Improving Patient Access to New Drugs in South Korea: Evaluation of the National Drug Formulary System
Abstract
:1. Introduction
1.1. Pharmaceutical Insurance System in South Korea
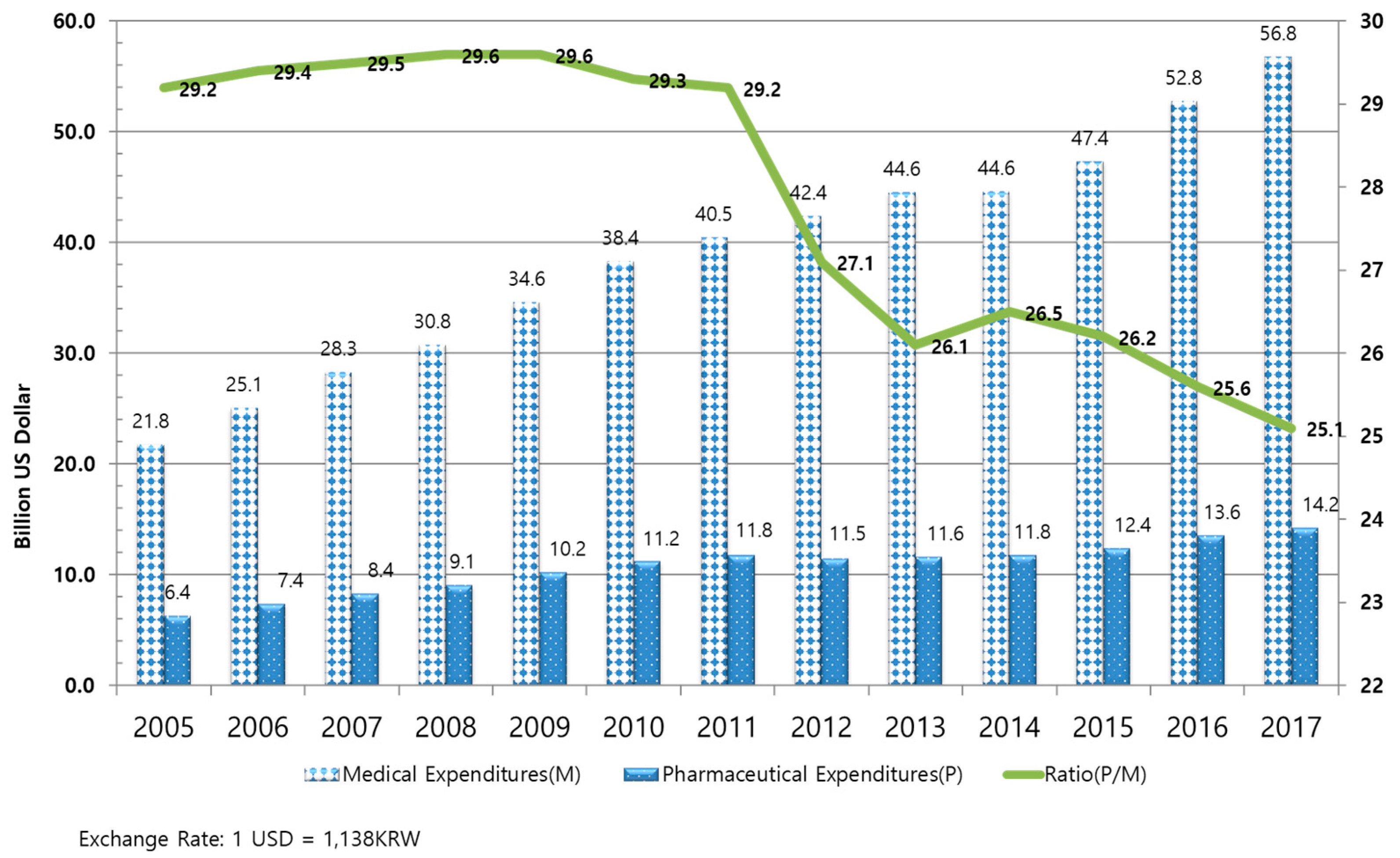
1.1.1. Drug Expenditures in South Korea
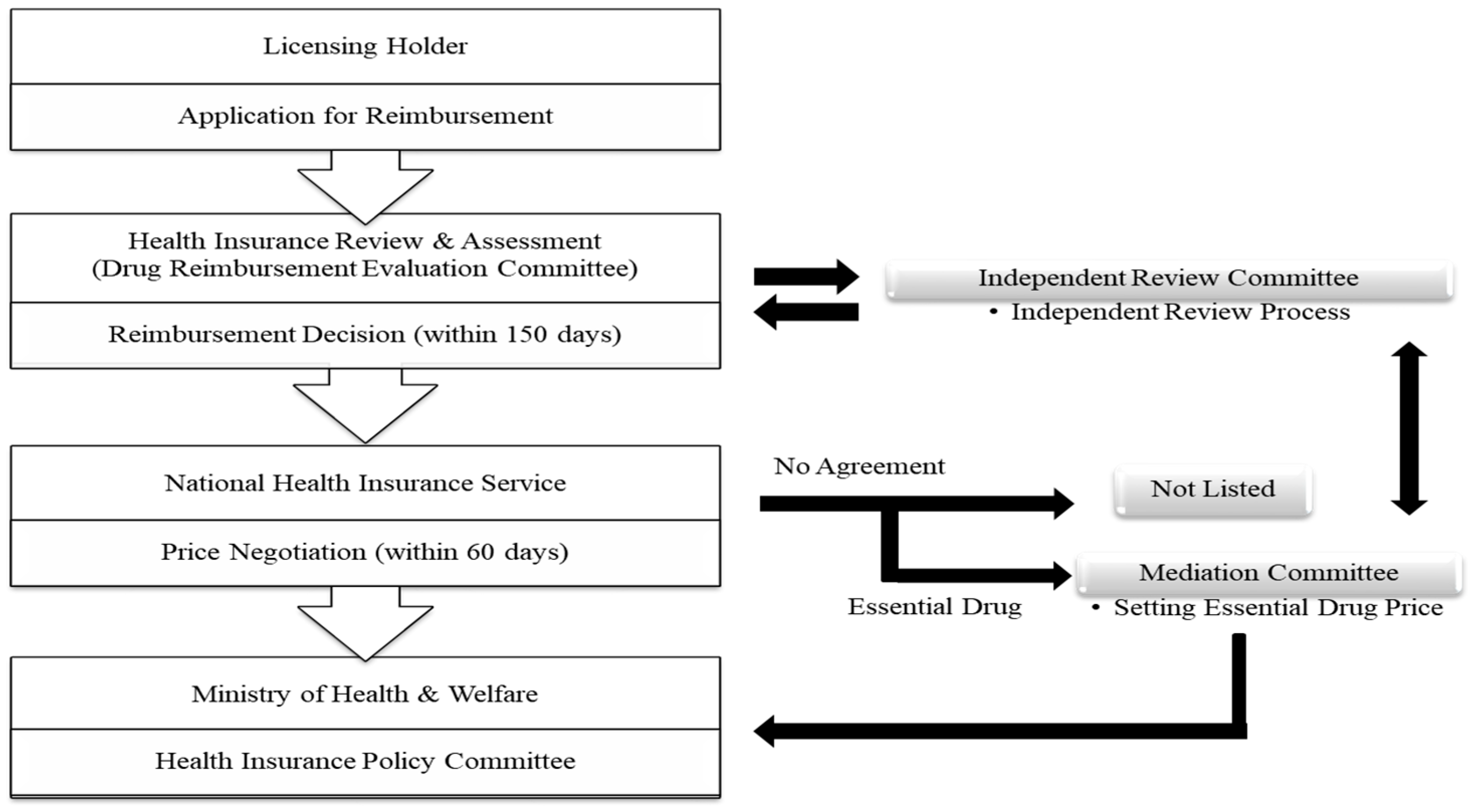
1.1.2. Listing System for the Price of New Drugs in South Korea
2. Ways of Improving Access to New Drugs in South Korea
2.1. Designation as Essential Drugs
2.2. The Risk-Sharing Agreement System
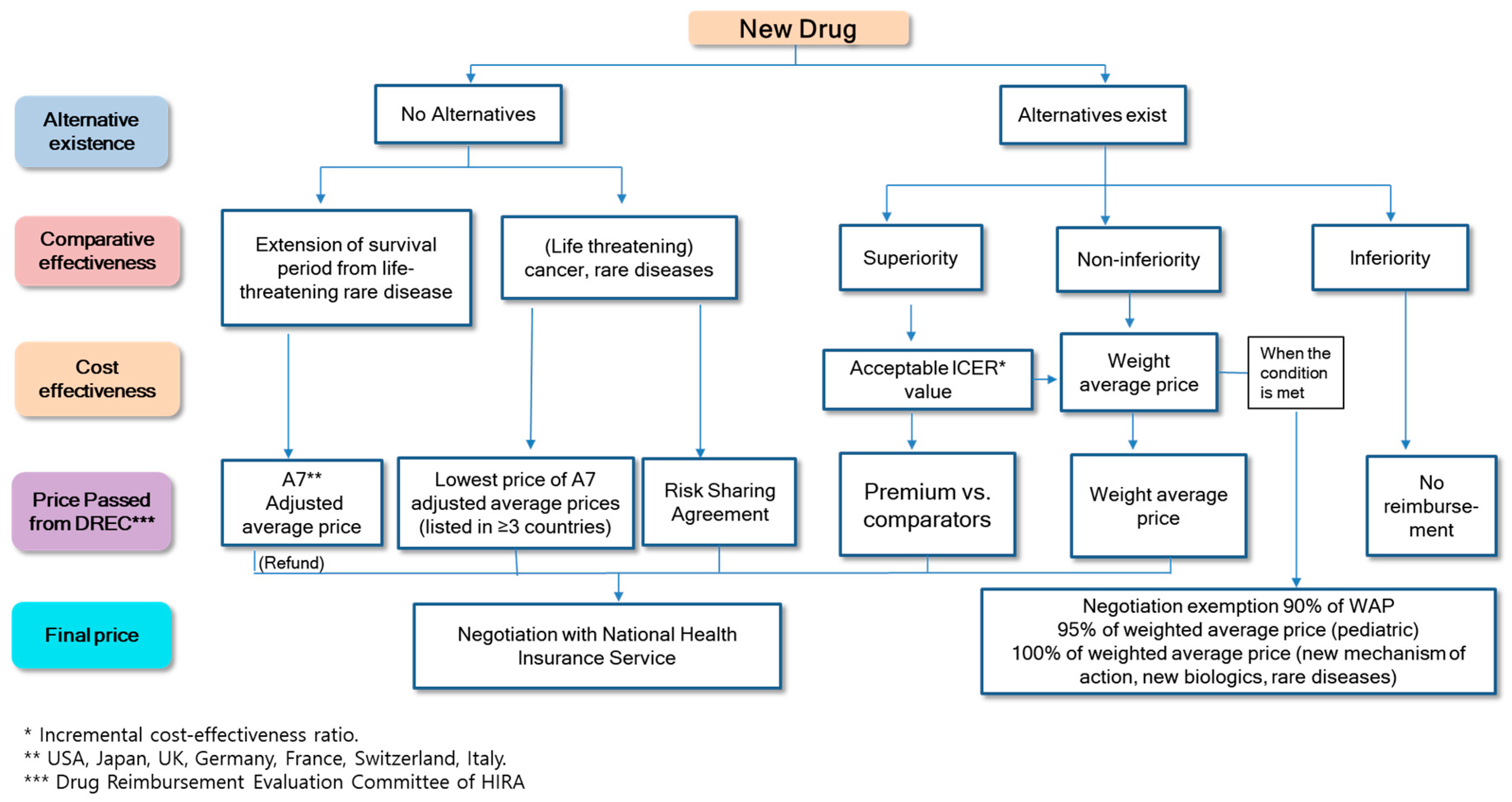
2.3. Cost-Effectiveness Analysis Waiver System
3. The Impact of Policy on Improvement of Patient Access to New Drugs
3.1. The Effects of Policies to Improve Patient Access
3.2. The Influence of Policies Aimed at Improving Patient Access to New Drugs on the Financial Resources of Health Insurance
4. Evaluation of the Policies by Stakeholders
4.1. Patients
4.2. Insured Persons
4.3. Pharmaceutical Companies
4.4. Insurance Payer (Government)
5. Discussion
5.1. Policy Suggestions
5.1.1. Increase the Transparency and Rationality of Cost-Effectiveness Analysis
5.1.2. Improvement of the Risk-Sharing Agreement System
5.1.3. Improvement of the Drug Price Listing Process
5.1.4. The Necessity of External Financial Resources for Orphan and Anticancer Drugs Independent of the NHIS
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vitry, A.; Roughead, E. Managed entry agreements for pharmaceuticals in Australia. Health Policy 2014, 117, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, C.; Drummond, M.; Bhuiyan Khan, B. Medical technology as a key driver of