Evaluation of the Burdening on the Czech Population by Brominated Flame Retardants
Abstract
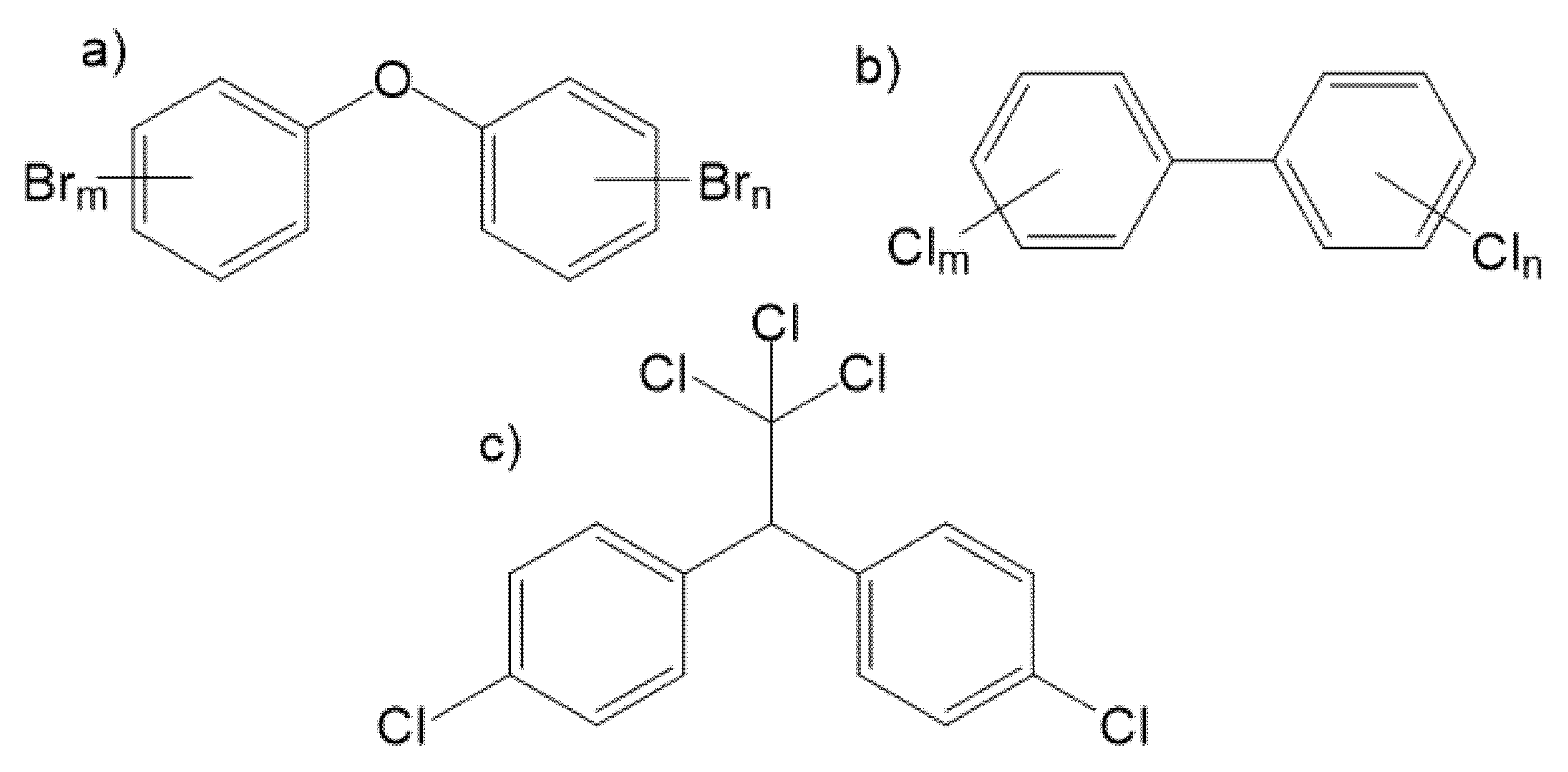
1. Introduction
2. Materials and Methods
2.1. Characteristics of Donors and Taking Fat Tissue Samples
2.2. Chemicals
2.3. Treatment of Clinical Samples
2.4. Gas Chromatography with Mass Spectrometry
3. Results and Discussions
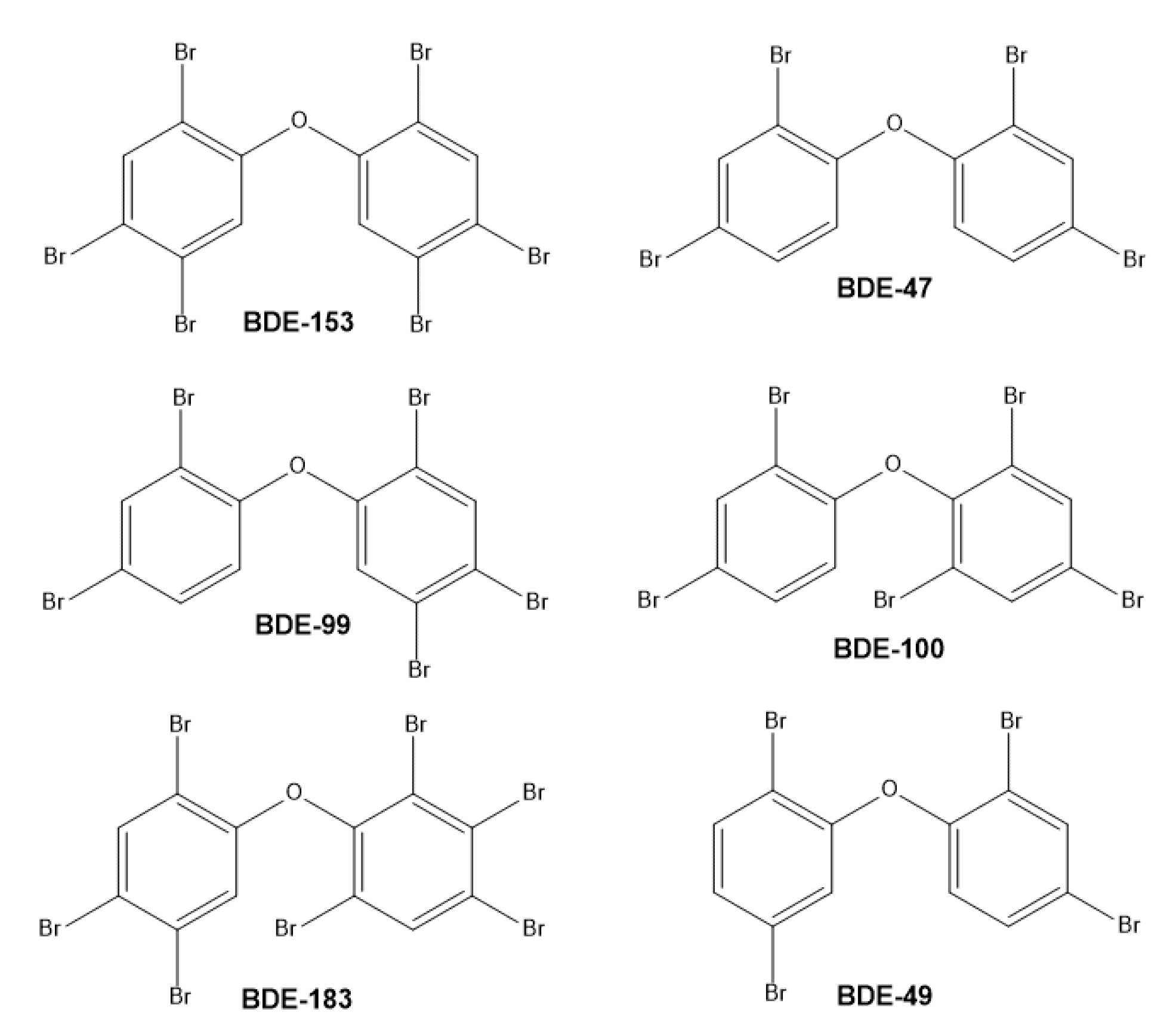
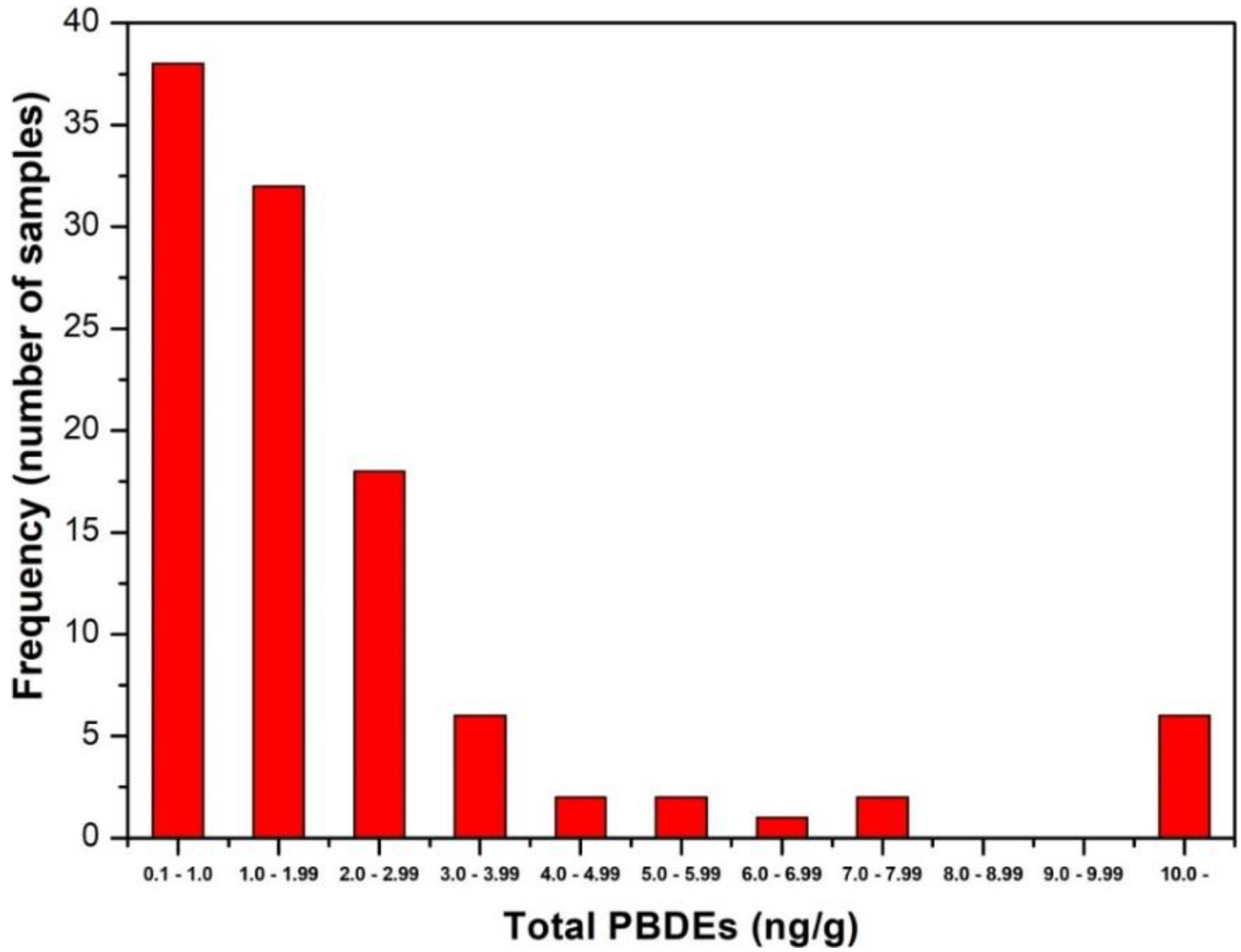
3.1. Polybrominated Diphenyl Ethers
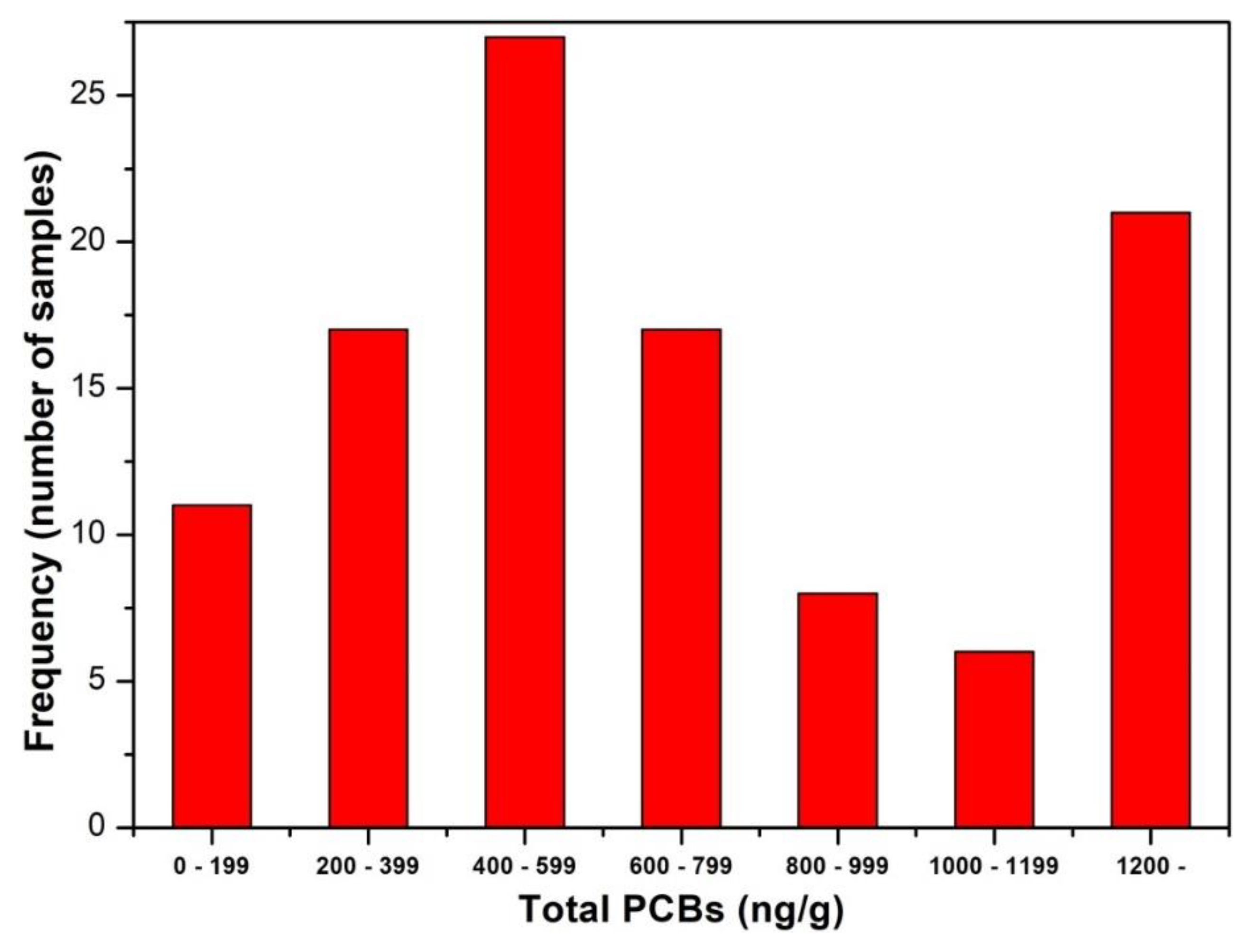
3.2. PCBs and DDTs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Environmental Health Criteria 192; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Brown, S.C.; Van den Ende, N.; Van der Veen, I. Flame retardants: Inorganic oxide and hydroxide systems. In Plastics Additives: An A–Z Reference; Pritchard, G., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 1998; pp. 287–296. [Google Scholar] [CrossRef]
- Bergman, A.; Ryden, A.; Law, R.J.; de Boer, J.; Covaci, A.; Alaee, M.; Birnbaum, L.; Petreas, M.; Rose, M.; Sakai, S.; et al. A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environ. Int. 2012, 49, 57–82. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef] [PubMed]
- Weschler, C.J.; Nazaroff, W.W. SVOC partitioning between the gas phase and settled dust indoors. Atmos. Environ. 2010, 44, 3609–3620. [Google Scholar] [CrossRef]
- Eskenazi, B.; Chevrier, J.; Rauch, S.A.; Kogut, K.; Harley, K.G.; Johnson, C.; Trujillo, C.; Sjodin, A.; Bradman, A. In Utero and Childhood Polybrominated Diphenyl Ether (PBDE) Exposures and Neurodevelopment in the CHAMACOS Study. Environ. Health Perspect. 2013, 121, 257–262. [Google Scholar] [CrossRef]
- Gomara, B.; Herrero, L.; Pacepavicius, G.; Ohta, S.; Alaee, M.; Gonzalez, M.J. Occurrence of co-planar polybrominated/chlorinated biphenyls (PXBs), polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in breast milk of women from Spain. Chemosphere 2011, 83, 799–805. [Google Scholar] [CrossRef]
- Kang, C.S.; Lee, J.H.; Kim, S.K.; Lee, K.T.; Lee, J.S.; Park, P.S.; Yun, S.H.; Kannan, K.; Yoo, Y.W.; Ha, J.Y.; et al. Polybrominated diphenyl ethers and synthetic musks in umbilical cord Serum, maternal serum, and breast milk from Seoul, South Korea. Chemosphere 2010, 80, 116–122. [Google Scholar] [CrossRef]
