Heart Rate Variability and Performance of Commercial Airline Pilots during Flight Simulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimentation
2.3. Data Analysis
3. Results
3.1. Influencing Factors of HRV
3.2. HRV and Pilot Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IATA. Iata Forecasts Passenger Demand to Double over 20 Years. Available online: http://www.iata.org/pressroom/pr/Pages/2016-10-18-02.aspx (accessed on 15 June 2017).
- FAA. Air Traffic by the Numbers. Available online: https://www.faa.gov/air_traffic/by_the_numbers/media/Air_Traffic_by_the_Numbers_2017_Final.pdf (accessed on 1 July 2018).
- Boeing. Statistical Summary of Commercial Jet Airplane Accidents, Worldwide Operations 1959–2016. Available online: https://www.boeing.com/resources/boeingdotcom/company/about_bca/pdf/statsum.pdf (accessed on 1 July 2018).
- FAA. Active Civil Airmen Statistics. 2017. Available online: https://www.faa.gov/data_research/aviation_data_statistics/civil_airmen_statistics/ (accessed on 1 July 2018).
- Wu, A.C.; Donnelly-McLay, D.; Weisskopf, M.G.; McNeely, E.; Betancourt, T.S.; Allen, J.G. Airplane pilot mental health and suicidal thoughts: A cross-sectional descriptive study via anonymous web-based survey. Environ. Health 2016, 15, 121. [Google Scholar] [CrossRef]
- Petrilli, R.M.; Roach, G.D.; Dawson, D.; Lamond, N. The sleep, subjective fatigue, and sustained attention of commercial airline pilots during an international pattern. Chronobiol. Int. 2006, 23, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.; Mestre, C.; Canhão, H.; Gradwell, D.; Paiva, T. Sleep complaints and fatigue of airline pilots. Sleep Sci. 2016, 9, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Roscoe, A.H. Assessing pilot workload. Why measure heart rate, hrv and respiration? Biol. Psychol. 1992, 34, 259–287. [Google Scholar] [CrossRef]
- Cavallari, J.M.; Fang, S.C.; Eisen, E.A.; Schwartz, J.; Hauser, R.; Herrick, R.F.; Christiani, D.C. Time course of heart rate variability decline following particulate matter exposures in an occupational cohort. Inhal. Toxicol. 2008, 20, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Magari, S.R.; Hauser, R.; Schwartz, J.; Williams, P.L.; Smith, T.J.; Christiani, D.C. Association of heart rate variability with occupational and environmental exposure to particulate air pollution. Circulation 2001, 104, 986–991. [Google Scholar] [CrossRef]
- Regula, M.; Socha, V.; Kutilek, P.; Socha, L.; Hana, K.; Hanakova, L.; Szabo, S. Study of heart rate as the main stress indicator in aircraft pilots. In Proceedings of the 2014 16th International Conference on Mechatronics-Mechatronika (ME), Brno, Czech Republic, 3–5 Decmeber 2014; pp. 639–643. [Google Scholar]
- Rotenberg, S.; McGrath, J.J. Inter-relation between autonomic and hpa axis activity in children and adolescents. Biol. Psychol. 2016, 117, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.G. Psychological stress and the autonomic nervous system. In Primer on the Autonomic Nervous System, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 291–293. [Google Scholar]
- Egliston, K.-A.; McMahon, C.; Austin, M.-P. Stress in pregnancy and infant hpa axis function: Conceptual and methodological issues relating to the use of salivary cortisol as an outcome measure. Psychoneuroendocrinology 2007, 32, 1–13. [Google Scholar] [CrossRef]
- Porges, S.W. The polyvagal perspective. Biol. Psychol. 2007, 74, 116–143. [Google Scholar] [CrossRef]
- McCorry, L.K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef]
