Field Research on Mixing Aeration in a Drinking Water Reservoir: Performance and Microbial Community Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites and Field Work
2.2. Physical and Chemical Analysis
2.3. High-Throughput Sequencing
2.4. Data Analysis
3. Results and Discussion
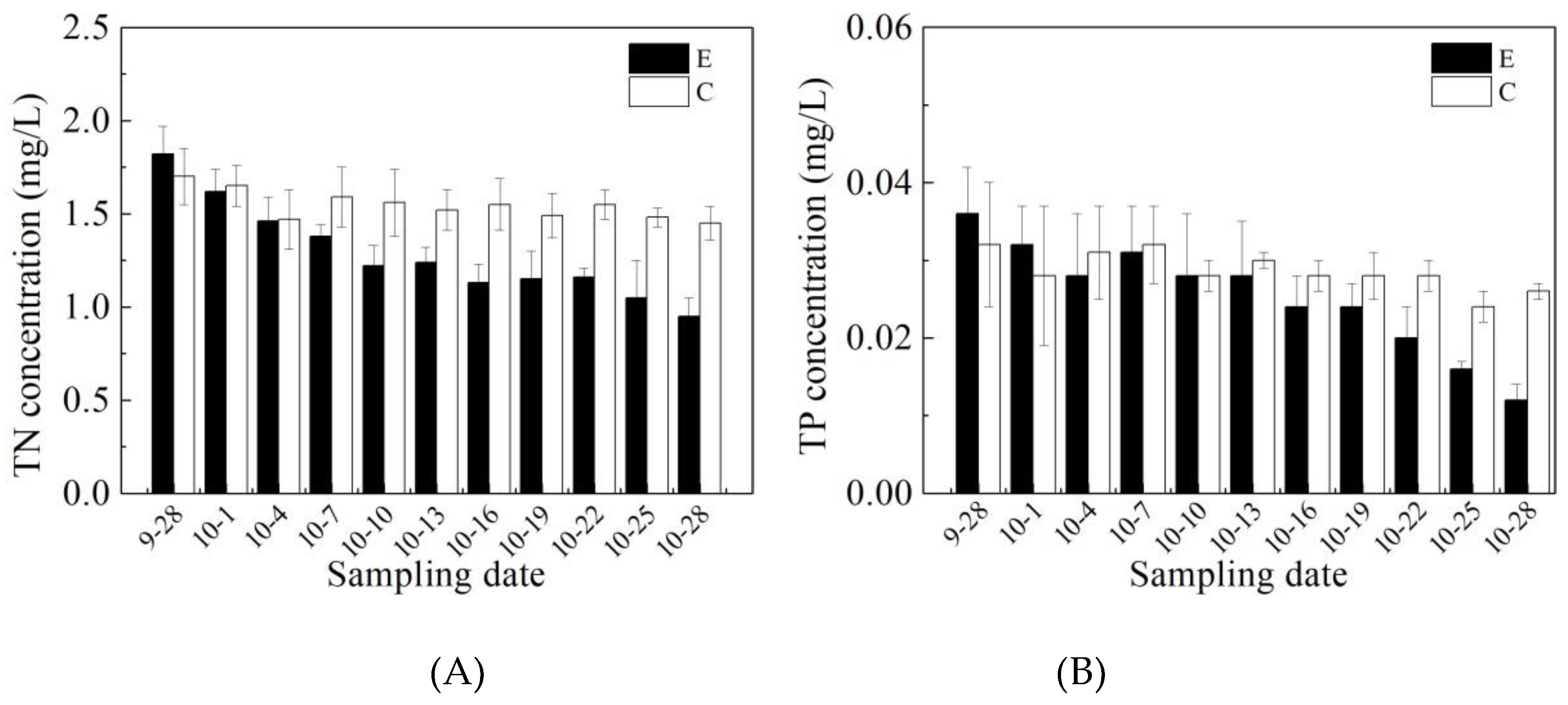
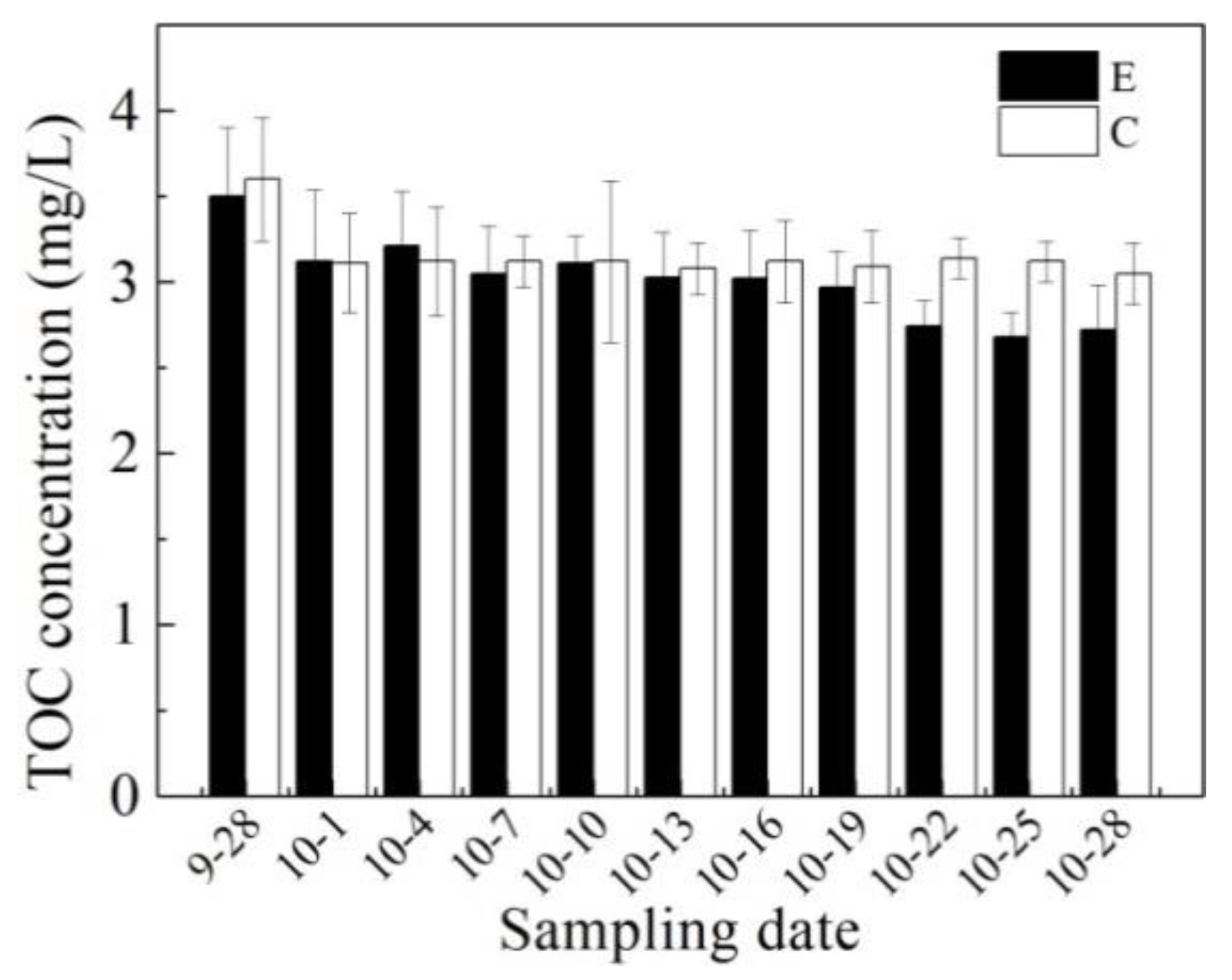
3.1. Effects of Mixing Aeration on Water Quality
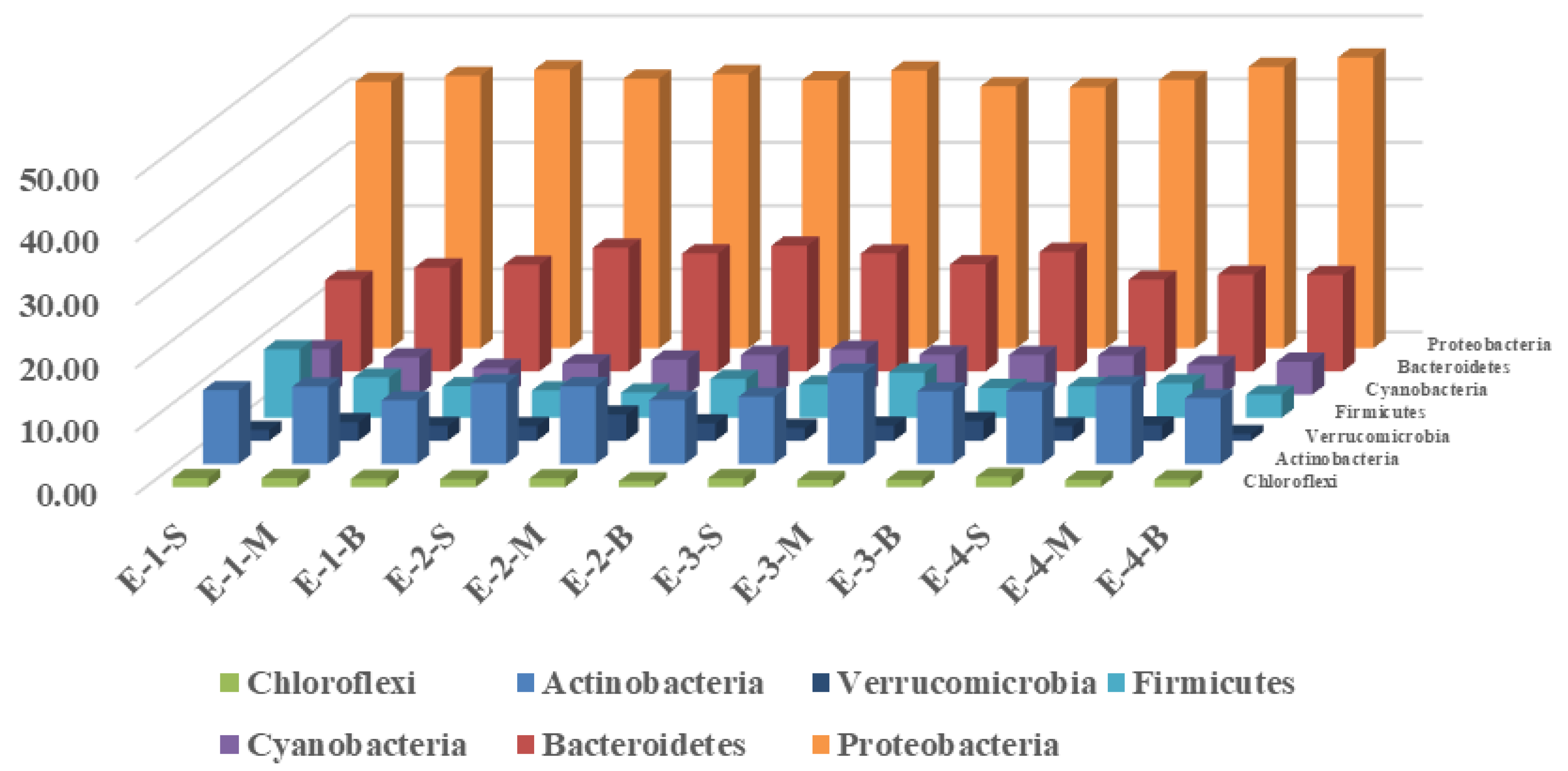
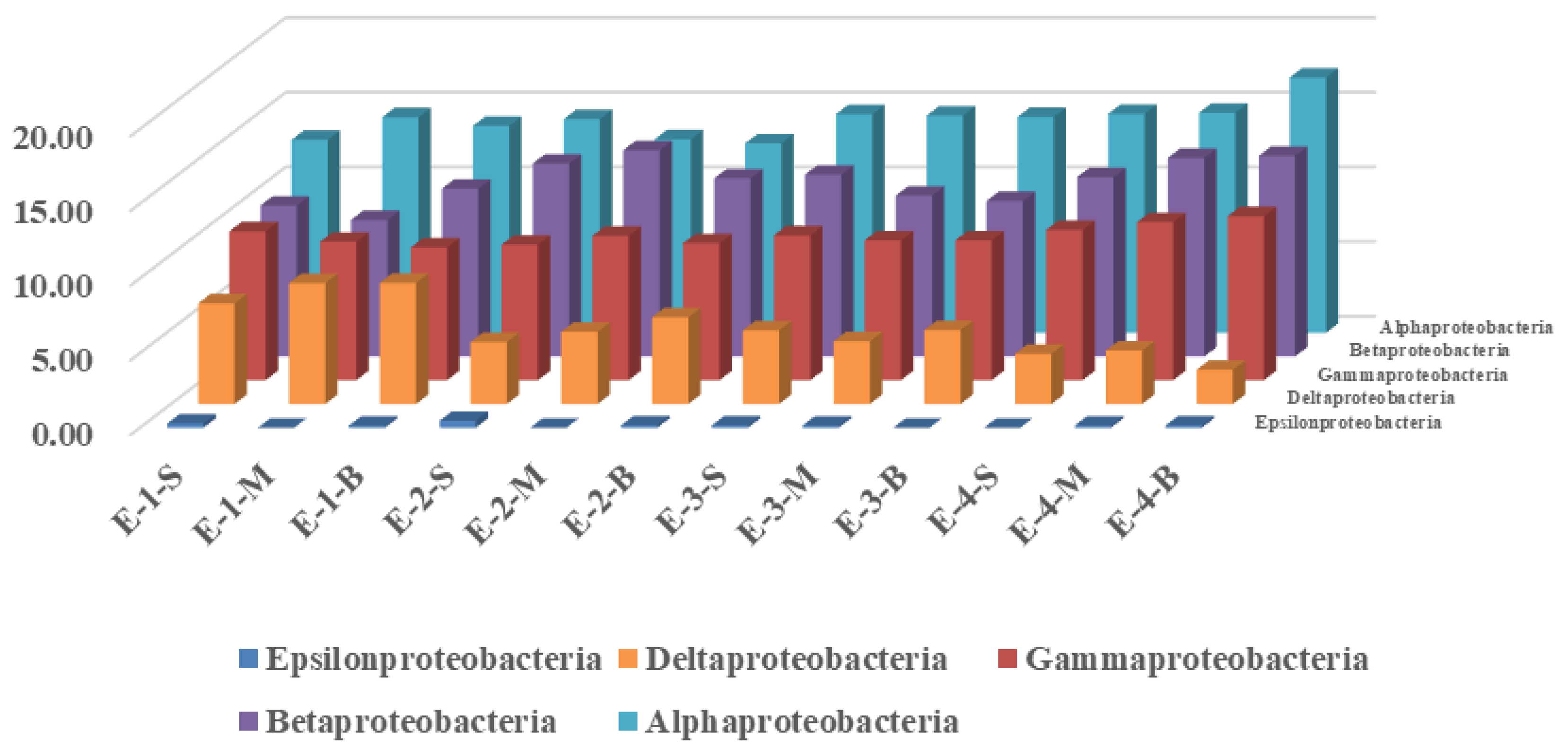
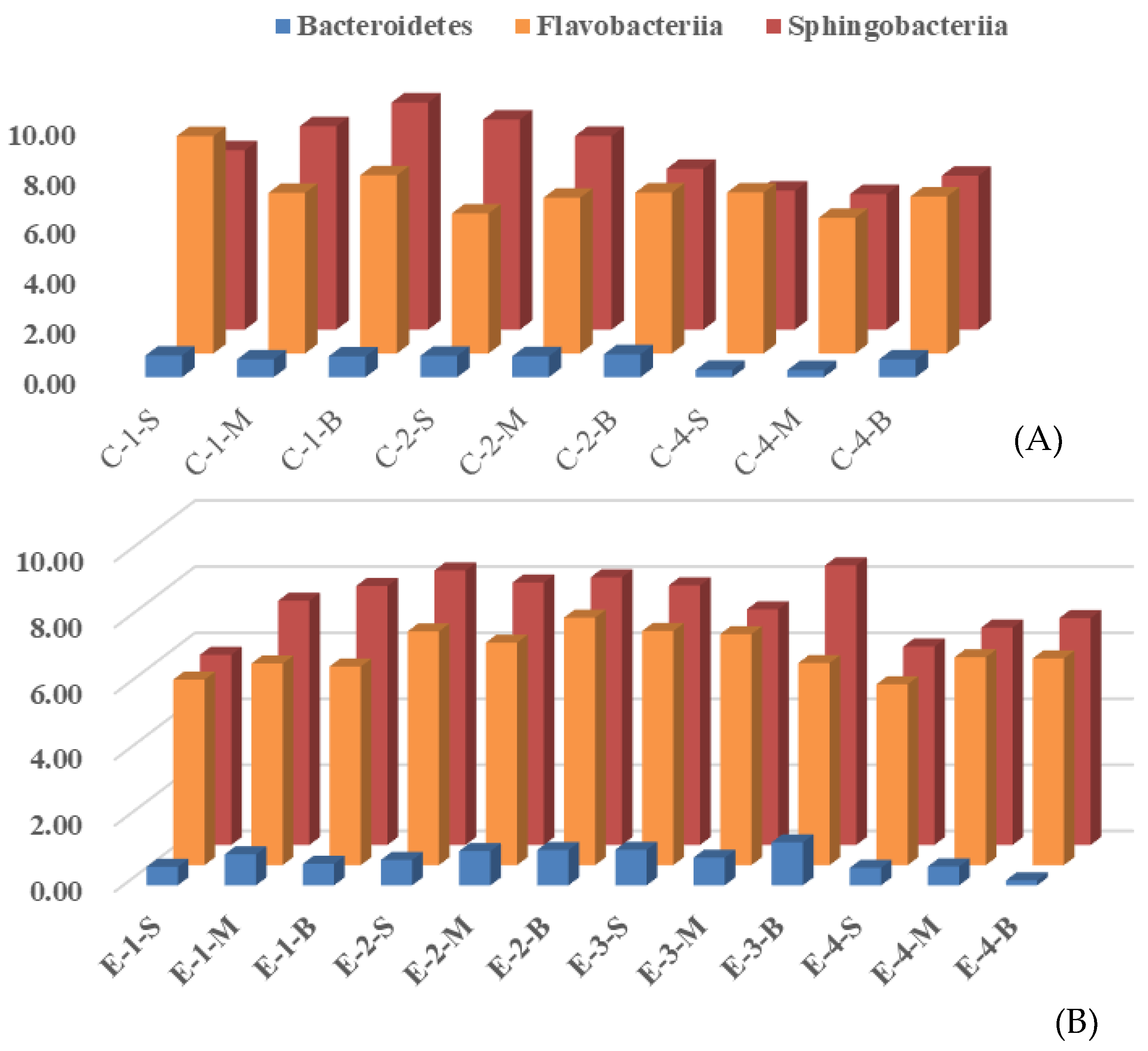
3.2. Effects of Mixing Aeration on Microbial Community Structure
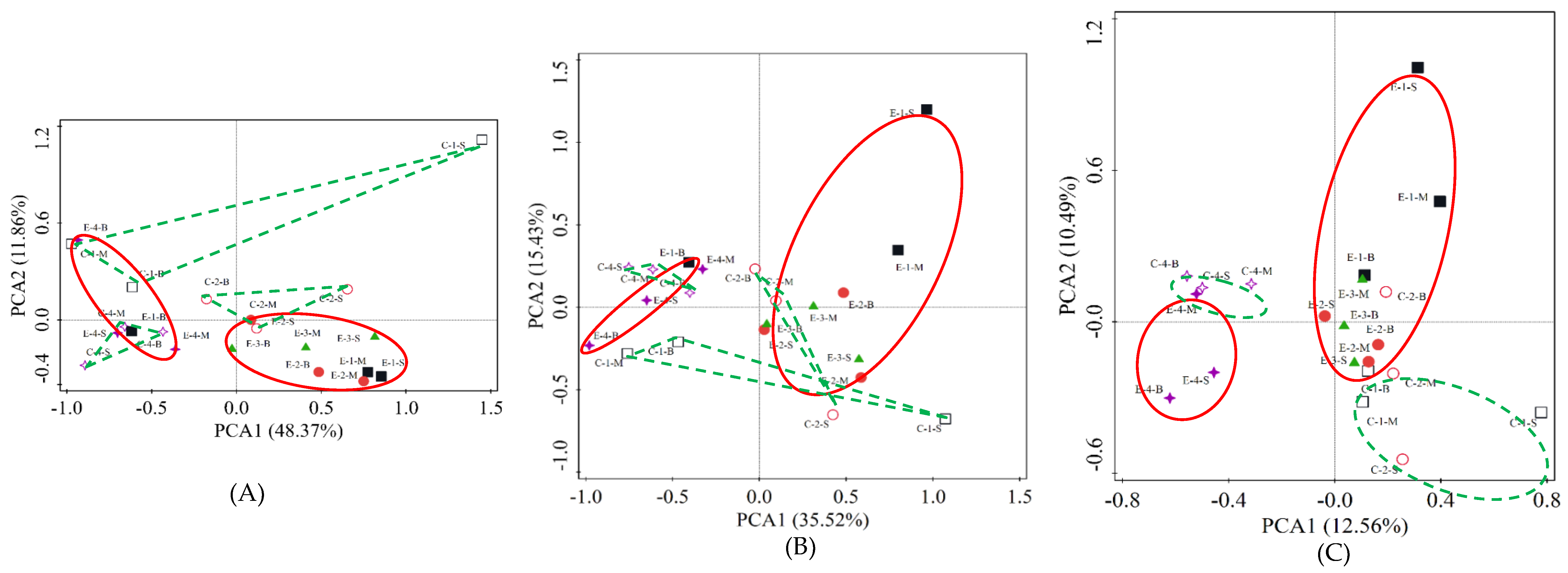
3.3. PCA of Microbial Community
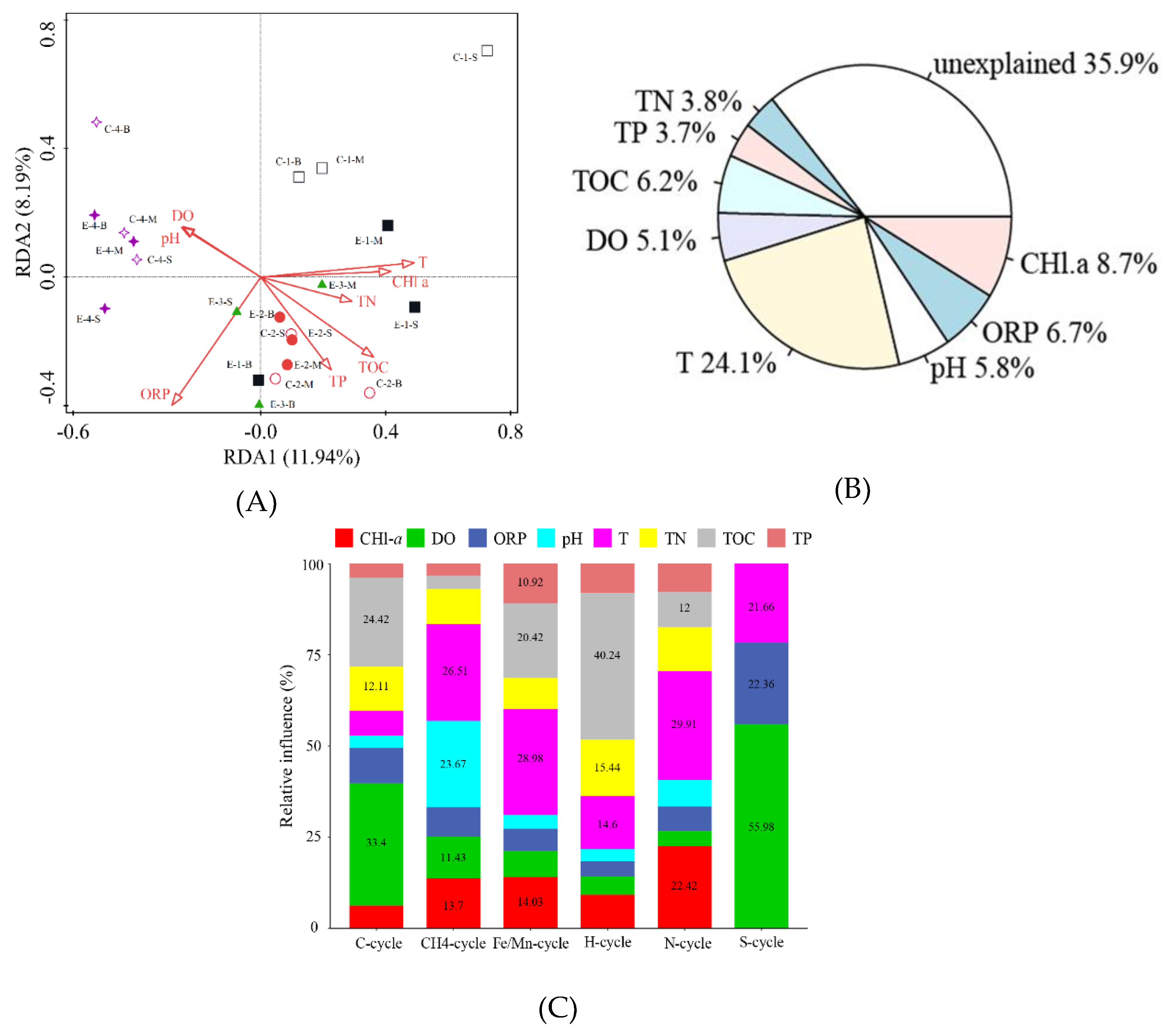
3.4. Analysis of the Relationship between Environmental Factors and Microbial Communities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, M.; Wu, F.; Yuan, M.; Li, Q.; Gu, Y.; Wang, Y.; Liu, Q. Antioxidant responses of triangle sail mussel Hyriopsis cumingii exposed to harmful algae Microcystis aeruginosa and hypoxia. Chemosphere 2015, 139, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zheng, B.; Qin, Y.; Ma, Y.; Yang, C.; Liu, Z.; Chi, M. Impact of upstream river inputs and reservoir operation on phosphorus fractions in water-particulate phases in the Three Gorges Reservoir. Sci. Total Environ. 2017, 610, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Huang, T.; Li, Y.; Ma, W.; Zhou, S.; Long, S. Sediment pollution characteristics and in situ control in a deep drinking water reservoir. J. Environ. Sci. 2017, 52, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Zhao, Z.; Cai, Y. Heavy metal pollution in reservoirs in the hilly area of southern China: Distribution, source apportionment and health risk assessment. Sci. Total Environ. 2018, 634, 158–169. [Google Scholar] [CrossRef]
