Intraoperative Blood Pressure Variability Predicts Postoperative Mortality in Non-Cardiac Surgery—A Prospective Observational Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lonjaret, L.; Lairez, O.; Minville, V.; Geeraerts, T. Optimal perioperative management of arterial blood pressure. Integr. Blood Press Control 2014, 7, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packiasabapathy, K.S.; Subramaniam, B. Optimal Perioperative Blood Pressure Management. Adv. Anesth. 2018, 36, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Kucewicz-Czech, E.; Krzych, Ł.J.; Ligowski, M. Perioperative haemodynamic optimisation in patients undergoing non-cardiac surgery—A position statement from the Cardiac and Thoracic Anaesthesia Section of the Polish Society of Anaesthesiology and Intensive Therapy. Part 1. Anaesthesiol. Intensive Ther. 2017, 49, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Spaite, D.W.; Hu, C.; Bobrow, B.J.; Chikani, V.; Sherrill, D.; Barnhart, B.; Gaither, J.B.; Denninghoff, K.R.; Viscusi, C.; Mullins, T.; et al. Mortality and Prehospital Blood Pressure in Major Traumatic Brain Injury: The Absence of a Hypotension Threshold. JAMA Surg. 2017, 152, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Monk, T.G.; Bronsert, M.R.; Henderson, W.G.; Mangione, M.P.; Sum-Ping, S.T.; Bentt, D.R.; Nguyen, J.D.; Richman, J.S.; Meguid, R.A.; Hammermeister, K.E. Association between Intraoperative Hypotension and Hypertension and 30-day Postoperative Mortality in Noncardiac Surgery. Anesthesiology 2015, 123, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.; McCormick, P.J.; Sessler, D.I.; Reich, D.L.; You, J.; Mascha, E.J.; Castillo, J.G.; Levin, M.A.; Duncan, A.E. Prolonged concurrent hypotension and low bispectral index (‘double low’) are associated with mortality, serious complications, and prolonged hospitalization after cardiac surgery. Br. J. Anaesth. 2017, 119, 40–49. [Google Scholar] [CrossRef]
- Balzer, F.; Aronson, S.; Campagna, J.A.; Ding, L.; Treskatsch, S.; Spies, C.; Sander, M. High Postoperative Blood Pressure After Cardiac Surgery Is Associated with Acute Kidney Injury and Death. J. Cardiothorac. Vasc. Anesth. 2016, 30, 1562–1570. [Google Scholar] [CrossRef]
- Martin, L.A.; Finlayson, S.R.G.; Brooke, B.S. Patient Preparation for Transitions of Surgical Care: Is Failing to Prepare Surgical Patients Preparing Them to Fail? World J. Surg. 2017, 41, 1447–1453. [Google Scholar] [CrossRef]
- Jinadasa, S.P.; Mueller, A.; Prasad, V.; Subramaniam, K.; Heldt, T.; Novack, V.; Subramaniam, B. Blood Pressure Coefficient of Variation and Its Association with Cardiac Surgical Outcomes. Anesth. Analg. 2018, 127, 832–839. [Google Scholar] [CrossRef]
- Aronson, S.; Stafford-Smith, M.; Phillips-Bute, B.; Shaw, A.; Gaca, J.; Newman, M. Intraoperative systolic blood pressure variability predicts 30-day mortality in aortocoronary bypass surgery patients. Anesthesiology 2010, 113, 305–312. [Google Scholar] [CrossRef]
- Levin, M.A.; Fischer, G.W.; Lin, H.M.; McCormick, P.J.; Krol, M.; Reich, D.L. Intraoperative arterial blood pressure lability is associated with improved 30-day survival. Br. J. Anaesth. 2015, 115, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Doyle, D.J.; Garmon, E.H. American Society of Anesthesiologists Classification (ASA Class). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, January 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441940/ (accessed on 19 January 2019).
- Guarracino, F.; Baldassarri, R.; Priebe, H.J. Revised ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management. Implications for preoperative clinical evaluation. Minerva Anestesiol. 2015, 81, 226–233. [Google Scholar] [PubMed]
- Act of 5 December 1996 on the Medical Profession. Available online: prawo.sejm.gov.pl: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU19970280152 (accessed on 28 October 2019).