- McCraty, R.; Shaffer, F. Heart rate variability: New perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Glob. Adv. Health Med. 2015, 4, 46–61. [Google Scholar] [CrossRef]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investig. 2018, 15, 235. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Task Force. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Task force of the european society of cardiology and the north american society of pacing and electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Kang, M.G.; Koh, S.B.; Cha, B.S.; Park, J.K.; Woo, J.M.; Chang, S.J. Association between job stress on heart rate variability and metabolic syndrome in shipyard male workers. Yonsei Med. J. 2004, 45, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Zeki Al Hazzouri, A.; Elfassy, T.; Carnethon, M.R.; Lloyd-Jones, D.M.; Yaffe, K. Heart rate variability and cognitive function in middle-age adults: The coronary artery risk development in young adults. Am. J. Hypertens. 2017, 31, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ernst, G. Heart-rate variability—More than heart beats? Front. Public Health 2017, 5, 240. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T., Jr. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef]
- Bekö, G.; Allen, J.G.; Weschler, C.J.; Vallarino, J.; Spengler, J.D. Impact of cabin ozone concentrations on passenger reported symptoms in commercial aircraft. PLoS ONE 2015, 10, e0128454. [Google Scholar] [CrossRef]
- Allen, J.G.; Stapleton, H.M.; Vallarino, J.; McNeely, E.; McClean, M.D.; Harrad, S.J.; Rauert, C.B.; Spengler, J.D. Exposure to flame retardant chemicals on commercial airplanes. Environ. Health 2013, 12, 17. [Google Scholar] [CrossRef]
- Zevitas, C.D.; Spengler, J.D.; Jones, B.; McNeely, E.; Coull, B.; Cao, X.; Loo, S.M.; Hard, A.-K.; Allen, J.G. Assessment of noise in the airplane cabin environment. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liu, J.; Pei, J.; Zhang, Y.; Li, J.; Zhu, X. 2d-piv measurement of aircraft cabin air distribution with a high spatial resolution. Build. Environ. 2014, 82, 9–19. [Google Scholar] [CrossRef]
- Cao, X.; Li, J.; Liu, J.; Yang, W. 2d-piv measurement of isothermal air jets from a multi-slot diffuser in aircraft cabin environment. Build. Environ. 2016, 99, 44–58. [Google Scholar] [CrossRef]
- Cao, X.; Zevitas, C.D.; Spengler, J.D.; Coull, B.; McNeely, E.; Jones, B.; Loo, S.M.; MacNaughton, P.; Allen, J.G. The on-board carbon dioxide concentrations and ventilation performance in passenger cabins of us domestic flights. Indoor Built Environ. 2018. [Google Scholar] [CrossRef]
- FAR. Part 25-Airworthiness Standards: Transport Category Airplanes; Federal Aviation Administration (FAA): Washington, DC, USA, 2013.
- EASA. Caq Preliminary Cabin Air Quality Measurement Campaign. Available online: https://www.easa.europa.eu/system/files/dfu/EASA%20CAQ%20Study%20Final%20Report_21.03.2017.pdf (accessed on 1 July 2018).
- Allen, J.G.; MacNaughton, P.; Satish, U.; Santanam, S.; Vallarino, J.; Spengler, J.D. Associations of cognitive function scores with carbon dioxide, ventilation, and volatile organic compound exposures in office workers: A controlled exposure study of green and conventional office environments. Environ. Health Perspect. 2016, 124, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wargocki, P.; Lian, Z.; Thyregod, C. Effects of exposure to carbon dioxide and bioeffluents on perceived air quality, self-assessed acute health symptoms, and cognitive performance. Indoor Air 2017, 27, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Satish, U.; Mendell, M.J.; Shekhar, K.; Hotchi, T.; Sullivan, D.; Streufert, S.; Fisk, W.J. Is CO2 an indoor pollutant? Direct effects of low-to-moderate CO2 concentrations on human decision-making performance. Environ. Health Perspect. 2012, 120, 1671. [Google Scholar] [CrossRef]
- MacNaughton, P.; Spengler, J.; Vallarino, J.; Santanam, S.; Satish, U.; Allen, J. Environmental perceptions and health before and after relocation to a green building. Build. Environ. 2016, 104, 138–144. [Google Scholar] [CrossRef]
- Allen, J.; MacNaughton, P.; Cedeno-Laurent, J.; Cao, X.; Flanigan, S.; Vallarino, J.; Rueda, F.; Donnelly-McLay, D.; Spengler, J. Airplane pilot flight performance on 21 maneuvers in a flight simulator under varying carbon dioxide concentrations. J. Expo. Sci. Environ. Epidemiol. 2018. [Google Scholar] [CrossRef]
- WAI. Current Statistics of Women in Aviation Careers in U.S. Available online: https://www.wai.org/resources/waistats (accessed on 1 July 2018).