- Schecter, A.; Colacino, J.; Sjodin, A.; Needham, L.; Birnbaum, L. Partitioning of polybrominated diphenyl ethers (PBDEs) in serum and milk from the same mothers. Chemosphere 2010, 78, 1279–1284. [Google Scholar] [CrossRef]
- Wang, H.S.; Jiang, G.M.; Chen, Z.J.; Du, J.; Man, Y.B.; Giesy, J.P.; Wong, C.K.C.; Wong, M.H. Concentrations and congener profiles of polybrominated diphenyl ethers (PBDEs) in blood plasma from Hong Kong: Implications for sources and exposure route. J. Hazard. Mater. 2013, 261, 253–259. [Google Scholar] [CrossRef]
- Roosens, L.; Cornelis, C.; D’Hollander, W.; Bervoets, L.; Reynders, H.; Van Campenhout, K.; Van Den Heuvel, R.; Neels, H.; Covaci, A. Exposure of the Flemish population to brominated flame retardants: Model and risk assessment. Environ. Int. 2010, 36, 368–376. [Google Scholar] [CrossRef]
- Bragigand, V.; Amiard-Triquet, C.; Parlier, E.; Boury, P.; Marchand, P.; El Hourch, M. Influence of biological and ecological factors on the bioaccumulation of polybrominated diphenyl ethers in aquatic food webs from French estuaries. Sci. Total Environ. 2006, 368, 615–626. [Google Scholar] [CrossRef]
- Bocio, A.; Llobet, J.M.; Domingo, J.L.; Corbella, J.; Teixido, A.; Casas, C. Polybrominated diphenyl ethers (PBDEs) in foodstuffs: Human exposure through the diet. J. Agric. Food Chem. 2003, 51, 3191–3195. [Google Scholar] [CrossRef] [PubMed]
- Covaci, A.; Roosens, L.; Dirtu, A.C.; Waegeneers, N.; Van Overmeire, I.; Neels, H.; Goeyens, L. Brominated flame retardants in Belgian home-produced eggs: Levels and contamination sources. Sci. Total Environ. 2009, 407, 4387–4396. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, M.; Vorkamp, K.; Thomsen, M.; Knudsen, L.E. Human internal and external exposure to PBDEs—A review of levels and sources. Int. J. Hyg. Environ. Health. 2009, 212, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Harrad, S.; Hazrati, S.; Ibarra, C. Concentrations of polychlorinated biphenyls in indoor air and polybrominated diphenyl ethers in indoor air and dust in Birmingham, United Kingdom: Implications for human exposure. Environ. Sci. Technol. 2006, 40, 4633–4638. [Google Scholar] [CrossRef] [PubMed]