- Cheng, W.; Zhang, J.; Wang, Z.; Wang, M.; Xie, S. Bacterial communities in sediments of a drinking water reservoir. Ann. Microbiol. 2014, 64, 875–878. [Google Scholar] [CrossRef]
- Cheng, W.P.; Chi, F.H.; Yu, R.F. Evaluating the efficiency of coagulation in the removal of dissolved organic carbon from reservoir water using fluorescence and ultraviolet photometry. Environ. Monit. Assess. 2004, 98, 421–431. [Google Scholar] [CrossRef]
- Jeppesen, E.; Brucet, S.; Naselli-Flores, L.; Papastergiadou, E.; Stefanidis, K.; Noges, T.; Bucak, T. Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia 2015, 750, 201–227. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Z.; Wang, X.; Yang, J.; Luo, S.; Tian, Y.; Zhen, X. Dynamic changes of dissolved organic matter during nitrate transport in a loose-pore geothermal reservoir. Chem. Geol. 2018, 487, 76–85. [Google Scholar]
- Ding, S.; Fang, D.; Pang, Z.; Luo, B.; Kuang, L.; Wang, H.; Ji, F. Immobilization of powdery calcium silicate hydrate via PVA covalent cross-linking process for phosphorus removal. Sci. Total Environ. 2018, 645, 937–945. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, T.; Zhang, H.; Zeng, M.; Liu, F.; Bai, S.; Yang, X. Nitrogen removal characteristics of enhanced in situ indigenous aerobic denitrification bacteria for micro-polluted reservoir source water. Bioresour. Technol. 2016, 201, 195–207. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, T.; Ngo, H.H.; Zhang, H.; Liu, F.; Zeng, M.; Qiu, X. Nitrogen removal characteristics of indigenous aerobic denitrifiers and changes in the microbial community of a reservoir enclosure system via in situ oxygen enhancement using water lifting and aeration technology. Bioresour. Technol. 2016, 214, 63–73. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, L.; Wang, X. Spatiotemporal variation of bacterial and archaeal communities in sediments of a drinking reservoir, Beijing, China. Appl. Microbiol. Biotechnol. 2016, 101, 1–13. [Google Scholar] [CrossRef]