- FAA. Airline Transport Pilot and Aircraft Type Rating Practical Test Standards for Airplane. Available online: https://www.faa.gov/training_testing/testing/test_standards/media/atp_pts.pdf (accessed on 1 July 2018).
- FAA. Pilot/Controller Glossary. Available online: https://www.faa.gov/air_traffic/publications/media/pcg_chg_2_9-13-18.pdf (accessed on 13 September 2018).
- Movisens. Ecgmove 3. Available online: https://www.movisens.com/en/products/ecg-and-activity-sensor-ecgmove-3/ (accessed on 1 July 2018).
- Nunan, D.; Sandercock, G.R.; Brodie, D.A. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin. Electrophysiol. 2010, 33, 1407–1417. [Google Scholar] [CrossRef]
- Voss, A.; Schroeder, R.; Heitmann, A.; Peters, A.; Perz, S. Short-term heart rate variability—Influence of gender and age in healthy subjects. PLoS ONE 2015, 10, e0118308. [Google Scholar] [CrossRef] [PubMed]
- Agelink, M.W.; Malessa, R.; Baumann, B.; Majewski, T.; Akila, F.; Zeit, T.; Ziegler, D. Standardized tests of heart rate variability: Normal ranges obtained from 309 healthy humans, and effects of age, gender, and heart rate. Clin. Auton. Res. 2001, 11, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Beckers, F.; Verheyden, B.; Aubert, A.E. Aging and nonlinear heart rate control in a healthy population. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2560–H2570. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.; Heitmann, A.; Schroeder, R.; Peters, A.; Perz, S. Short-term heart rate variability—Age dependence in healthy subjects. Physiol. Meas. 2012, 33, 1289. [Google Scholar] [CrossRef]
- Greiser, K.H.; Kluttig, A.; Schumann, B.; Swenne, C.A.; Kors, J.A.; Kuss, O.; Haerting, J.; Schmidt, H.; Thiery, J.; Werdan, K. Cardiovascular diseases, risk factors and short-term heart rate variability in an elderly general population: The carla study 2002–2006. Eur. J. Epidemiol. 2009, 24, 123. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.K.; Barzilay, J.I.; Chaves, P.H.; Domitrovich, P.P.; Gottdiener, J.S. Heart rate variability and its changes over 5 years in older adults. Age Ageing 2009, 38, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Porta, A.; Faes, L.; Bari, V.; Marchi, A.; Bassani, T.; Nollo, G.; Perseguini, N.M.; Milan, J.; Minatel, V.; Borghi-Silva, A. Effect of age on complexity and causality of the cardiovascular control: Comparison between model-based and model-free approaches. PLoS ONE 2014, 9, e89463. [Google Scholar] [CrossRef]
- Davy, K.P.; Orr, J.S. Sympathetic nervous system behavior in human obesity. Neurosci. Biobehav. Rev. 2009, 33, 116–124. [Google Scholar] [CrossRef]
- Molfino, A.; Fiorentini, A.; Tubani, L.; Martuscelli, M.; Fanelli, F.R.; Laviano, A. Body mass index is related to autonomic nervous system activity as measured by heart rate variability. Eur. J. Clin. Nutr. 2009, 63, 1263. [Google Scholar] [CrossRef]
- Millis, R.M.; Austin, R.E.; Hatcher, M.D.; Bond, V.; Faruque, M.U.; Goring, K.L.; Hickey, B.M.; DeMeersman, R.E. Association of body fat percentage and heart rate variability measures of sympathovagal balance. Life Sci. 2010, 86, 153–157. [Google Scholar] [CrossRef]