- Harrad, S.; Ibarra, C.; Abdallah, M.A.E.; Boon, R.; Neels, H.; Covaci, A. Concentrations of brominated flame retardants in dust from United Kingdom cars, homes, and offices: Causes of variability and implications for human exposure. Environ. Int. 2008, 34, 1170–1175. [Google Scholar] [CrossRef]
- Meironyte, D.; Noren, K.; Bergman, A. Analysis of polybrominated diphenyl ethers in Swedish human milk. A time-related trend study, 1972–1997. J. Toxicol. Env. Health Part A 1999, 58, 329–341. [Google Scholar] [CrossRef]
- Stapleton, H.M.; Dodder, N.G.; Offenberg, J.H.; Schantz, M.M.; Wise, S.A. Polybrominated diphenyl ethers in house dust and clothes dryer lint. Environ. Sci. Technol. 2005, 39, 925–931. [Google Scholar] [CrossRef]
- Jones-Otazo, H.A.; Clarke, J.P.; Diamond, M.L.; Archbold, J.A.; Ferguson, G.; Harner, T.; Richardson, G.M.; Ryan, J.J.; Wilford, B. Is house dust the missing exposure pathway for PBDEs? An analysis of the urban fate and human exposure to PBDEs. Environ. Sci. Technol. 2005, 39, 5121–5130. [Google Scholar] [CrossRef]
- Kazda, R.; Hajslova, J.; Poustka, J.; Cajka, T. Determination of polybrominated diphenyl ethers in human milk samples in the Czech Republic—Comparative study of negative chemical ionisation mass spectrometry and time-of-flight high-resolution mass spectrometry. Anal. Chim. Acta 2004, 520, 237–243. [Google Scholar] [CrossRef]
- Schecter, A.; Harris, T.R.; Shah, N.; Musurnbal, A.; Papke, O. Brominated flame retardants in US food. Mol. Nutr. Food Res. 2008, 52, 266–272. [Google Scholar] [CrossRef]
- Dingemans, M.M.L.; de Groot, A.; van Kleef, R.; Bergman, A.; van den Berg, M.; Vijverberg, H.P.M.; Westerink, R.H.S. Hydroxylation increases the neurotoxic potential of BDE-47 to affect exocytosis and calcium homeostasis in PC12 cells. Environ. Health Perspect. 2008, 116, 637–643. [Google Scholar] [CrossRef] [PubMed]
- International Environment House. Stockholm Convention on Persistant Organic Pollutants; International Environment House: Geneva, Switzerland, 2001. [Google Scholar]