- Lin, J.; Hao, B.; Cao, G.; Wang, J.; Feng, Y.; Tan, X.; Wang, W. A study on the microbial community structure in oil reservoirs developed by water flooding. J. Pet. Sci. Eng. 2014, 122, 354–359. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, Z.; Xia, P.; Xiang, H.; Xiang, S.; Jiang, Z. Effects of wet and dry seasons on the aquatic bacterial community structure of the Three Gorges Reservoir. World J. Microbiol. Biotechnol. 2013, 29, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Cho, K.; Bae, H.; Keshvardoust, P.; Rice, S.A.; Vigneswaran, S.; Leiknes, T. Effect of microbial community structure on organic removal and biofouling in membrane adsorption bioreactor used in seawater pretreatment. Chem. Eng. J. 2016, 294, 30–39. [Google Scholar] [CrossRef]
- Fan, Z.; She, Y.H.; Zhang, X.T.; Shu, F.C.; Wang, Z.L.; Yu, L.J.; Hou, D.J. Impact of an indigenous microbial enhanced oil recovery field trial on microbial community structure in a high pour-point oil reservoir. Appl. Microbiol. Biot. 2012, 95, 811–821. [Google Scholar]
- Long, Y.; Bing, Y.; Zhang, Z.; Cui, K.; Pan, X.; Yan, X.; Guo, Q. Influence of plantation on microbial community in porous concrete treating polluted surface water. Int. Biodeter. Biodegr. 2017, 117, 8–13. [Google Scholar] [CrossRef]