- Kuck, K.; Baker, P.D. Perioperative Noninvasive Blood Pressure Monitoring. Anesth. Analg. 2018, 127, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Whittle, J. Blood Pressure Variability Predicts Clinical Outcomes: Now What? Hypertension 2017, 69, 584–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesan, S.; Myles, P.R.; Manning, H.J.; Mozid, A.M.; Andersson, C.; Jørgensen, M.E.; Hardman, J.G.; Moonesinghe, S.R.; Foex, P.; Mythen, M.; et al. Cohort study of preoperative blood pressure and risk of 30-day mortality after elective non-cardiac surgery. Br. J. Anaesth. 2017, 119, 65–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascha, E.J.; Yang, D.; Weiss, S.; Sessler, D.I. Intraoperative Mean Arterial Pressure Variability and 30-day Mortality in Patients Having Noncardiac Surgery. Anesthesiology 2015, 123, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Vernooij, L.M.; van Klei, W.A.; Machina, M.; Pasma, W.; Beattie, W.S.; Peelen, L.M. Different methods of modelling intraoperative hypotension and their association with postoperative complications in patients undergoing non-cardiac surgery. Br. J. Anaesth. 2018, 120, 1080–1089. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.Y.; Chung, A.M.; Farkouh, M.E.; van Diepen, S.; Weinberger, J.; Bourke, M.; Ruel, M. Defining an Intraoperative Hypotension Threshold in Association with Stroke in Cardiac Surgery. Anesthesiology. 2018, 129, 440–447. [Google Scholar] [CrossRef]