rising health expenditure: Disentangling the relationship. Clin. Outcomes Res. 2013, 5, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Rotar, A.M.; Preda, A.; Löblová, O.; Benkovic, V.; Zawodnik, S.; Gulacsi, L.; Niewada, M.; Boncz, I.; Petrova, G.; Dimitrova, M.; et al. Rationalizing the introduction and use of pharmaceutical products: The role of managed entry agreements in central and eastern european countries. Health Policy 2018, 122, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, A.; Kanavos, P. Dealing with uncertainty and high prices of new medicines: A comparative analysis of the use of managed entry agreements in belgium, england, the netherlands and sweden. Soc. Sci. Med. 2015, 124, 39–47. [Google Scholar] [CrossRef]
- Walker, S.; Sculpher, M.; Claxton, K.; Palmer, S. Coverage with evidence development, only in research, risk sharing, or patient access scheme? A framework for coverage decisions. Value Health 2012, 15, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Golec, J.; Vernon, J.A. Financial effects of pharmaceutical price regulation on r&d spending by eu versus us firms. Pharmacoeconomics 2010, 28, 615–628. [Google Scholar]
- Ermisch, M.; Bucsics, A.; Vella Bonanno, P.; Arickx, F.; Bybau, A.; Bochenek, T.; van de Casteele, M.; Diogene, E.; Fürst, J.; Garuolienė, K.; et al. Payers’ views of the changes arising through the possible adoption of adaptive pathways. Front. Pharmacol. 