- Comte, J.; Jacquet, S.; Viboud, S.; Fontvieille, D.; Millery, A.; Paolini, G.; Domaizon, I. Microbial community structure and dynamics in the largest natural French lake (lake bourget). Microb. Ecol. 2006, 52, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Zhao, G.; Xu, X.; Zhang, Q.; Shen, Q.; Fang, Z.; Ji, F. Microbial community structures and functions of wastewater treatment systems in plateau and cold regions. Bioresour. Technol. 2018, 249, 684. [Google Scholar] [CrossRef] [PubMed]
- Zhiting, H.; Bing, X.; Qi, Y.; Weiqing, X.; Jun, L. Microbial community study in newly established Qingcaosha Reservoir of Shanghai, China. Appl. Microbiol. Biot. 2014, 98, 9849–9858. [Google Scholar]
- Xu, Z.; Woodhouse, J.N.; Shu, H.T.; Gin, Y.H.; He, Y.; Xu, C.; Chen, L. Seasonal variation in the bacterial community composition of a large estuarine reservoir and response to cyanobacterial proliferation. Chemosphere 2018, 202, 576–585. [Google Scholar] [CrossRef]
- Li, Y.; Huang, T.; Tan, X.; Zhou, Z.; Ma, W. Destratification and oxygenation efficiency of a water-lifting aerator system in a deep reservoir: Implications for optimal operation. J. Environ. Sci. 2018, 73, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.X.; Huang, T.L.; Li, X. Study of the application of the water-lifting aerators to improve the water quality of a stratified, eutrophicated reservoir. Ecol. Eng. 2015, 83, 281–290. [Google Scholar] [CrossRef]
- Zhou, Z.Z.; Huang, T.L.; Ma, W.X.; Li, Y.; Zeng, K. Impacts of water quality variation and rainfall runoff on Jinpen Reservoir, in Northwest China. Water Sci. Eng. 2015, 8, 301–308. [Google Scholar]
- Yao, S.; Ni, J.; Chen, Q.; Borthwick, A.G.L. Enrichment and characterization of a bacteria consortium capable of heterotrophic nitrification and aerobic denitrification at low temperature. Bioresour. Technol. 2013, 127, 151–157. [Google Scholar] [CrossRef]
- Yu, W.; Hua-Fang, S.; Yan, H.; Jin-Ya, W.; Yun-Xia, J.; Nora Fung-Yee, T.; Hong-Wei, Z. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl. Environ. Microb. 2012, 78, 8264–8271. [Google Scholar]