- Wendon, J. Critical care “normality”: Individualized versus protocolized care. Crit. Care Med. 2010, 38 (Suppl. 10), 590–599. [Google Scholar] [CrossRef]
- Sessler, D.I.; Khanna, A.K. Perioperative myocardial injury and the contribution of hypotension. Intensive Care Med. 2018, 44, 811–822. [Google Scholar] [CrossRef]
- Sun, L.Y.; Wijeysundera, D.N.; Tait, G.A.; Beattie, W.S. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology 2015, 123, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Parati, G.; Ochoa, J.E.; Lombardi, C.; Bilo, G. Blood pressure variability: Assessment, predictive value, and potential as a therapeutic target. Curr. Hypertens. Rep. 2015, 17, 537. [Google Scholar] [CrossRef] [PubMed]
- Gajniak, D.; Mendrala, K.; Gierek, D.; Krzych, Ł.J. Inter-arm differences in blood pressure among subjects with disseminated atherosclerosis scheduled for vascular surgery. Anaesthesiol. Intensive Ther. 2018, 50, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Checketts, M.R.; Alladi, R.; Ferguson, K.; Gemmell, L.; Handy, J.M.; Klein, A.A.; Love, N.J.; Misra, U.; Morris, C.; Nathanson, M.H.; et al. Recommendations for standards of monitoring during anaesthesia and recovery 2015: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 2016, 71, 85–93. [Google Scholar] [CrossRef]