2016, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Carrera, P.M.; Kantarjian, H.M.; Blinder, V.S. The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J. Clin. 2018, 68, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Degtiar, I. A review of international coverage and pricing strategies for personalized medicine and orphan drugs. Health Policy 2017, 121, 1240–1248. [Google Scholar] [CrossRef]
- Ronfard, V.; Vertès, A.A.; May, M.H.; Dupraz, A.; van Dyke, M.E.; Bayon, Y. Evaluating the past, pesent, and future of regenerative medicine: A global view. Tissue Eng. Part B Rev. 2017, 23, 199–210. [Google Scholar] [CrossRef]
- Garrison, L.P.; Towse, A. Value-based pricing and reimbursement in personalised healthcare: Introduction to the basic health economics. J. Pers. Med. 2017, 7, 10. [Google Scholar] [CrossRef]
- Mahalatchimy, A. Reimbursement of cell-based regenerative therapy in the uk and france. Med. Law Rev. 2016, 24, 234–258. [Google Scholar] [CrossRef]
- Adamski, J.; Godman, B.; Ofierska-Sujkowska, G.; Osińska, B.; Herholz, H.; Wendykowska, K.; Laius, O.; Jan, S.; Sermet, C.; Zara, C.; et al. Risk sharing arrangements for pharmaceuticals: Potential considerations and recommendations for european payers. BMC Health Serv. Res. 2010, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Klemp, M.; Frønsdal, K.B.; Facey, K. What principles should govern the use of managed entry agreements? Int. J. Technol. Assess. Health Care 2011, 27, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Wonder, M.; Backhouse, M.E.; Sullivan, S.D. Australian managed entry scheme: A new manageable process for the reimbursement of new medicines? Value Health 2012, 15, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.J.; Bliss, S.K.; Chambers, J.D. Therapies for advanced cancers pose a special challenge for health technology assessment organizations in many countries. Health Aff. (Millwood) 2012, 31, 700–708. [Google Scholar] [CrossRef]