- Oberauner, L.; Zachow, C.; Lackner, S.; Hã Genauer, C.; Smolle, K.H.; Berg, G. The ignored diversity: Complex bacterial communities in intensive care units revealed by 16S pyrosequencing. Sci Rep. 2013, 3, 1413. [Google Scholar] [CrossRef]
- Jami, E.; Israel, A.; Kotser, A.; Mizrahi, I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef]
- Hartmank, K.; Van Der Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.C.; Schlaeppi, K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2018, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ling, N.; Chen, H.; Zhu, C.; Kong, Y.; Wang, M.; Guo, S. Distinct drivers of activity, abundance, diversity and composition of ammonia-oxidizers: Evidence from a long-term field experiment. Soil Biol. Biochem. 2017, 115, 403–414. [Google Scholar] [CrossRef]
- Cubas, F.J.; Holbrook, R.D.; Novak, J.T.; Godrej, A.N.; Grizzard, T.J. Effective depth controls the nitrate removal rates in a water supply reservoir with a high nitrate load. Sci. Total Environ. 2019, 673, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Z.; Kang, P.; Wang, Y.; Feng, J.; Jia, J.; Zhang, Z. Biological nitrogen removal and metabolic characteristics of a novel aerobic denitrifying fungus, Hanseniaspora uvarum, strain KPL108. Bioresour. Technol. 2018, 267, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Kamjunke, N.; Oosterwoud, M.R.; Herzsprung, P.; Tittel, J. Bacterial production and their role in the removal of dissolved organic matter from tributaries of drinking water reservoirs. Sci. Total Environ. 2016, 548, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhou, J.; Yang, J.; Yu, X.; Liu, L. Vertical distribution of diazotrophic bacterial community associated with temperature and oxygen gradients in a subtropical reservoir. Hydrobiologia 2014, 741, 69–77. [Google Scholar] [CrossRef]