- Rinehart, J.; Ma, M.; Calderon, M.D.; Bardaji, A.; Hafiane, R.; Van der Linden, P.; Joosten, A. Blood pressure variability in surgical and intensive care patients: Is there a potential for closed-loop vasopressor administration? Anaesth. Crit. Care Pain Med. 2019, 38, 69–71. [Google Scholar] [CrossRef]
- Fowler, A.J.; Ahmad, T.; Abbott, T.E.F.; Torrance, H.D.; Wouters, P.F.; De Hert, S.; Lobo, S.M.; Rasmussen, L.S.; Della Rocca, G.; Beattie, W.S.; et al. Association of preoperative anaemia with postoperative morbidity and mortality: An observational cohort study in low-, middle-, and high-income countries. Br. J. Anaesth. 2018, 121, 1227–1235. [Google Scholar] [CrossRef]
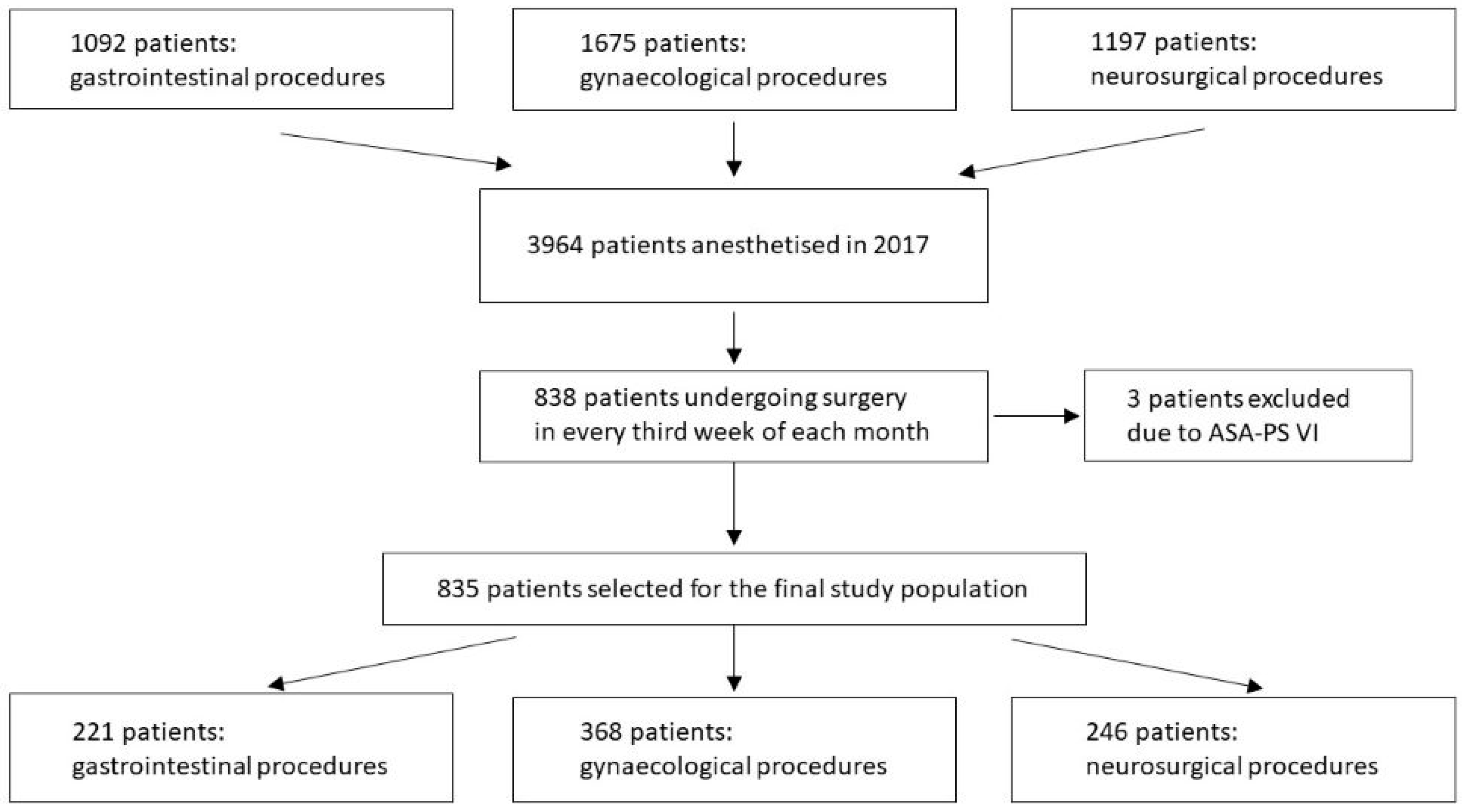

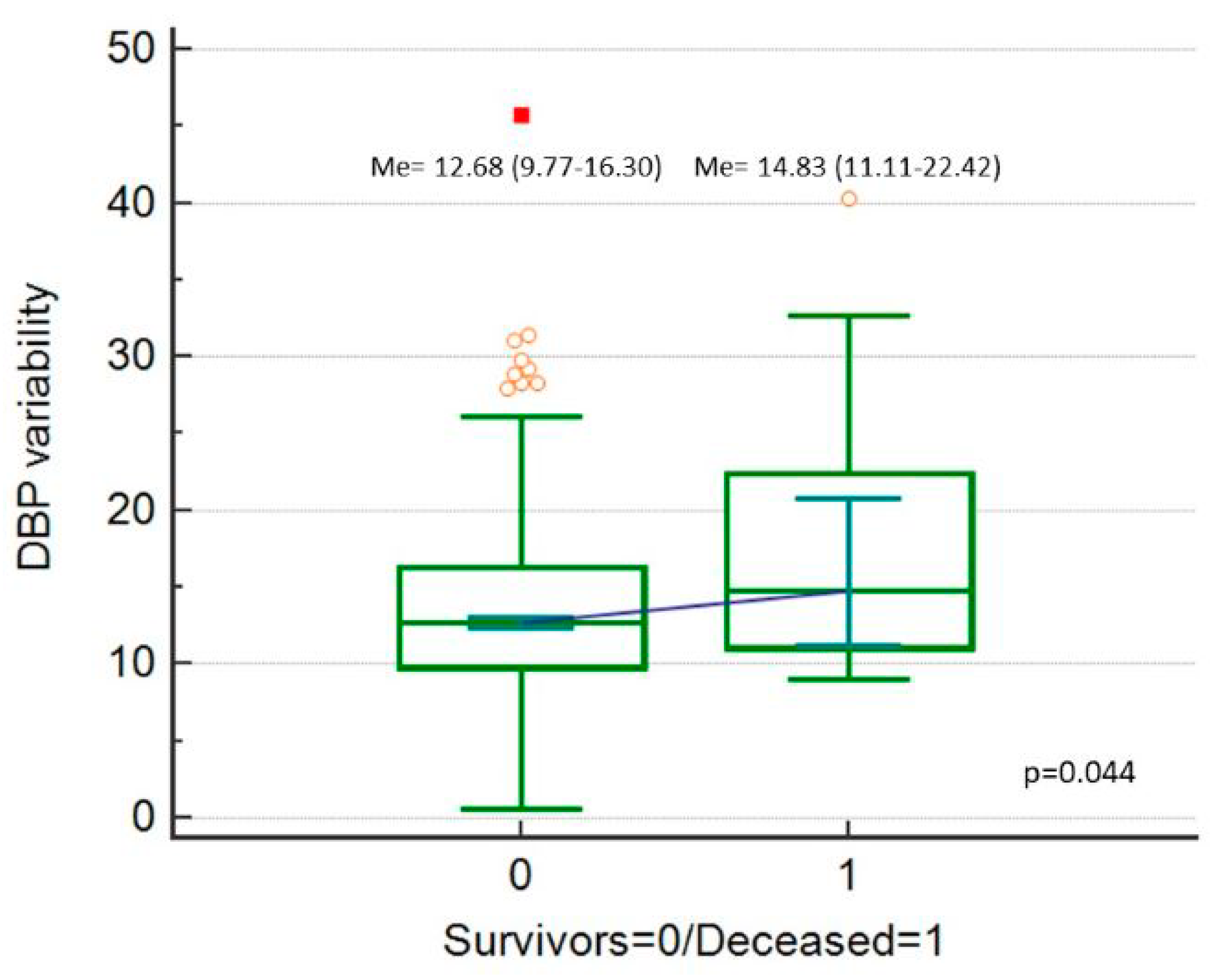
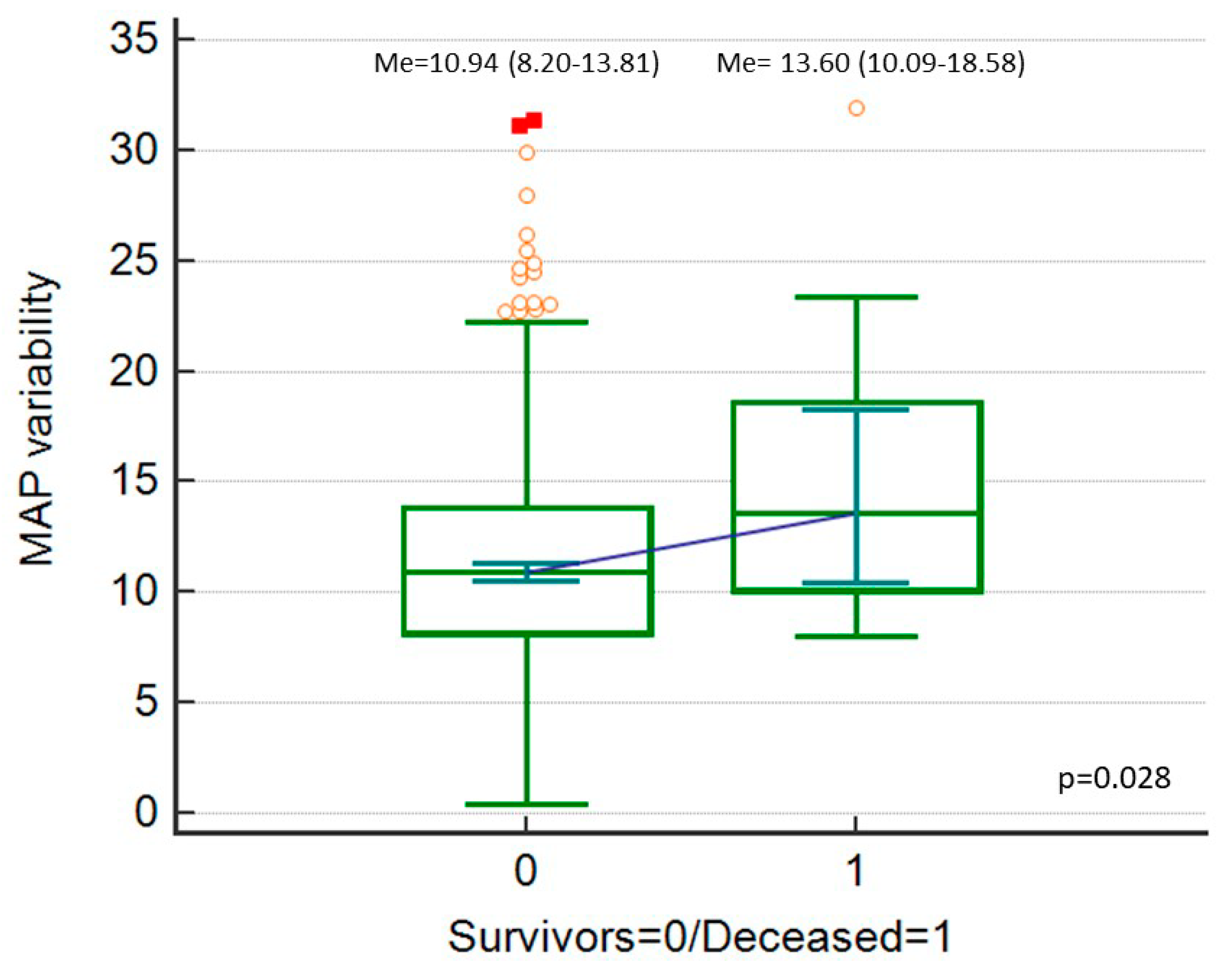
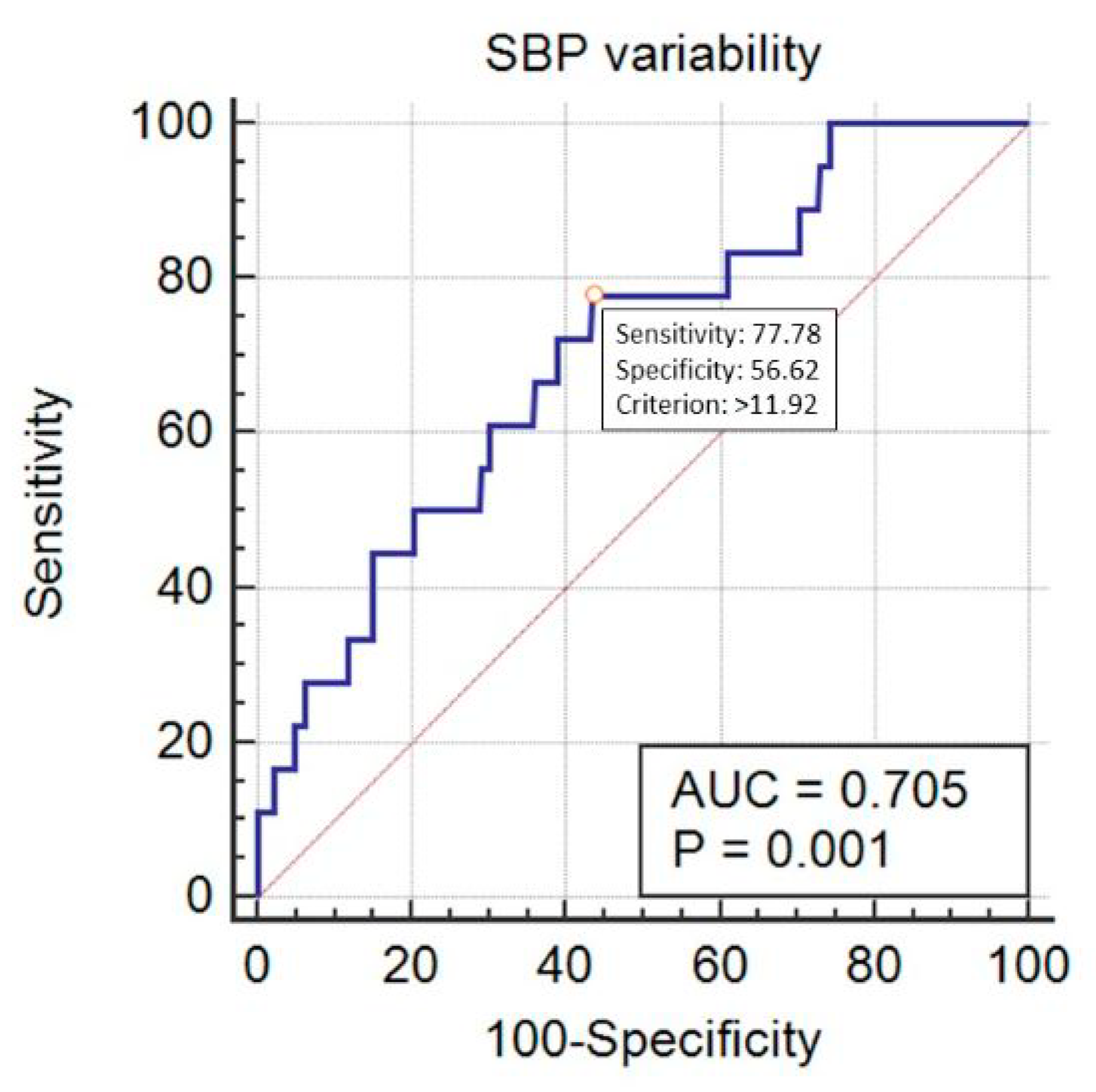
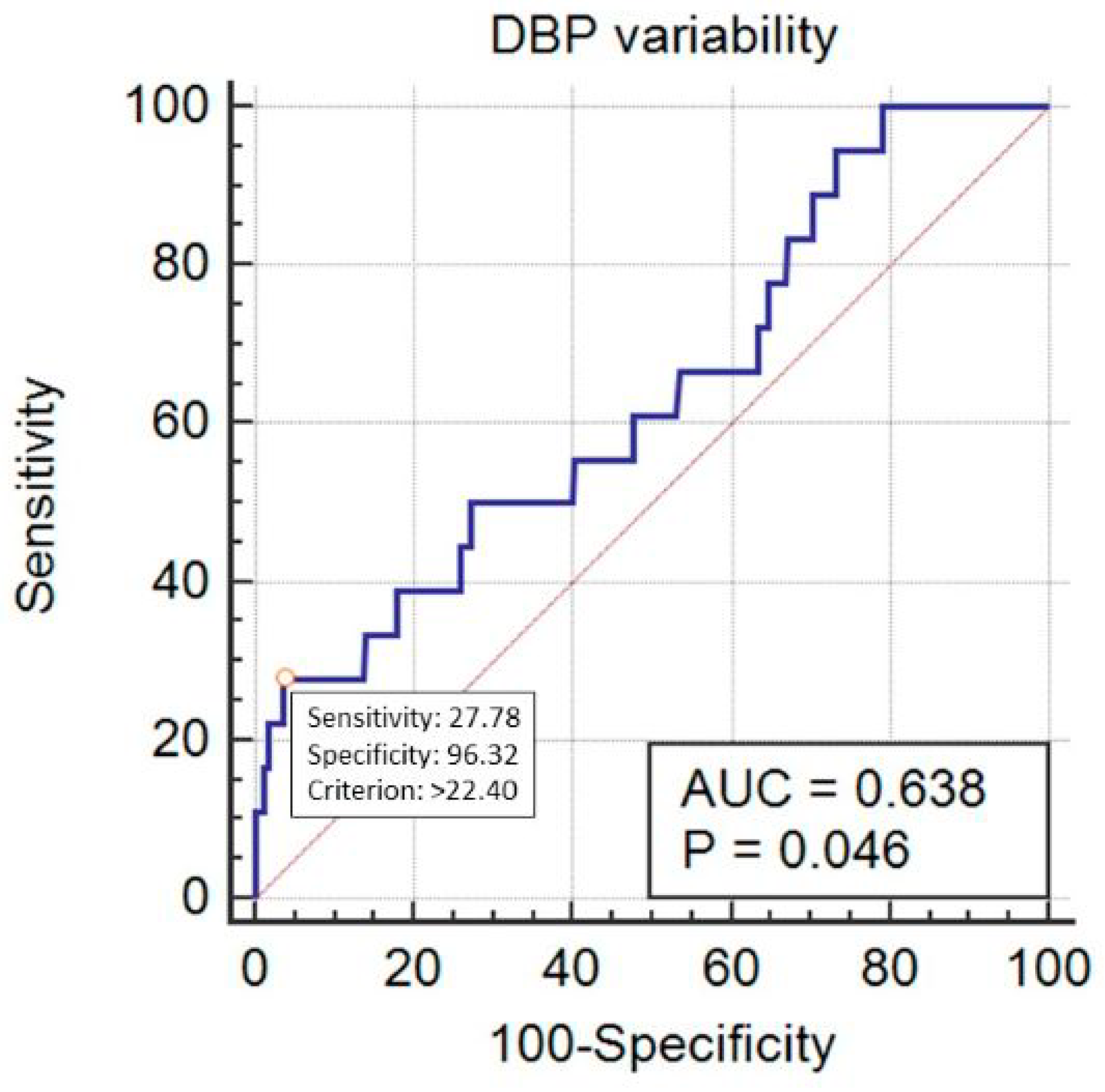
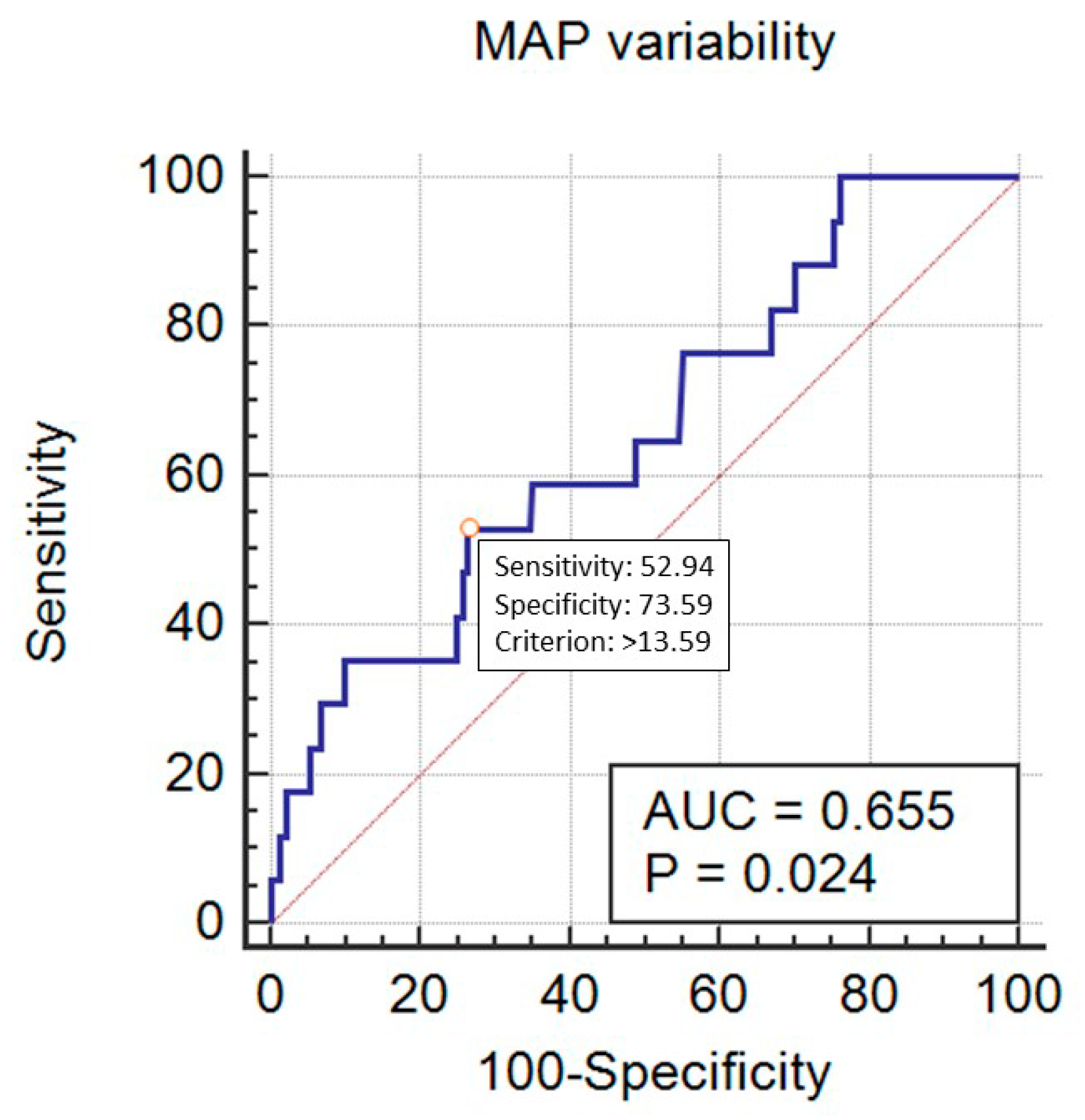
| Variable | Category | Value |
|---|---|---|
| Sex | Males | 231 (27.7%) |
| Females | 604 (72.3%) | |
| Age | (years) | 48 (34–62) |
| Body Mass Index | (kg/m2) | 26.0 (22.9–29.6) |
| American Society of Anesthesiology Physical Status (ASA-PS) class | Median class (IQR) | 2 (2–3) |
| Class I | 204 (24.4%) | |
| Class II | 356 (42.6%) | |
| Class III | 203 (24.3%) | |
| Class IV | 65 (7.8%) | |
| Class V | 7 (0.8%) | |
| Urgency of surgery | Emergency surgery | 137 (16.4%) |
| Non-emergency surgery (elective + urgent) | 698 (83.6%) | |
| Length of anaesthesia | (minutes) | 100 (60–160) |
| Type of anaesthesia | Volatile | 556 (66.6%) |
| TIVA | 92 (11.0%) | |
