females, 25 males); Age = 30 to 38 years | ** L. acidophilus CUL-60, L. acidophilus CUL-21, B. bifidum CUL-20, B. animalis subsp. lactis CUL-34 | Total 30 × 1010 CFU per day; 12 weeks | ↓ Endotoxin units ↓ GI symptoms Maintenance of intestinal permeability | Roberts et al. [33] |
| Trained athletes (triathletes, runners, cyclists); n = 23 males; Age = 30 to 45 years | B. bifidum W23, B. lactis W51, Enterococcus faecium W54, L. acidophilus W22, L. brevis W63, and L. lactis W58. | 1010 CFU per day; 14 weeks | Improves intestinal permeability ↓ Exercise-induced protein oxidation ↓ TNF-α level | Lamprecht et al. [34] |
| Trained athletes; n = 29 (16 females, 13 males); Age = 20 to 35 years | B. bifidum W23, B. lactis W51, E. faecium W54, L. acidophilus W22, L. brevis W63, L. lactis W58 | 1010 CFU per day; 12 weeks | ↓ Incidence of URTI ↓ Rate of exercise-induced tryptophan degradation | Strasser et al. [35] |
| Endurance athletes; n = 66 (38 female, 28 male); Age = 19 to 29 years | L. salivarius | 2 × 1010 CFU per day; 4 months | No influence on URTI, and mucosal immunity | Gleeson et al. [36] |
| Healthy marathon runners; n = 139 (16 females, 123 males); Age = 30 to 50 years | L. rhamnosus GG | 4 × 1010 CFU or 1010 CFU per day; 3 months | No significant change in the incidence of allergic diseases | Moreira et al. [37] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Effect of Probiotics Supplementations on Health Status of Athletes. Int. J. Environ. Res. Public Health 2019, 16, 4469. https://doi.org/10.3390/ijerph16224469
Sivamaruthi BS, Kesika P, Chaiyasut C. Effect of Probiotics Supplementations on Health Status of Athletes. International Journal of Environmental Research and Public Health. 2019; 16(22):4469. https://doi.org/10.3390/ijerph16224469
Chicago/Turabian StyleSivamaruthi, Bhagavathi Sundaram, Periyanaina Kesika, and Chaiyavat Chaiyasut. 2019. "Effect of Probiotics Supplementations on Health Status of Athletes" International Journal of Environmental Research and Public Health 16, no. 22: 4469. https://doi.org/10.3390/ijerph16224469
APA StyleSivamaruthi, B. S., Kesika, P., & Chaiyasut, C. (2019). Effect of Probiotics Supplementations on Health Status of Athletes. International Journal of Environmental Research and Public Health, 16(22), 4469. https://doi.org/10.3390/ijerph16224469







