Involvement of Akt/mTOR in the Neurotoxicity of Rotenone-Induced Parkinson’s Disease Models
Abstract
1. Introduction
2. Experimental Procedure
2.1. Chemicals and Antibodies
2.2. Animals
2.3. Behavioral Tests
2.4. Nissl Staining
2.5. TH Immunofluorescent Staining
2.6. Cell Culture and Treatment
2.7. PTMScan Multipathway Analysis
2.8. Western Blot Analysis
2.9. Cell Viability Measurement
2.10. Transmission Electron Microscopy (TEM)
2.11. Statistical Analysis
3. Results
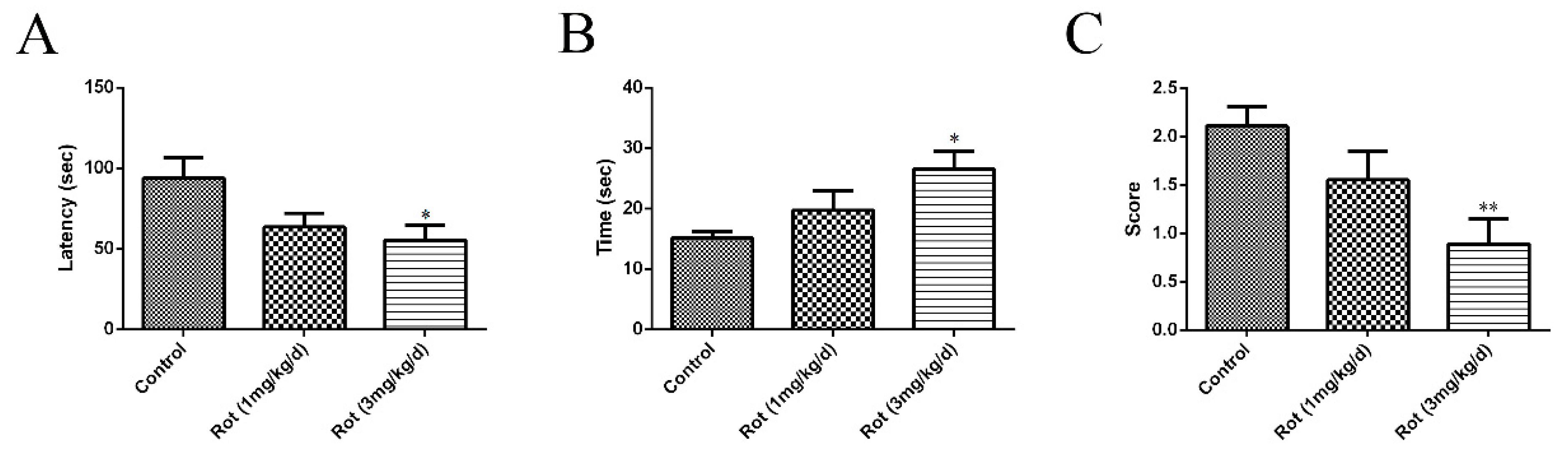
3.1. Rotenone-Reduced Motor Functions of Mice
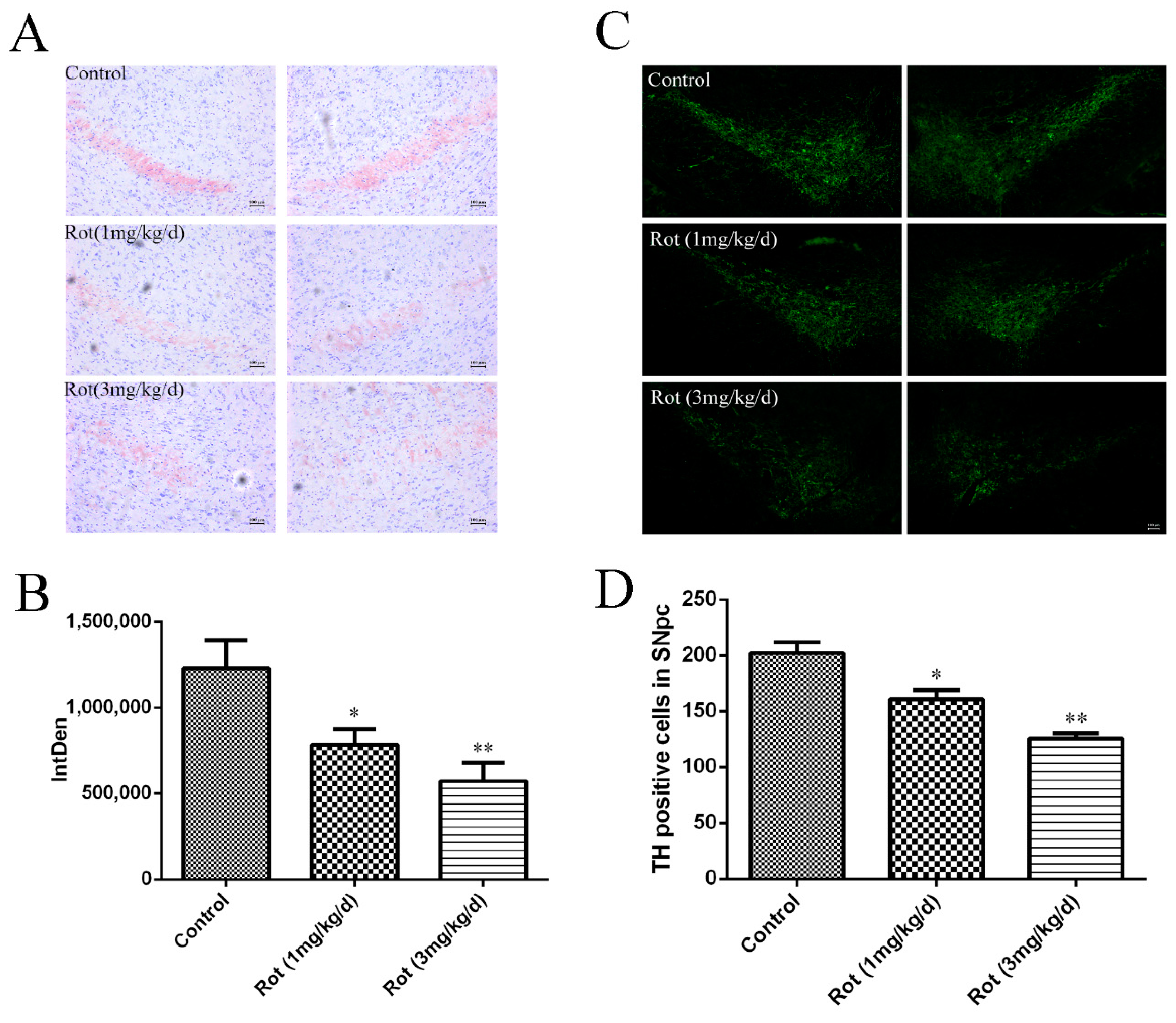
3.2. Rotenone-Induced Neuronal Loss in SNpc
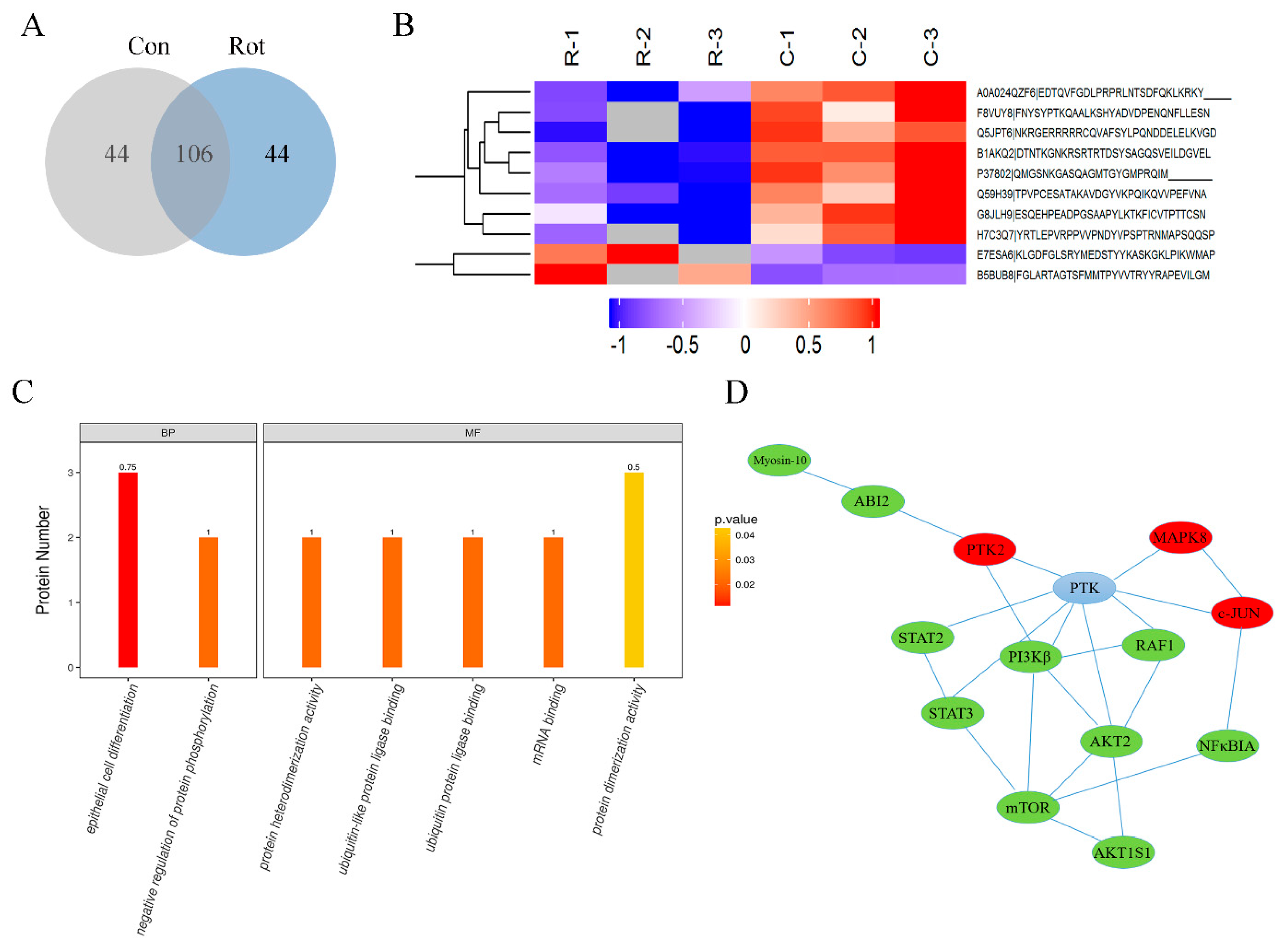
3.3. Global Changes in Phosphorylation Proteomic Profiles in Rotenone-Injured SH-SY5Y Cells
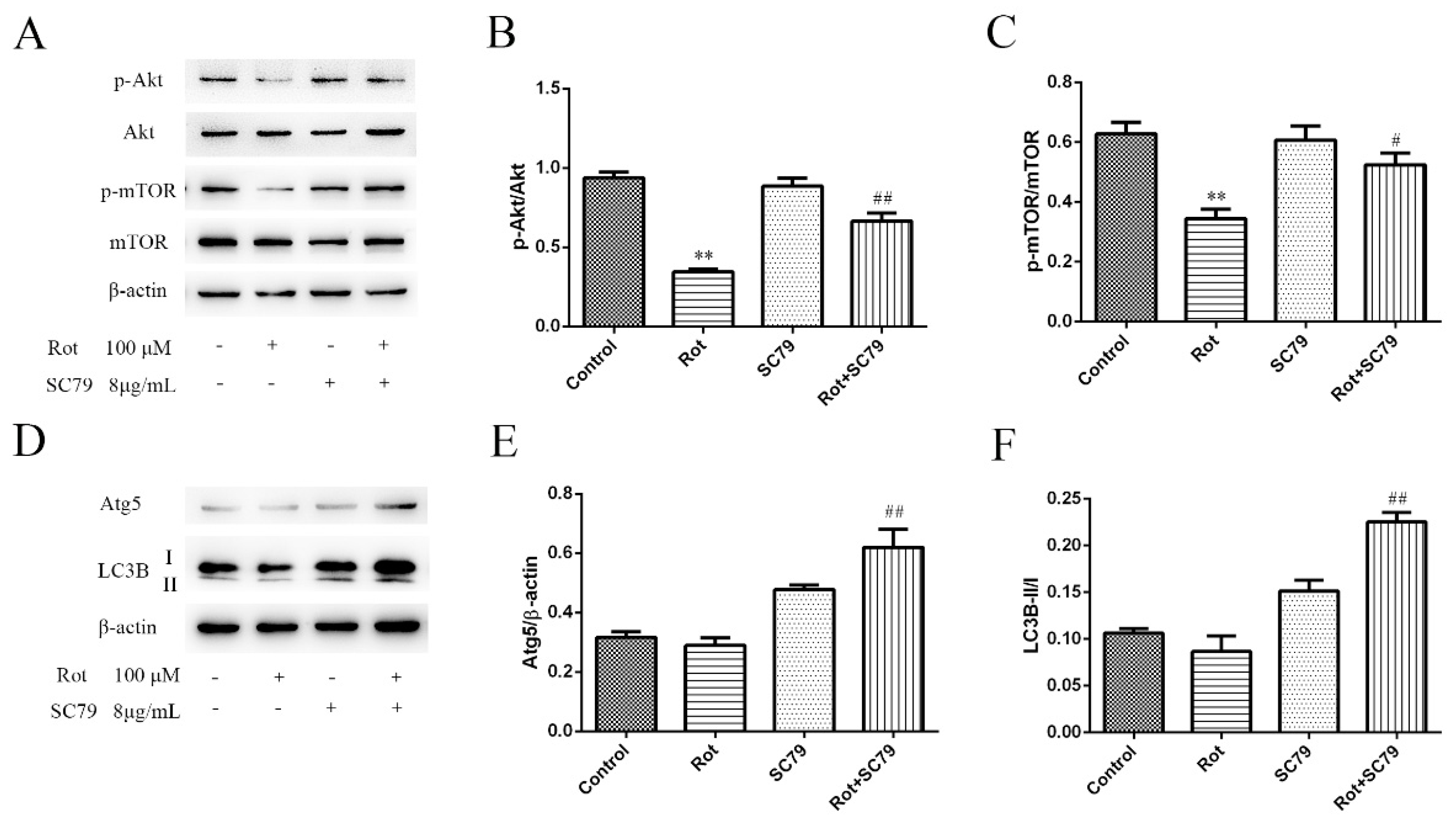
3.4. The Phosphorylation of Akt/mTOR Signaling Decreased in Rotenone-Injured SH-SY5Y Cells
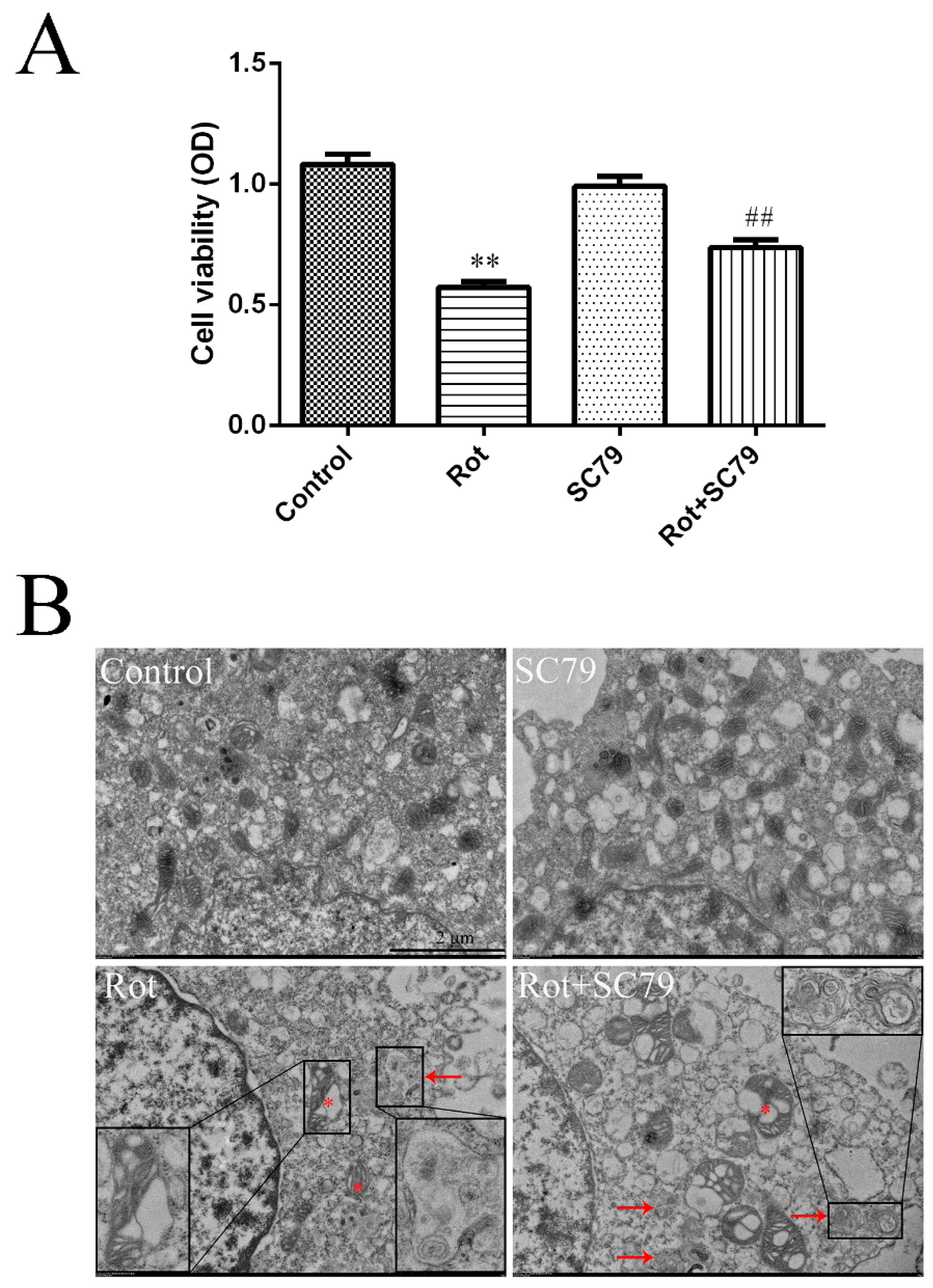
3.5. The Activation of Akt/mTOR Reduced the Neurotoxicity of Rotenone and Induced Autophagy
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cannon, J.R.; Tapias, V.; Na, H.M.; Honick, A.S.; Drolet, R.E.; Greenamyre, J.T. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol. Dis. 2009, 34, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.E.; Bobrovskaya, L. An update on the rotenone models of Parkinson’s disease: Their ability to reproduce the features of clinical disease and model gene-environment interactions. Neurotoxicology 2015, 46, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Imaizumi, Y.; Sugawara, M.; Andoh-Noda, T.; Banno, S.; Chai, M.; Sone, T.; Yamazaki, K.; Ito, M.; Tsukahara, K.; et al. T-type Calcium Channels Determine the Vulnerability of Dopaminergic Neurons to Mitochondrial Stress in Familial Parkinson Disease. Stem. Cell. Rep. 2018, 11, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chen, S.; Shen, M.; He, Q.; Zhang, Y.; Shi, Y.; Ding, F.; Zhang, Q. Mitochondrial