| Regional or combined (regional + general) | 187 (22.4%) | |
| Surgical position | Supine | 350 (41.9%) |
| Gynecological | 352 (42.1%) | |
| Prone | 82 (9.8%) | |
| Supine + lateral | 24 (2.9%) | |
| Sitting | 13 (1.6%) | |
| Lateral | 9 (1.1%) | |
| Risk of the procedure according to ESC/ESA | Low | 243 (29.1%) |
| Intermediate | 509 (61.0%) | |
| High | 83 (9.9%) |
| Variable | Category | SBP_Cv (%) | DBP_Cv (%) | MAP_Cv (%) |
|---|---|---|---|---|
| Sex | Males | 11.45 (8.88–14.70) | 12.48 (10.15–16.07) | 10.58 (8.68–14.04) |
| Females | 11.10 (8.08–14.47) | 12.76 (9.59–16.43) | 11.10 (7.98–13.78) | |
| ‘p’ | 0.042 | 0.803 | 0.740 | |
| ASA-PS class | Class I | 9.34 (6.68–12.07) | 11.79 (8.27–14.77) | 9.67 (6.91–12.55) |
| Class II | 10.99 (8.08–13.94) | 12.40 (9.61–16.07) | 10.58 (8.32–13.68) | |
| Class III | 13.43 (10.51–15.89) | 13.66 (11.06–17.02) | 12.12 (9.76–14.78) | |
| Class IV | 13.72 (9.97–17.27) | 13.70 (11.05–18.39) | 12.55 (9.44–16.10) | |
| Class V | 10.13 (9.24–29.75) | 17.81 (12.28–31.33) | 13.31 (7.71–30.36) | |
| ‘p’ | <0.001 | <0.001 | <0.001 | |
| Urgency of surgery | Emergency | 11.00 (8.49–14.73) | 12.75 (10.43–17.15) | 11.05 (8.30–13.85) |
| Other | 11.28 (8.39–14.59) | 12.68 (9.77–16.19) | 10.94 (8.25–13.85) | |
| ‘p’ | 0.8 | 0.4 | 0.499 | |
| Type of anaesthesia | TIVA | 8.04 (4.82–11.13) | 8.98 (5.76–13.01) | 7.45 (4.41–11.15) |
| Other | 11.55 (8.81–14.89) | 13.01 (10.30–16.51) | 11.35 (8.81–14.13) | |