- WEEE. Waste from Electrical and Electronics Equipment a RoHS (Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment); European Commission: Brussels, Belgium, 2003; Volume 2003/11/EC. [Google Scholar]
- Schecter, A.; Papke, O.; Tung, K.C.; Joseph, J.; Harris, T.R.; Dahlgren, J. Polybrominated diphenyl ether flame retardants in the US population: Current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J. Occup. Environ. Med. 2005, 47, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Mazdai, A.; Dodder, N.G.; Abernathy, M.P.; Hites, R.A.; Bigsby, R.M. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ. Health Perspect. 2003, 111, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.F.; Araque, P.; Kiviranta, H.; Molina-Molina, J.M.; Rantakokko, P.; Laine, O.; Vartiainen, T.; Olea, N. PBDEs and PBBs in the adipose tissue of women from Spain. Chemosphere 2007, 66, 377–383. [Google Scholar] [CrossRef]
- Guvenius, D.M.; Bergman, A.; Noren, K. Polybrominated diphenyl ethers in Swedish human liver and adipose tissue. Arch. Environ. Contam. Toxicol. 2001, 40, 564–570. [Google Scholar] [CrossRef]
- Johnson-Restrepo, B.; Kannan, K.; Rapaport, D.P.; Rodan, B.D. Polybrominated diphenyl ethers and polychlorinated biphenyls in human adipose tissue from New York. Environ. Sci. Technol. 2005, 39, 5177–5182. [Google Scholar] [CrossRef]
- Schecter, A.; Johnson-Welch, S.; Tung, K.C.; Harris, T.R.; Papke, O.; Rosen, R. Polybrominated diphenyl ether (PBDE) levels in livers of US human fetuses and newborns. J. Toxicol. Env. Health Part A 2007, 70, 1–6. [Google Scholar] [CrossRef]
- Herbstman, J.B.; Sjodin, A.; Apelberg, B.J.; Witter, F.R.; Halden, R.U.; Patterson, D.G.; Panny, S.R.; Needham, L.L.; Goldman, L.R. Birth delivery mode modifies the associations between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ. Health Perspect. 2008, 116, 1376–1382. [Google Scholar] [CrossRef]
- Akutsu, K.; Takatori, S.; Nozawa, S.; Yoshiike, M.; Nakazawa, H.; Hayakawa, K.; Makino, T.; Iwamoto, T. Polybrominated diphenyl ethers in human serum and sperm quality. Bull. Environ. Contam. Toxicol. 2008, 80, 345–350. [Google Scholar] [CrossRef]