- Chim, L.; Salkeld, G.; Kelly, P.; Lipworth, W.; Hughes, D.A.; Stockler, M.R. Societal perspective on access to publicly subsidised medicines: A cross sectional survey of 3080 adults in australia. PLoS ONE 2017, 12, e0172971. [Google Scholar] [CrossRef] [PubMed]
- Cookson, R. Can the nice “end-of-life premium” be given a coherent ethical justification? J. Health Politcs Policy Law 2013, 38, 1129–1148. [Google Scholar] [CrossRef]
- Brock, D.W. Ethical and value issues in insurance coverage for cancer treatment. Oncologist 2010, 15 (Suppl. 1), 36–42. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Fojo, T.; Chamberlain, C.; Davis, C.; Sullivan, R. Do patient access schemes for high-cost cancer drugs deliver value to society?-lessons from the nhs cancer drugs fund. Ann. Oncol. 2017, 28, 1738–1750. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P.; Chamberlain, C.; Hollingworth, W. Did it matter that the cancer drugs fund was not nice? A retrospective review. Value Health 2016, 19, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Jan, S.; Thompson, K. Funding therapies for rare diseases: An ethical dilemma with a potential solution. Aust. Health Rev. 2018, 42, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Sylvia, P.A. Comparative study on the system of rare medicine by country. Health Soc. Welf. Rev. 2013, 33, 525–548. [Google Scholar]