regulation by pyrroloquinoline quinone prevents rotenone-induced neurotoxicity in Parkinson’s disease models. Neurosci. Lett. 2018, 687, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.M.; Kamel, F.; Ross, G.W.; Hoppin, J.A.; Goldman, S.M.; Korell, M.; Marras, C.; Bhudhikanok, G.S.; Kasten, M.; Chade, A.R.; et al. Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef]
- Pajarillo, E.; Rizor, A.; Lee, J.; Aschner, M.; Lee, E. The role of posttranslational modifications of alpha-synuclein and LRRK2 in Parkinson’s disease: Potential contributions of environmental factors. Biochim. Biophys. Acta Mol. Basis. Dis. 2019, 1865, 1992–2000. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- Zhang, Z.N.; Zhang, J.S.; Xiang, J.; Yu, Z.H.; Zhang, W.; Cai, M.; Li, X.T.; Wu, T.; Li, W.W.; Cai, D.F. Subcutaneous rotenone rat model of Parkinson’s disease: Dose exploration study. Brain Res. 2017, 1655, 104–113. [Google Scholar] [CrossRef]
- Lin, T.K.; Cheng, C.H.; Chen, S.D.; Liou, C.W.; Huang, C.R.; Chuang, Y.C. Mitochondrial dysfunction and oxidative stress promote apoptotic cell death in the striatum via cytochrome c/caspase-3 signaling cascade following chronic rotenone intoxication in rats. Int. J. Mol. Sci. 2012, 13, 8722–8739. [Google Scholar] [CrossRef]
- Emmrich, J.V.; Hornik, T.C.; Neher, J.J.; Brown, G.C. Rotenone induces neuronal death by microglial phagocytosis of neurons. FEBS J. 2013, 280, 5030–5038. [Google Scholar] [CrossRef]
- Lin, D.; Liang, Y.; Zheng, D.; Chen, Y.; Jing, X.; Lei, M.; Zeng, Z.; Zhou, T.; Wu, X.; Peng, S.; et al. Novel biomolecular information in rotenone-induced cellular model of Parkinson’s disease. Gene 2018, 647, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Chen, M.; Du, T.; Duan, C.; Gao, G.; Yang, H. The novel mechanism of rotenone-induced alpha-synuclein phosphorylation via reduced protein phosphatase 2A activity. Int. J. Biochem. Cell. Biol. 