- Darnerud, P.O. Brominated flame retardants as possible endocrine disrupters. Int. J. Androl. 2008, 31, 152–160. [Google Scholar] [CrossRef]
- Meeker, J.D.; Johnson, P.I.; Camann, D.; Hauser, R. Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in men. Sci. Total Environ. 2009, 407, 3425–3429. [Google Scholar] [CrossRef] [PubMed]
- Hajslova, J.; Pulkrabova, J.; Poustka, J.; Cajka, T.; Randak, T. Brominated flame retardants and related chlorinated persistent organic pollutants in fish from river Elbe and its main tributary Vltava. Chemosphere 2007, 69, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Pulkrabova, J.; Hajslova, J.; Poustka, J.; Kazda, R. Fish as Biomonitors of Polybrominated Diphenyl Ethers and Hexabromocyclododecane in Czech Aquatic Ecosystems: Pollution of the Elbe River Basin. Environ. Health Perspect. 2007, 115, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Longnecker, M.P.; Rogan, W.J.; Lucier, G. The human health effects of DDT (dichlorodiphenyl-trichloroethane) and PCBS (polychlorinated biphenyls) and an overview of organochlorines in public health. Annu. Rev. Public Health 1997, 18, 211–244. [Google Scholar] [CrossRef] [PubMed]
- Pulkrabova, J.; Hradkova, P.; Hajslova, J.; Poustka, J.; Napravnikova, M.; Polacek, V. Brominated flame retardants and other organochlorine pollutants in human adipose tissue samples from the Czech Republic. Environ. Int. 2009, 35, 63–68. [Google Scholar] [CrossRef]
- Carrizo, D.; Grimalt, J.O. Influence of breastfeeding in the accumulation of polybromodiphenyl ethers during the first years of child growth. Environ. Sci. Technol. 2007, 41, 4907–4912. [Google Scholar] [CrossRef]
- Naert, C.; Piette, M.; Bruneel, N.; Van Peteghem, C. Occurrence of polychlorinated biphenyls and polybrominated diphenyl ethers in Belgian human adipose tissue samples. Arch. Environ. Contam. Toxicol. 2006, 50, 290–296. [Google Scholar] [CrossRef]
- Covaci, A.; Voorspoels, S.; Roosens, L.; Jacobs, W.; Blust, R.; Neels, H. Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in human liver and adipose tissue samples from Belgium. Chemosphere 2008, 73, 170–175. [Google Scholar] [CrossRef]