- Ministry of Health and Welfare. Policy Report: Drug Expenditure Optimization System in National Health Insurance Services; Ministry of Health and Welfare: SeJong, Korea, 2006.
- Bae, E.-Y.; Hong, J.-M.; Kwon, H.-Y.; Jang, S.; Lee, H.-J.; Bae, S.; Yang, B.-M. Eight-year experience of using hta in drug reimbursement: South korea. Health Policy 2016, 120, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare. Policy Report: A Study on the Improvement of Drug Price System for the Improvement of Accessibility of New Products; Ministry of Health and Welfare: Seoul, Korea, 2014.
- OECD. Health at a Glance 2017; OECD: Paris, France, 2017. [Google Scholar]
- National Health Insurance Service. Ppri Pharma Profile Report: Korean Pharmaceutical Pricing and Repayment Policies; National Health Insurance Service: Won-ju, Korea, 2017. [Google Scholar]
- National Health Insurance Service. Policy Report: A Study on the Introduction of Risk Sharing Contract; National Health Insurance Service: SeJong, Korea, 2012. [Google Scholar]
- Health Insurance Review Assessment Service. Hira Notification: Detailed Evaluation Criteria for New Drugs Subject to Negotiation; Health Insurance Review Assessment Service: Seoul, Korea, 2017. [Google Scholar]
- Korean Pharmaceutical Manufacturers Association. Kpma Policy Report: Korean Pharmaceutical Manufacturers Association; Korean Pharmaceutical Manufacturers Association: Seoul, Korea, 2016. [Google Scholar]
- Health Insurance Review Assessment Service. List of Reimbursable Drugs. 2018. Available online: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA030014050000 (accessed on 30 November 2018).