| ‘p’ | <0.001 | <0.001 | <0.001 | |
| Type of surgery | Gastrointestinal | 12.32 (9.60–14.64) | 12.86 (10.47–16.23) | 11.15 (9.00–13.77) |
| Gynecological | 9.86 (6.95–13.29) | 11.98 (8.46–15.84) | 10.39 (6.94–13.29) | |
| Neurosurgery | 12.32 (9.27–16.02) | 13.35 (10.62–17.40) | 11.53 (8.94–15.05) | |
| ‘p’ | <0.001 | <0.001 | <0.001 | |
| Surgical position | Sitting/gynecological | 9.78 (6.97–12.97) | 11.89 (8.55–15.87) | 10.37 (6.97–13.37) |
| Horizontal | 12.38 (9.47–15.46) | 13.06 (10.48–16.65) | 11.42 (9.00–14.47) | |
| ‘p’ | <0.001 | <0.001 | <0.001 | |
| Risk of the procedure | Low | 8.84 (6.13–12.15) | 11.24 (7.11–15.20) | 9.65 (5.99–13.10) |
| Intermediate | 11.69 (9.09–14.93) | 13.22 (10.60–16.51) | 11.41 (8.88–14.10) | |
| High | 12.78 (10.93–15.46) | 13.36 (10.43–15.93) | 11.57 (9.31–13.74) | |
| ‘p’ | <0.001 | <0.001 | <0.001 |
| Variable | SBP_Cv (%) | DBP_Cv (%) | MAP_Cv (%) |
|---|---|---|---|
| Age (years) | R = 0.386 p < 0.0001 | R = 0.125 p = 0.0003 | R = 0.217 P < 0.0001 |
| BMI (kg/m2) | R = 0.130 p = 0.0001 | R = 0.103 p = 0.004 | R = 0.141 P < 0.001 |
| Duration of anaesthesia (min) | R = 0.228 p < 0.0001 | R = 0.156 p < 0.0001 | R = 0.106 P = 0.002 |
| Variable | Mortality Prediction by SBP Variability | Mortality Prediction by DBP Variability | Mortality Prediction by MAP Variability |
|---|---|---|---|
| SBP_Cv (per 1%) | OR = 1.10; 95% CI 1.00–1.21; p = 0.05 | - | - |
| DBP_Cv (per 1%) | - | OR = 1.10; 95% CI 1.01–1.21; p = 0.03 | - |