- She, J.W.; Petreas, M.; Winkler, J.; Visita, P.; McKinney, M.; Kopec, D. PBDEs in the San Francisco Bay Area: Measurements in harbor seal blubber and human breast adipose tissue. Chemosphere 2002, 46, 697–707. [Google Scholar] [CrossRef]
- Kunisue, T.; Takayanagi, N.; Isobe, T.; Takahashi, S.; Nose, M.; Yamada, T.; Komori, H.; Arita, N.; Ueda, N.; Tanabe, S. Polybrominated diphenyl ethers and persistent organochlorines in Japanese human adipose tissues. Environ. Int. 2007, 33, 1048–1056. [Google Scholar] [CrossRef]
- Petreas, M.; Nelson, D.; Brown, F.R.; Goldberg, D.; Hurley, S.; Reynolds, P. High concentrations of polybrominated diphenylethers (PBDEs) in breast adipose tissue of California women. Environ. Int. 2011, 37, 190–197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, Y.F.; Peng, L.; Zhang, W.C.; Liu, C.X.; Yang, Q.T.; Zheng, S.K.; Bao, M.; Huang, Y.N.; Wu, K.S. Adipose tissue levels of polybrominated diphenyl ethers and breast cancer risk in Chinese women: A case-control study. Environ. Res. 2018, 167, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, C.; Stigum, H.; Froshaug, M.; Broadwell, S.L.; Becher, G.; Eggesbo, M. Determinants of brominated flame retardants in breast milk from a large scale Norwegian study. Environ. Int. 2010, 36, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Hoopmann, M.; Albrecht, U.V.; Gierden, E.; Huppmann, R.; Suchenwirth, R. Time trends and individual characteristics associated with polybrominated diphenyl ethers in breast milk samples 2006–2009 in Lower Saxony, Germany. Int. J. Hyg. Environ. Health 2012, 215, 352–359. [Google Scholar] [CrossRef]
- Linderholm, L.; Biague, A.; Mansson, F.; Norrgren, H.; Bergman, A.; Jakobsson, K. Human exposure to persistent organic pollutants in West Africa—A temporal trend study from Guinea-Bissau. Environ. Int. 2010, 36, 675–682. [Google Scholar] [CrossRef]
- Turyk, M.E.; Anderson, H.A.; Steenport, D.; Buelow, C.; Imm, P.; Knobeloch, L. Longitudinal biomonitoring for polybrominated diphenyl ethers (PBDEs) in residents of the Great Lakes basin. Chemosphere 2010, 81, 517–522. [Google Scholar] [CrossRef]
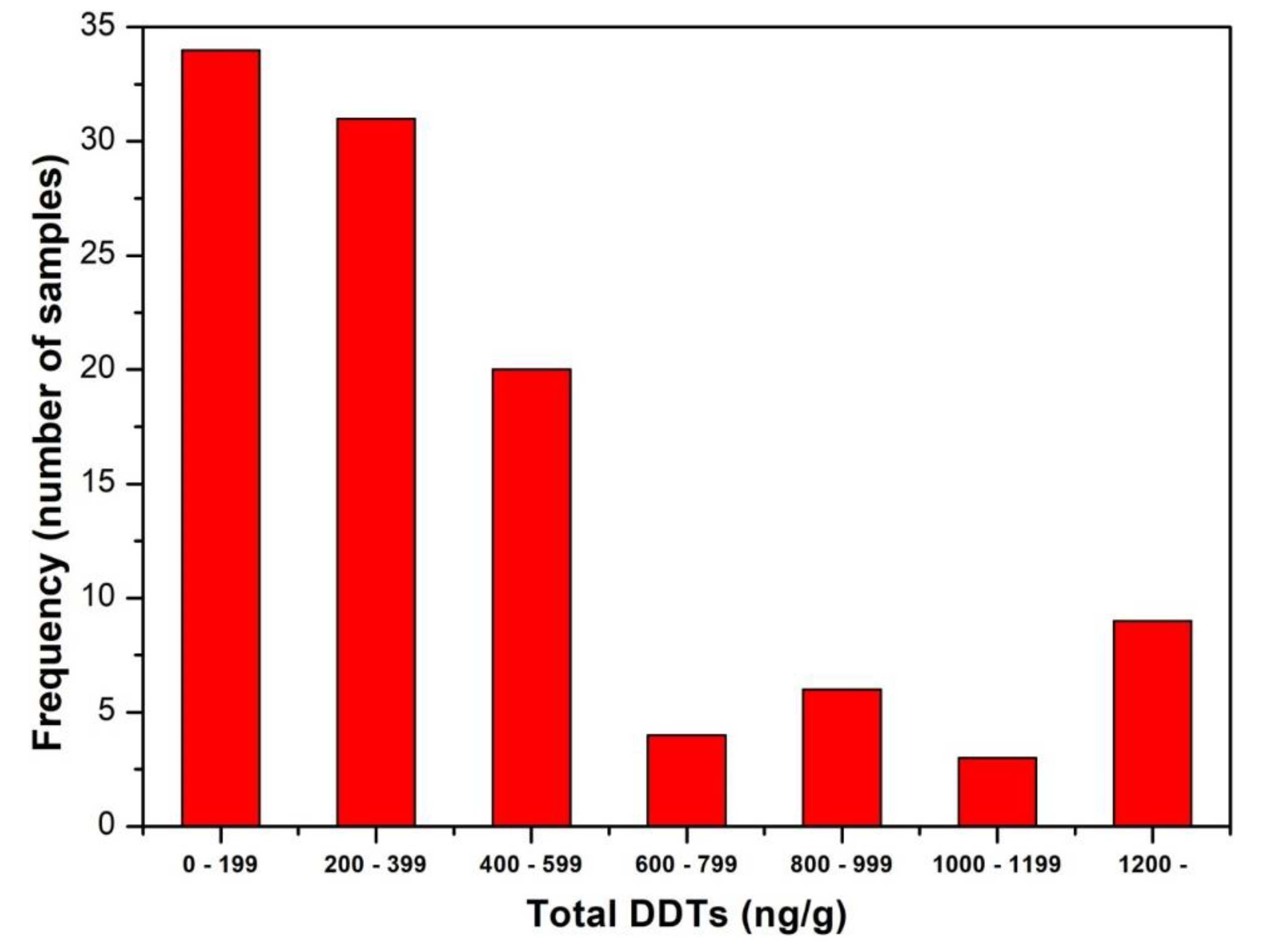