- Luo, J.; Liang, H.; Yan, L.; Ma, J.; Yang, Y.; Li, G. Microbial community structures in a closed raw water distribution system biofilm as revealed by 454-pyrosequencing analysis and the effect of microbial biofilm communities on raw water quality. Bioresour. Technol. 2013, 148, 189–195. [Google Scholar] [CrossRef]
- Park, Y.; Cho, H.; Yu, J.; Min, B.; Hong, S.K.; Kim, B.G.; Lee, T. Response of microbial community structure to pre-acclimation strategies in microbial fuel cells for domestic wastewater treatment. Bioresour. Technol. 2017, 233, 176–183. [Google Scholar] [CrossRef]
- Niu, A.; Song, L.Y.; Xiong, Y.H.; Lu, C.J.; Junaid, M.; Pei, D.S. Impact of water quality on the microbial diversity in the surface water along the Three Gorge Reservoir (TGR), China. Ecotoxicol. Environ. Saf. 2019, 181, 412–418. [Google Scholar] [CrossRef]
- Hassan, S.S.; Anjum, K.; Abbas, S.Q.; Akhter, N.; Shagufta, B.I.; Shah, S.A.; Tasneem, U. Emerging biopharmaceuticals from marine actinobacteria. Environ. Toxicol. Pharm. 2016, 49, 34. [Google Scholar] [CrossRef]
- Tatenda, D.; Wasserman, R.J. Cyanobacteria dynamics in a small tropical reservoir: Understanding spatio-temporal variability and influence of environmental variables. Sci. Total Environ. 2018, 643, 835–841. [Google Scholar]
- Beaver, J.R.; Tausz, C.E.; Scotese, K.C.; Pollard, A.I.; Mitchell, R.M. Environmental factors influencing the quantitative distribution of microcystin and common potentially toxigenic cyanobacteria in U.S. lakes and reservoirs. Harmful Algae 2018, 78, 118–128. [Google Scholar] [CrossRef]