| MAP_Cv (per 1%) | - | - | OR = 1.10; 95% CI 0.99–1.23, p = 0.06 |
| Sex (Female = 0/Male = 1) | OR = 1.05; 95% CI 0.34–3.28; p = 0.9 | OR = 1.04; 95% CI 0.33–3.28, p = 0.9 | OR = 1.00; 95% CI 0.31–3.21, p = 0.9 |
| Age (per 1 year) | OR = 1.01; 95% CI 0.97–1.05; p = 0.7 | OR =1.01; 95% CI 0.98–1.05; p = 0.4 | OR=1.01; 95% CI 0.97–1.05, p = 0.5 |
| BMI (per 1 kg/m2) | OR = 0.90; 95% CI 0.81–1.00; p = 0.05 | OR = 0.894; 95% CI 0.80–1.00; p = 0.04 | OR = 0.897; 95% CI 0.8–1.0, p = 0.05 |
| Patient’s risk (ASA-PS class I–V) | OR = 2.21; 95% CI 1.02–4.79; p = 0.04 | OR = 2.10; 95% CI 0.97–4.53; p = 0.06 | OR = 2.12; 95% CI 0.96–4.69, p = 0.06 |
| Urgency of surgery (Emergency=1/Non-emergency = 0) | OR = 2.61; 95% CI 0.64–10.70; p = 0.18 | OR = 2.80; 95% CI 0.69–11.32; p = 0.1 | OR = 2.66; 95% CI 0.62–11.3, p = 0.18 |
| Duration of anesthesia (per 1 min) | OR = 1.00; 95% CI 0.99–1.01; p = 0.3 | OR = 1.00; 95% CI 0.99–1.01; p = 0.4 | OR = 1.00; 95% CI 0.99–1.01, p = 0.2 |
| Type of anesthesia (TIVA = 1/Other = 0) | OR = 1.09; 95% CI 0.12–10.01; p = 0.9 | OR = 1.10; 95% CI 0.12–10.30; p = 0.9 | OR = 1.07; 95% CI 0.11–1.05, p = 0.9 |
| Surgical position (Sitting or gynecological = 0/Horizontal = 1) | OR = 1.55; 95% CI 0.28–8.56; p = 0.6 | OR = 1.56; 95% CI 0.28–8.64; p = 0.6 | OR = 1.59; 95% CI 0.28–8.95, p = 0.6 |
| Procedure’s risk (Low = 0/Intermediate = 1/High = 2 | OR = 1.02; 95% CI 0.21–5.04; p = 0.9 | OR = 1.15; 95% CI 0.23–5.74; p = 0.8 | OR = 0.93; 95% CI 0.18–4.79, p = 0.9 |