- Lee, W.-Y.; Shaw, I. The impact of out-of-pocket payments on health care inequity: The case of national health insurance in south korea. Int. J. Environ. Res. Public Health 2014, 11, 7304–7318. [Google Scholar] [CrossRef]
- Korea Institute for Health Social Affairs. Policy Report: Policy Measures to Enhance Access to Drugs for Rare Diseases; Korea Institute for Health Social Affairs: SeJong, Korea, 2010. [Google Scholar]
- Korean Research-based Pharmaceutical Industry Association. A Study on the Improvement of Drug Price System for the Improvement of Pharmaceutical Industry and the Improvement of Patient Access; Korea Institute for Health Social Affairs: SeJong, Korea, 2016. [Google Scholar]
- Korea Institute for Health Social Affairs. Policy Report: A Study on the Rationalization of Drug Price Management System; Korea Institute for Health Social Affairs: SeJong, Korea, 2015. [Google Scholar]
- Korea Institute for Health Social Affairs. Policy Report: The Problems and Improvement of the Payment System; Korea Institute for Health Social Affairs: SeJong, Korea, 2014. [Google Scholar]
- Ministry of Health and Welfare. A Proposal for the Reform of the Drug Price System to Strengthen Health Insurance Coverage (Moon Care); Ministry of Health and Welfare: SeJong, Korea, 2018.
- Health Insurance Policy Institute. Policy Report: A Study on the Health Insurance High-Cost Medicine; Health Insurance Policy Institute: Won-Ju, Korea, 2017. [Google Scholar]
- Leech, A.A.; Kim, D.D.; Cohen, J.T.; Neumann, P.J. Use and misuse of cost-effectiveness analysis thresholds in low- and middle-income countries: Trends in cost-per daly studies. Value Health 2018, 21, 759–761. [Google Scholar] [CrossRef]
- Wang, S.; Gum, D.; Merlin, T. Comparing the icers in medicine reimbursement submissions to nice and pbac-does the presence of an explicit threshold affect the icer proposed? Value Health 2018, 21, 938–943. [Google Scholar] [CrossRef]
- National Health Insurance Service. A Study on the Improvement of Drug Price Negotiation and Drug Price Management System; National Health Insurance Service: Seoul, Korea, 2013. [Google Scholar]
- National Health Insurance Service. Policy Report: A Study on the Promotion of Transparency in Price Negotiation; National Health Insurance Service: Seoul, Korea, 2013. [Google Scholar]
- Health Insurance Policy Institute. Policy Report: A Study on the Establishment of Roadmap for Systematic Management of Health Insurance Pharmaceuticals; Health Insurance Policy Institute: Seoul, Korea, 2012. [Google Scholar]
| Brand Name | Active Substance | Indication | Evaluation Year |
|---|---|---|---|
| Cystadane | Betaine anhydrous | Homocystinuria | 2007 |
| Sprycel | Dasatinib | Leukemia | 2007 |
| Elaprase | Idursulfase | Mucopolysaccharidosis type II | 2008 |
| Naglazyme | Galsulfase | Mucopolysaccharidosis type VI | 2008 |
| Myozyme | Alglucosidase alpha | Pompe disease | 2008 |
| Zavesca | Miglustat | Gaucher’s disease | 2009 |
| Inovelon | Rufinamide | Lennox–Gastaut syndrome | 2010 |
| Remodulin | Treprostinil | Pulmonary hypertension | 2010 |
| Soliris | Eculizumab | Paroxysmal nocturnal hemoglobinuria | 2011 |
| Carbaglu | Carglumic acid | Hyperammonemia | 2014 |
| Product (Active Substance) | Indication | Risk-Sharing Agreement Type | Cost Effectiveness Analysis (CEA) |