2016, 75, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Mendivil-Perez, M.; Velez-Pardo, C.; Jimenez-Del-Rio, M. Neuroprotective Effect of the LRRK2 Kinase Inhibitor PF-06447475 in Human Nerve-Like Differentiated Cells Exposed to Oxidative Stress Stimuli: Implications for Parkinson’s Disease. Neurochem. Res. 2016, 41, 2675–2692. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, X.; Liu, Z.; Zhu, S.; Liu, H.; Fan, W.; Hu, Y.; Hu, T.; Yu, Y.; Li, Y.; et al. Resveratrol Suppresses Rotenone-induced Neurotoxicity Through Activation of SIRT1/Akt1 Signaling Pathway. Anat. Rec. 2018, 301, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.Y.; Lee, M.K.; Hong, J.T. Lack of CCR5 modifies glial phenotypes and population of the nigral dopaminergic neurons, but not MPTP-induced dopaminergic neurodegeneration. Neurobiol. Dis. 2013, 49, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.S.; Zhang, X.P.; Yao, H.B.; Wang, G.; Chen, S.W.; Gao, W.W.; Yao, H.J.; Sun, Y.R.; Xi, C.H.; Ji, Y.D. Tetranectin knockout mice develop features of Parkinson disease. Cell. Physiol. Biochem. 2014, 34, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, S.; Yu, S.; Qin, J.; Zhang, J.; Cheng, Q.; Ke, K.; Ding, F. Neuroprotective effects of pyrroloquinoline quinone against rotenone injury in primary cultured midbrain neurons and in a rat model of Parkinson’s disease. Neuropharmacology 2016, 108, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Stokes, M.P.; Farnsworth, C.L.; Moritz, A.; Silva, J.C.; Jia, X.; Lee, K.A.; Guo, A.; Polakiewicz, R.D.; Comb, M.J. PTMScan direct: Identification and quantification of peptides from critical signaling proteins by immunoaffinity enrichment coupled with LC-MS/MS. Mol. Cell. Proteom. 