| Variable | Mean | SD | Median | IQR | Min | Max |
|---|---|---|---|---|---|---|
| Age (years) | 43.2 | 12.4 | 41.0 | 16.5 | 19.0 | 76.0 |
| Weight (kg) | 75.2 | 15.2 | 74.0 | 18.0 | 49.0 | 150.0 |
| Length (cm) | 167.4 | 7.27 | 166.0 | 6.0 | 150.0 | 190.0 |
| BMI (kg/m2) | 26.8 | 4.88 | 26.0 | 6.0 | 18.7 | 47.3 |
| Analyte | Mean | CV | Median | IQR | P5 | P95 | Min | Max |
|---|---|---|---|---|---|---|---|---|
| BDE 47 | 0.59 | 1.64 | 0.30 | 0.56 | 0.05 | 2.47 | 0.05 | 7.03 |
| BDE 49 | 0.22 | 3.04 | 0.03 | 0.16 | 0.05 | 0.80 | 0.05 | 6.33 |
| BDE 99 | 0.49 | 2.46 | 0.14 | 0.24 | 0.05 | 2.18 | 0.05 | 8.23 |
| BDE 100 | 0.19 | 1.37 | 0.11 | 0.17 | 0.05 | 0.76 | 0.05 | 1.66 |
| BDE 153 | 1.02 | 1.78 | 0.67 | 0.64 | 0.10 | 2.05 | 0.10 | 16.6 |
| BDE 183 | 0.70 | 1.93 | 0.10 | 0.29 | 0.10 | 3.73 | 0.10 | 7.91 |
| PCB 28 | 1.88 | 2.32 | 0.99 | 1.52 | 0.03 | 4.03 | 0.03 | 35.5 |
| PCB 52 | 0.16 | 1.22 | 0.07 | 0.19 | 0.03 | 0.47 | 0.03 | 1.06 |
| PCB 101 | 0.61 | 1.31 | 0.33 | 0.66 | 0.03 | 1.93 | 0.03 | 4.34 |
| PCB 118 | 12.9 | 0.85 | 9.51 | 10.9 | 2.59 | 31.9 | 1.60 | 68.8 |
| PCB 138 | 193.9 | 1.05 | 142.6 | 155.7 | 33.2 | 530.3 | 11.4 | 1473 |
| PCB 153 | 237.6 | 0.89 | 177.5 | 254.3 | 35.7 | 577.8 | 19.0 | 1114 |
| PCB 180 | 329.1 | 0.84 | 241.4 | 244.7 | 53.2 | 827.3 | 20.9 | 1652 |
| HCB | 151.5 | 1.32 | 65.6 | 127.9 | 11.4 | 533.2 | 8.68 | 1152 |
| α-HCH | 0.53 | 1.06 | 0.49 | 0.68 | 0.03 | 1.21 | 0.03 | 3.87 |
| β-HCH | 29.5 | 1.26 | 14.64 | 30.8 | 2.70 | 127.0 | 1.40 | 190.4 |
| Lindane | 0.39 | 0.74 | 0.44 | 0.66 | 0.03 | 0.71 | 0.03 | 0.89 |
| o,p’-DDE | 0.37 | 1.02 | 0.24 | 0.70 | 0.03 | 0.74 | 0.03 | 2.48 |
| p,p’-DDE | 409.6 | 0.99 | 252.3 | 363.7 | 70.9 | 1267 | 45.5 | 2217 |
| o,p’-DDD | 0.47 | 1.07 | 0.23 | 0.85 | 0.05 | 0.94 | 0.05 | 3.39 |
| p,p’-DDD | 10.0 | 2.35 | 2.73 | 7.41 | 0.25 | 32.63 | 0.03 | 165.6 |
| o,p’-DDT | 1.41 | 1.61 | 0.87 | 1.25 | 0.20 | 4.25 | 0.11 | 18.6 |
| p,p’-DDT | 45.5 | 1.80 | 25.6 | 36.7 | 4.15 | 133.9 | 0.50 | 617 |
| Current Study (n = 107) | Previous Study (n = 98) | p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | Mean | CV | Median | P5 | P95 | Mean | CV | Median | P5 | P95 | |