- Nemani, V.A.; Mckie, M.J.; Taylor-Edmonds, L.; Andrews, R.C. Impact of biofilter operation on microbial community structure and performance. J. Water Process. Eng. 2018, 24, 35–41. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, T.; Gong, W.; Li, Y.; Liu, Y.; Zhao, F.; Ma, W. In Situ Water Quality Improvement Mechanism (Nitrogen Removal) by Water-Lifting Aerators in a Drinking Water Reservoir. Water 2018, 10, 1051. [Google Scholar] [CrossRef]
- He, W.; Luo, J.; Xing, L.; Yu, X.; Zhang, J.; Chen, S. Effects of temperature-control curtain on algae biomass and dissolved oxygen in a large stratified reservoir: Sanbanxi Reservoir case study. J. Environ. Manag. 2019, 248, 109250. [Google Scholar] [CrossRef] [PubMed]


| Sampling Time | Site (Water Depth) | Enhanced Area | Control Area | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reads | 0.97 Level | Reads | 0.97 Level | ||||||||||||
| OTUs | Diversity | Coverage | Richness | OTUs | Diversity | Coverage | Richness | ||||||||
| ACE | Chao1 | Shannon | Simpson | ACE | Chao1 | Shannon | Simpson | ||||||||
| 09–28 | 0.5 m | 24,639 | 712 | 931 | 923 | 0.9916 | 4.55 | 0.0275 | 42,439 | 461 | 617 | 616 | 0.9968 | 3.59 | 0.0566 |
| 45 m | 39,804 | 852 | 1034 | 1021 | 0.9951 | 4.85 | 0.0198 | 31,783 | 702 | 948 | 934 | 0.9932 | 4.09 | 0.0473 | |
| 90 m | 40,082 | 765 | 1019 | 1029 | 0.9943 | 4.38 | 0.0313 | 29,379 | 717 | 1205 | 1065 | 0.9914 | 4.15 | 0.0405 | |
| 10–15 | 0.5 m | 27,963 | 650 | 1105 | 1004 | 0.9920 | 4.32 | 0.029 | 27,036 | 465 | 600 | 641 | 0.9952 | 4.04 | 0.0441 |
| 45 m | 29,817 | 579 | 740 | 780 | 0.9947 | 4.22 | 0.0338 | 33,172 | 595 | 758 | 773 | 0.9953 | 4.23 | 0.0379 | |
| 90 m | 30,418 | 654 | 858 | 877 | 0.9938 | 4.45 | 0.0259 | 39,454 | 654 | 820 | 839 | 0.9958 | 4.38 | 0.0333 | |
| 10–29 | 0.5 m | 33,431 | 649 | 953 | 843 | 0.9946 | 4.47 | 0.0256 | -- | -- | -- | -- | -- | -- | -- |