| AUC for the final logistic model | 0.884; 95% CI 0.859–0.906; p < 0.0001 | 0.897; 95% CI 0.873–0.918; p < 0.0001 | 0.892; 95% CI 0.867–0.913 0.0003 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiórek, A.; Krzych, Ł.J. Intraoperative Blood Pressure Variability Predicts Postoperative Mortality in Non-Cardiac Surgery—A Prospective Observational Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 4380. https://doi.org/10.3390/ijerph16224380
Wiórek A, Krzych ŁJ. Intraoperative Blood Pressure Variability Predicts Postoperative Mortality in Non-Cardiac Surgery—A Prospective Observational Cohort Study. International Journal of Environmental Research and Public Health. 2019; 16(22):4380. https://doi.org/10.3390/ijerph16224380
Chicago/Turabian StyleWiórek, Agnieszka, and Łukasz J. Krzych. 2019. "Intraoperative Blood Pressure Variability Predicts Postoperative Mortality in Non-Cardiac Surgery—A Prospective Observational Cohort Study" International Journal of Environmental Research and Public Health 16, no. 22: 4380. https://doi.org/10.3390/ijerph16224380
APA StyleWiórek, A., & Krzych, Ł. J. (2019). Intraoperative Blood Pressure Variability Predicts Postoperative Mortality in Non-Cardiac Surgery—A Prospective Observational Cohort Study. International Journal of Environmental Research and Public Health, 16(22), 4380. https://doi.org/10.3390/ijerph16224380