| PBDEs1 | 3.31 | 1.26 | 1.87 | 0.45 | 12.2 | 4.4 | 1.39 | 3.1 | 0.9 | 9.1 | >0.05 <0.1 |
| PCBs2 | 776.0 | 0.80 | 563.0 | 161.9 | 2136 | 625.5 | 0.6 | 595.0 | 214.6 | 1285 | <0.025 |
| DDTs3 | 467.4 | 0.94 | 301.5 | 108.6 | 1502 | 615.6 | 0.71 | 509.7 | 137.7 | 1668 | <0.01 |
| Analyte | Geometric Mean | LCI | UCI |
|---|---|---|---|
| BDE 47 | 0.27 | 0.22 | 0.35 |
| BDE 99 | 0.15 | 0.12 | 0.20 |
| BDE 100 | 0.11 | 0.10 | 0.14 |
| BDE 153 | 0.65 | 0.55 | 0.77 |
| PCB 28 | 0.72 | 0.54 | 0.58 |
| PCB 52 | 0.079 | 0.063 | 0.100 |
| PCB 101 | 0.24 | 0.18 | 0.32 |
| PCB 118 | 9.33 | 8.01 | 10.9 |
| PCB 138 | 134.2 | 113.6 | 158.5 |
| PCB 153 | 162.8 | 136.4 | 194.2 |
| PCB 180 | 247.4 | 213.1 | 287.2 |
| HCB | 73.7 | 58.4 | 92.9 |
| α-HCH | 0.26 | 0.19 | 0.34 |
| β-HCH | 15.6 | 12.5 | 19.4 |
| Lindane | 0.20 | 0.15 | 0.27 |
| o,p’-DDE | 0.17 | 0.13 | 0.22 |
| p,p’-DDE | 282.0 | 239.0 | 331.9 |
| o,p’-DDD | 0.21 | 0.16 | 0.28 |
| p,p’-DDD | 3.06 | 2.24 | 4.19 |
| o,p’-DDT | 0.78 | 0.64 | 0.95 |
| p,p’-DDT | 24.4 | 19.5 | 30.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logerová, H.; Tůma, P.; Stupák, M.; Pulkrábová, J.; Dlouhý, P. Evaluation of the Burdening on the Czech Population by Brominated Flame Retardants. Int. J. Environ. Res. Public Health 2019, 16, 4105. https://doi.org/10.3390/ijerph16214105
Logerová H, Tůma P, Stupák M, Pulkrábová J, Dlouhý P. Evaluation of the Burdening on the Czech Population by Brominated Flame Retardants. International Journal of Environmental Research and Public Health. 2019; 16(21):4105. https://doi.org/10.3390/ijerph16214105
Chicago/Turabian StyleLogerová, Hana, Petr Tůma, Michal Stupák, Jana Pulkrábová, and Pavel Dlouhý. 2019. "Evaluation of the Burdening on the Czech Population by Brominated Flame Retardants" International Journal of Environmental Research and Public Health 16, no. 21: 4105. https://doi.org/10.3390/ijerph16214105
APA StyleLogerová, H., Tůma, P., Stupák, M., Pulkrábová, J., & Dlouhý, P. (2019). Evaluation of the Burdening on the Czech Population by Brominated Flame Retardants. International Journal of Environmental Research and Public Health, 16(21), 4105. https://doi.org/10.3390/ijerph16214105