| 45 m | 31,302 | 715 | 960 | 981 | 0.9930 | 4.47 | 0.0261 | -- | -- | -- | -- | -- | -- | -- | |
| 90 m | 38,056 | 768 | 1007 | 1018 | 0.9942 | 4.33 | 0.0397 | -- | -- | -- | -- | -- | -- | -- | |
| 11–28 | 0.5 m | 19,696 | 566 | 905 | 810 | 0.9905 | 4.15 | 0.0402 | 26,986 | 685 | 918 | 908 | 0.9924 | 4.41 | 0.0327 |
| 45 m | 46,082 | 699 | 1045 | 952 | 0.9957 | 4.34 | 0.0305 | 34,268 | 702 | 914 | 947 | 0.9941 | 4.37 | 0.034 | |
| 90 m | 45,379 | 655 | 1041 | 882 | 0.9955 | 3.95 | 0.0502 | 30,516 | 846 | 1111 | 1122 | 0.9918 | 4.91 | 0.0171 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Huang, T.; Gong, W.; Li, Y.; Liu, Y.; Zhou, S. Field Research on Mixing Aeration in a Drinking Water Reservoir: Performance and Microbial Community Structure. Int. J. Environ. Res. Public Health 2019, 16, 4221. https://doi.org/10.3390/ijerph16214221
Zhou Z, Huang T, Gong W, Li Y, Liu Y, Zhou S. Field Research on Mixing Aeration in a Drinking Water Reservoir: Performance and Microbial Community Structure. International Journal of Environmental Research and Public Health. 2019; 16(21):4221. https://doi.org/10.3390/ijerph16214221
Chicago/Turabian StyleZhou, Zizhen, Tinlin Huang, Weijin Gong, Yang Li, Yue Liu, and Shilei Zhou. 2019. "Field Research on Mixing Aeration in a Drinking Water Reservoir: Performance and Microbial Community Structure" International Journal of Environmental Research and Public Health 16, no. 21: 4221. https://doi.org/10.3390/ijerph16214221
APA StyleZhou, Z., Huang, T., Gong, W., Li, Y., Liu, Y., & Zhou, S. (2019). Field Research on Mixing Aeration in a Drinking Water Reservoir: Performance and Microbial Community Structure. International Journal of Environmental Research and Public Health, 16(21), 4221. https://doi.org/10.3390/ijerph16214221