|---|---|---|---|
| Eboltra (clofarabine) | Acute lymphoblastic leukemia | Coverage with evidence development | X |
| Erbitux (cetuximab) | Colorectal cancer | Refund | O |
| Revlimid (lenalidomide) | Multiple myeloma | Refund | X |
| Xtandi (enzalutamide) | Prostate cancer | Refund | O |
| Xalkori (crizotinib) | Non-small cell lung carcinoma | Refund | O |
| Pirespa (pirfenidone) | Idiopathic pulmonary fibrosis | Refund | O |
| Soliris (eculizumab) | Paroxysmal nocturnal hemoglobinuria | Refund | X (essential drug) |
| Caprelsa (vandetinib) | Thyroid gland cancer | Expenditure cap | X (waiver of CEA) |
| Naglazyme (galsulfase) | Mucopolysaccharidosis | Refund | X (essential drug) |
| Stivarga (regorafenib) | Gastrointestinal tumors | Refund | O |
| Vimizim (elosulfase alfa) | Morquio syndrome | Expenditure cap | X (waiver of CEA) |
| Diterin (sapropterin) | Phenylketonuria | Expenditure cap | X (waiver of CEA) |
| Pomalyst (pomalidomide) | Multiple myeloma | Refund | O |
| Defitelio (defibrotide) | Hepatic veno-occlusive disease | Expenditure cap | X (waiver of CEA) |
| Perjeta (pertuzumab) | Breast cancer | Utilization cap per patient | O |
| Zelboraf (vemurafenib) | Melanoma | Expenditure cap | X (waiver of CEA) |
| Kadcyla (trastuzumab emtansine) | Breast cancer | Utilization cap per patient | O |
| Keytruda (pembrolizumab) | Non-small cell lung carcinoma | Refund/ Expenditure cap | O |
| Opdivo (nivolumab) | Non-small cell lung carcinoma | Refund/ Expenditure cap | O |
| Lynparza (olaparib) | Ovarian cancer | Expenditure cap | X (waiver of CEA) |
| Meqsel (trametinib) | Melanoma | Expenditure cap | X (waiver of CEA) |
| Ibrance (palbociclib) | Breast cancer | Refund | O |
| Olita (olmutinib) | Non-small cell lung carcinoma | Expenditure cap | X (waiver of CEA) |
| Tagrisso (osimertinib) | Non-small cell lung carcinoma | Refund | O |
| Rafinlar (dabrafenib) | Melanoma | Expenditure cap | X (waiver of CEA) |
| Alecensa (alectinib hydrochloride) | Non-small cell lung carcinoma | Expenditure cap | X (waiver of CEA) |
| Tecentriq (atezolizumab) | Non-small cell lung carcinoma | Expenditure cap | X (waiver of CEA) |
| Sylvant (siltuximab) | Castleman’s disease | Expenditure cap | X (waiver of CEA) |
| Kyprolis (carfilzomib) | Multiple myeloma | Refund | O |
| Lartruvo (olaratumab) | Soft tissue tumors and sarcomas | Expenditure cap | X (waiver of CEA) |
| Iclusig (ponatinib) | Leukemia | Expenditure cap | X (waiver of CEA) |
| Imbruvica (ibrutinib) | Mantle cell lymphoma | Expenditure cap | X (waiver of CEA) |
| Cyramza (ramucirumab) | Gastric cancer | Refund | O |
| Risk-Sharing Agreement Type | Cancer Drug | Cancer/Orphan Drug | Orphan Drug | Total Number of Drugs | (%) |
|---|---|---|---|---|---|
| Coverage with additional evidence | 0 | 1 | 0 | 1 | (3.0) |
| Expenditure cap | 2 | 10 | 3 | 15 | (45.5) |
| Refund | 6 | 3 | 3 | 12 | (36.4) |
| Utilization cap per patient | 2 | 1 | 0 | 3 | (9.1) |
| Refund/Expenditure cap | 2 | - | - | 2 | (6.1) |
| Total | 12 | 15 | 6 | 33 | (100.0) |
| Product | Active Ingredient | Indication | Reimbursed Year | Risk-Sharing Agreement Type |
|---|---|---|---|---|
| Caprelsa | Vandetanib | Thyroid gland cancer | 2015 | Expenditure cap |
| Adcetris | Brentuximab vedotin | Hodgkin’s lymphoma | 2016 | Not applied |