2012, 11, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mo, M.; Wang, Y.; Yu, W.; Song, C.; Wang, X.; Chen, S.; Liu, Y. Activation of the immunoproteasome protects SH-SY5Y cells from the toxicity of rotenone. Neurotoxicology 2019, 73, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Di Rita, A.; D’Acunzo, P.; Simula, L.; Campello, S.; Strappazzon, F.; Cecconi, F. AMBRA1-Mediated Mitophagy Counteracts Oxidative Stress and Apoptosis Induced by Neurotoxicity in Human Neuroblastoma SH-SY5Y Cells. Front. Cell. Neurosci. 2018, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Stokes, M.P.; Silva, J.C.; Jia, X.; Lee, K.A.; Polakiewicz, R.D.; Comb, M.J. Quantitative profiling of DNA damage and apoptotic pathways in UV damaged cells using PTMScan Direct. Int. J. Mol. Sci. 2012, 14, 286–307. [Google Scholar] [CrossRef] [PubMed]
- Krishna, A.; Biryukov, M.; Trefois, C.; Antony, P.M.; Hussong, R.; Lin, J.; Heinaniemi, M.; Glusman, G.; Koglsberger, S.; Boyd, O.; et al. Systems genomics evaluation of the SH-SY5Y neuroblastoma cell line as a model for Parkinson’s disease. BMC Genom. 2014, 15, 1154. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Heras-Sandoval, D.; Perez-Rojas, J.M.; Hernandez-Damian, J.; Pedraza-Chaverri, J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell. Signal. 2014, 26, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhou, T.; Chen, Y.; Lin, D.; Jing, X.; Peng, S.; Zheng, D.; Zeng, Z.; Lei, M.; Wu, X.; et al. Rifampicin inhibits rotenone-induced microglial inflammation via enhancement of autophagy. Neurotoxicology 2017, 63, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Shang, Y.C.; Wang, S.; Maiese, K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog. Neurobiol. 2012, 99, 128–148. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Wu, Y.Y.; Wu, D.; Luo, W.F.; Zhang, Z.Q.; Liu, C.F. SC79, a novel Akt activator, protects dopaminergic neuronal cells from MPP(+) and rotenone. Mol. Cell. Biochem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.Q.; Huang, W.; Li, K.R.; Liu, Y.Y.; Cao, G.F.; Cao, C.; Jiang, Q. SC79 protects retinal pigment epithelium cells from UV radiation via activating Akt-Nrf2 signaling. Oncotarget 2016, 7, 60123–60132. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Mondal, S.; Tan, D.; Nagata, E.; Takizawa, S.; Sharma, A.K.; Hou, Q.; Shanmugasundaram, K.; Prasad, A.; Tung, J.K.; et al. Small molecule-induced cytosolic activation of protein kinase Akt rescues ischemia-elicited neuronal death. Proc. Natl. Acad. Sci. USA 2012, 109, 10581–10586. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Rong, X.; Li, Y.; Zhou, J.; Lu, L. Neuroprotective Role of the PI3 Kinase/Akt Signaling Pathway in Zebrafish. Front. Endocrinol. 2017, 8, 21. [Google Scholar] [CrossRef]
- Xiong, N.; Xiong, J.; Jia, M.; Liu, L.; Zhang, X.; Chen, Z.; Huang, J.; Zhang, Z.; Hou, L.; Luo, Z.; et al. The role of autophagy in Parkinson’s disease: Rotenone-based modeling. Behav. Brain Funct. 2013, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; Graziani, I.; De Zio, D.; Dini, L.; Centonze, D.; Rotilio, G.; Ciriolo, M.R. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: Possible implications for Parkinson’s disease. Neurobiol. Aging 2012, 33, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, D.A. Influence of Normal Aging on Brain Autophagy: A Complex Scenario. Front. Aging Neurosci. 2019, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Su, L.Y.; Luo, R.; Liu, Q.; Su, J.R.; Yang, L.X.; Ding, Y.Q.; Xu, L.; Yao, Y.G. Atg5- and Atg7-dependent autophagy in dopaminergic neurons regulates cellular and behavioral responses to morphine. Autophagy 2017, 13, 1496–1511. [Google Scholar] [CrossRef] [PubMed]
- Otomo, C.; Metlagel, Z.; Takaesu, G.; Otomo, T. Structure of the human ATG12~ATG5 conjugate required for LC3 lipidation in autophagy. Nat. Struct. Mol. Biol. 2013, 20, 59–66. [Google Scholar] [CrossRef]
| Protein Name | Gene Name | Position | Amino Acid | Sequence Window | Charge | Average R a | Average C b | R/C c |