| Imbruvica | Ibrutinib | Mantle cell lymphoma | 2016 | Not applied |
| Vimizim | Elosulfase alfa | Morquio syndrome | 2016 | Expenditure cap |
| Zykadia | Ceritinib | Non-small cell lung carcinoma | 2016 | Not applied |
| Blincyto | Blinatumomab | Lymphocytic leukemia | 2016 | Not applied |
| Diterin | Sapropterin | Phenylketonuria | 2017 | Expenditure cap |
| Defitelio | Defibrotide | Hepatic veno-occlusive disease | 2017 | Expenditure cap |
| Zelboraf | Vemurafenib | Melanoma | 2017 | Expenditure cap |
| Lynparza | Olaparib | Ovarian cancer | 2017 | Expenditure cap |
| Meqsel | Trametinib | Melanoma | 2017 | Expenditure cap |
| Olita | Olmutinib | Non-small cell lung carcinoma | 2017 | Expenditure cap |
| Sylvant | Siltuximab | Castleman’s disease | 2018 | Expenditure cap |
| Lartruvo | Olaratumab | Soft tissue tumors and sarcomas | 2018 | Expenditure cap |
| Iclusig | Ponatinib | Leukemia | 2018 | Expenditure cap |
| Category | 2015 | 2016 | 2017 | ||||
|---|---|---|---|---|---|---|---|
| Amount | (%) | Amount | (%) | Amount | (%) | ||
| Total drug expenditures | (million KRW) | 14,098,500 | (100) | 15,428,600 | (100) | 16,209,800 | (100) |
| (million USD) | 12,389 | 13,558 | 14,244 | ||||
| Essential drugs | (million KRW) | 53,522 | (0.38) | 60,753 | (0.39) | 62,604 | (0.39) |
| (million USD) | 47.0 | 53.4 | 55.0 | ||||
| No. of patients | 1506 | 1719 | 1812 | ||||
| Risk-sharing agreement drugs | (million KRW) | 103,518 | (0.74) | 161,358 | (1.05) | 260,360 | (1.61) |
| (million USD) | 91.0 | 141.8 | 228.8 | ||||
| No. of patients | 5125 | 7861 | 13,112 | ||||
| CEA waiver drugs | (million KRW) | 58 | (0.00) | 13,516 | (0.09) | 39,672 | (0.25) |
| (million USD) | 0.05 | 11.9 | 34.9 | ||||
| No. of patients | 12 | 343 | 936 | ||||
| Total amount saved by patients * | Total (million KRW) | 148,033 | (1.05) | 221,414 | (1.44) | 341,140 | (2.10) |
| Total (million USD) | 130.1 | 194.6 | 299.8 | ||||
| Amount per person (million KRW) | 22.3 | 22.3 | 21.5 | ||||
| Amount per person (million USD) | 0.02 | 0.02 | 0.02 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, S.-L.; Kim, D.-J.; Lee, S.-M.; Kang, W.-G.; Kim, S.-Y.; Lee, J.H.; Suh, D.-C. Improving Patient Access to New Drugs in South Korea: Evaluation of the National Drug Formulary System. Int. J. Environ. Res. Public Health 2019, 16, 288. https://doi.org/10.3390/ijerph16020288
Yoo S-L, Kim D-J, Lee S-M, Kang W-G, Kim S-Y, Lee JH, Suh D-C. Improving Patient Access to New Drugs in South Korea: Evaluation of the National Drug Formulary System. International Journal of Environmental Research and Public Health. 2019; 16(2):288. https://doi.org/10.3390/ijerph16020288
Chicago/Turabian StyleYoo, Seung-Lai, Dae-Jung Kim, Seung-Mi Lee, Won-Gu Kang, Sang-Yoon Kim, Jong Hyuk Lee, and Dong-Churl Suh. 2019. "Improving Patient Access to New Drugs in South Korea: Evaluation of the National Drug Formulary System" International Journal of Environmental Research and Public Health 16, no. 2: 288. https://doi.org/10.3390/ijerph16020288
APA StyleYoo, S.-L., Kim, D.-J., Lee, S.-M., Kang, W.-G., Kim, S.-Y., Lee, J. H., & Suh, D.-C. (2019). Improving Patient Access to New Drugs in South Korea: Evaluation of the National Drug Formulary System. International Journal of Environmental Research and Public Health, 16(2), 288. https://doi.org/10.3390/ijerph16020288