|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase (fragment) | MAPK8 | 185 | Y | FGLARTAGTSFMMTPYVVTRYYRAPEVILGM | 2 | 899,695,000 | 60,972,333 | 14.76 |
| Focal adhesion kinase 1 | PTK2 | 620 | Y | KLGDFGLSRYMEDSTYYKASKGKLPIKWMAP | 2 | 32,010,500 | 11,210,166 | 2.86 |
| Transcription factor AP-1 | JUN | 73 | S | SDLLTSPDVGLLKLASPELERLIIQSSNGHI | 3 | 218,540,000 | 75,921,666 | 2.88 |
| Akt1 substrate 1 (proline-rich), isoform CRA_a | Akt1S1 | 246 | T | EDTQVFGDLPRPRLNTSDFQKLKRKY | 2 | 996,283,333 | 2,161,533,333 | 0.46 |
| Signal transducer and activator of transcription | STAT3 | 607 | Y | ESQEHPEADPGSAAPYLKTKFICVTPTTCSN | 3 | 95,216,000 | 231,736,666 | 0.41 |
| Abl interactor 2 (fragment) | ABI2 | 79 | Y | YRTLEPVRPPVVPNDYVPSPTRNMAPSQQSP | 3 | 21,101,000 | 53,364,000 | 0.40 |
| GIG10 | 108 | S | NKRGERRRRRCQVAFSYLPQNDDELELKVGD | 2 | 70,922,000 | 202,776,666 | 0.35 | |
| Signal transducer and activator of transcription (fragment) | 700 | Y | TPVPCESATAKAVDGYVKPQIKQVVPEFVNA | 2 | 83,187,000 | 238,250,000 | 0.35 | |
| Sodium-coupled neutral amino acid transporter 2 | SLC38A2 | 41 | Y | FNYSYPTKQAALKSHYADVDPENQNFLLESN | 2 | 15,504,000 | 45,813,333 | 0.34 |
| Transgelin-2 | TAGLN2 | 192 | Y | QMGSNKGASQAGMTGYGMPRQIM | 2 | 22,752,666 | 68,413,666 | 0.33 |
| Serine/threonine-protein kinase mTOR (fragment) | MTOR | 104 | S | DTNTKGNKRSRTRTDSYSAGQSVEILDGVEL | 2 | 35,380,000 | 176,786,666 | 0.20 |
| Tubulin alpha-1B chain | TUBA1B | 223 | T | AIYDICRRNLDIERPTYTNLNRLIGQIVSSI | 3 | 15,692,500 | — d | |
| La-related protein 1 | LARP1 | 631 | S | MDGRKNTFTAWSDEESDYEIDDRDVNKILIV | 3 | 21,394,333 | — | |
| La-related protein 1 | LARP1 | 627 | S | EMEQMDGRKNTFTAWSDEESDYEIDDRDVNK | 3 | 21,394,333 | — | |
| cDNA FLJ60109, highly similar to RUN and SH3 domain-containing protein 2 | 543 | S | PAAMAGPGSPPRRVTSFAELAKGRKKTGGSG | 2 | 21,557,500 | — | ||
| cDNA FLJ40872 fis, clone TUTER2000283, highly similar to Homo sapiens transformer-2-beta (SFRS10) gene | 230 | S | DRYEDYDYRYRRRSPSPYYSRYRSRSRSRSY | 3 | 64,762,500 | — | ||
| cDNA FLJ40872 fis, clone TUTER2000283, highly similar to Homo sapiens transformer-2-beta (SFRS10) gene | 228 | S | GWRAAQDRDQIYRRRSPSPYYSRGGYRSRSR | 3 | 64,762,500 | — | ||
| Rho-related GTP-binding protein RhoC (fragment) | RHOC | 34 | Y | TCLLIVFSKDQFPEVYVPTVFENYIADIEVD | 2 | 90,055,500 | — | |
| ADAM metallopeptidase domain 10, isoform CRA_b | ADAM10 | 740 | S | PPQPIQQPQRQRPRESYQMGHMRR | 4 | — | 11,429,000 | |
| Tyrosine-protein kinase | 32 | Y | VDLKTQPVRNTERTIYVRDPTSNKQQRPVPE | 2 | — | 11,667,133 | ||
| Serine/threonine-protein kinase mTOR (fragment) | MTOR | 110 | S | NKRSRTRTDSYSAGQSVEILDGVELGEPAHK | 4 | — | 46,053,666 | |
| Histone H2B | HIST1H2BI | 65 | S | HPDTGISSKAMGIMNSFVNDIFERIAGEASR | 2 | — | 180,860,000 | |
| Kin of IRRE-like protein 1 | KIRREL | 637 | S | EAYDPIGKYATATRFSYTSQHSDYGQRFQQR | 3 | — | 21,853,500 | |
| cDNA FLJ51708, highly similar to phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta isoform | 29 | Y | KVKTKKSTKTINPSKYQTIRKAGKVHYPVAW | 2 | — | 17,646,400 | ||
| cDNA FLJ58463, highly similar to myosin-10 | 512 | S | TKTFTPCERLEKRRTSFLEGTLRRSFRTGSV | 3 | — | 42,488,333 | ||
| cDNA FLJ50355, highly similar to RAF proto-oncogene serine/threonine-protein kinase | 123 | S | GTQEKNKIRPRGQRDSSYYWEIEASEVMLST | 3 | — | 68,606,333 | ||
| Tripartite motif-containing 3, isoform CRA_f (fragment) | TRIM3 | 308 | S | SPFRVRALRPGDLPPSPDDVKRRVKSPGGPG | 3 | — | 8,615,450 | |
| NF-kappa-B inhibitor alpha | NFKBIA | 32 | S | RDGLKKERLLDDRHDSGLDSMKDEEYEQMVK | 3 | — | 14,951,000 | |
| AP complex subunit beta | AP2B1 | 276 | Y | KVLMKFLELLPKDSDYYNMLLKKLAPPLVTL | 2 | — | 36,866,666 | |
| RAC-beta serine/threonine-protein kinase (fragment) | AKT2 | 165 | T | FGLCKEGISDGATMKTFCGTPEYLAPEVLED | 3 | — | 256,295,000 | |
| ARF GTPase-activating protein GIT1 | GIT1 | 545 | Y | RLQPFHSTELEDDAIYSVHVPAGLYRIRKGV | 3 | — | 46,371,500 | |
| Aurora kinase C (fragment) | AURKC | 26 | T | SEKLDEQRTATVRRKTMCGTLDYLPPEMIEG | 2 | — | 148,152,333 | |
| Serine/threonine-protein kinase N1 | PKN1 | 914 | T | TDVSNFDEEFTGEAPTLSPPRDARPLTAAEQ | 3 | — | 965,445,000 | |
| MYO1E variant protein | MYO1E | 989 | Y | YPHAPGSQRSNQKSLYTSMARPPLPRQQSTS | 3 | — | 7,483,433 | |
| Enhancer of mRNA-decapping protein 3 | EDC3 | 150 | S | QQCSKSYVDRHMESLSQSKSFRRRHNSWSSS | 2 | — | 11,110,500 | |
| PCDHGC3 protein | 24 | Y | PQFTLQHVPDYRQNVYIPGSNATLTNAAGKR | 3 | — | 19,835,000 | ||
| Ubiquinol-cytochrome-c reductase complex assembly factor 2 (fragment) | UQCC2 | 67 | Y | ACDQMYESLARLHSNYYKHKYPRPRDTSFSG | 3 | — | 6,781,933 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Guo, H.; Guo, X.; Ge, D.; Shi, Y.; Lu, X.; Lu, J.; Chen, J.; Ding, F.; Zhang, Q. Involvement of Akt/mTOR in the Neurotoxicity of Rotenone-Induced Parkinson’s Disease Models. Int. J. Environ. Res. Public Health 2019, 16, 3811. https://doi.org/10.3390/ijerph16203811
Zhang Y, Guo H, Guo X, Ge D, Shi Y, Lu X, Lu J, Chen J, Ding F, Zhang Q. Involvement of Akt/mTOR in the Neurotoxicity of Rotenone-Induced Parkinson’s Disease Models. International Journal of Environmental Research and Public Health. 2019; 16(20):3811. https://doi.org/10.3390/ijerph16203811
Chicago/Turabian StyleZhang, Yu, Hui Guo, Xinyu Guo, Denfeng Ge, Yue Shi, Xiyu Lu, Jinli Lu, Juan Chen, Fei Ding, and Qi Zhang. 2019. "Involvement of Akt/mTOR in the Neurotoxicity of Rotenone-Induced Parkinson’s Disease Models" International Journal of Environmental Research and Public Health 16, no. 20: 3811. https://doi.org/10.3390/ijerph16203811
APA StyleZhang, Y., Guo, H., Guo, X., Ge, D., Shi, Y., Lu, X., Lu, J., Chen, J., Ding, F., & Zhang, Q. (2019). Involvement of Akt/mTOR in the Neurotoxicity of Rotenone-Induced Parkinson’s Disease Models. International Journal of Environmental Research and Public Health, 16(20), 3811. https://doi.org/10.3390/ijerph16203811