- Kaye, J.; Buchanan, F.; Kendrick, A.; Johnson, P.; Lowry, C.; Bailey, J.; Nutt, D.; Lightman, S. Acute carbon dioxide exposure in healthy adults: Evaluation of a novel means of investigating the stress response. J. Neuroendocrinol. 2004, 16, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Pöyhönen, M.; Syväoja, S.; Hartikainen, J.; Ruokonen, E.; Takala, J. The effect of carbon dioxide, respiratory rate and tidal volume on human heart rate variability. Acta Anaesthesiol. Scand. 2004, 48, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Y.; Chuang, H.-C.; Liu, I.-J.; Chen, H.-W.; Chuang, K.-J. Reducing indoor air pollution by air conditioning is associated with improvements in cardiovascular health among the general population. Sci. Total Environ. 2013, 463, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Hansen, A.L.; Saus-Rose, E.; Johnsen, B.H. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. 2009, 37, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.F.; Torres, R.V. The renaissance of heart rate variability as a predictor of cognitive functioning. Am. J. Hypertens. 2017, 31, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.D.; McRae, K.; Reiman, E.M.; Chen, K.; Ahern, G.L.; Thayer, J.F. Neural correlates of heart rate variability during emotion. Neuroimage 2009, 44, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Lane, R.D. Claude bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.; Goldman-Rakic, P.S. Noise stress impairs prefrontal cortical cognitive function in monkeys: Evidence for a hyperdopaminergic mechanism. Arch. Gen. Psychiatry 1998, 55, 362–368. [Google Scholar] [CrossRef]
- Thayer, J.F.; Brosschot, J.F. Psychosomatics and psychopathology: Looking up and down from the brain. Psychoneuroendocrinology 2005, 30, 1050–1058. [Google Scholar] [CrossRef]
- Hansen, A.L.; Johnsen, B.H.; Thayer, J.F. Vagal influence on working memory and attention. Int. J. Psychophysiol. 2003, 48, 263–274. [Google Scholar] [CrossRef]
- Hansen, A.L.; Johnsen, B.H.; Sollers, J.J.; Stenvik, K.; Thayer, J.F. Heart rate variability and its relation to prefrontal cognitive function: The effects of training and detraining. Eur. J. Appl. Physiol. 2004, 93, 263–272. [Google Scholar] [CrossRef]
- Frewen, J.; Finucane, C.; Savva, G.M.; Boyle, G.; Coen, R.F.; Kenny, R.A. Cognitive function is associated with impaired heart rate variability in ageing adults: The irish longitudinal study on ageing wave one results. Clin. Auton. Res. 2013, 23, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, S.P.; Gurja, J.P.; Sharma, R. Does heart rate variability predict human cognitive performance at higher memory loads? Indian J. Physiol. Pharm. 2017, 61, 14–22. [Google Scholar]
- Plessow, F.; Kiesel, A.; Kirschbaum, C. The stressed prefrontal cortex and goal-directed behaviour: Acute psychosocial stress impairs the flexible implementation of task goals. Exp. Brain Res. 2012, 216, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, L.; Tegenthoff, M.; Höffken, O.; Wolf, O.T. Simultaneous glucocorticoid and noradrenergic activity disrupts the neural basis of goal-directed action in the human brain. J. Neurosci. 2012, 32, 10146–10155. [Google Scholar] [CrossRef] [PubMed]
- Luft, C.D.B.; Takase, E.; Darby, D. Heart rate variability and cognitive function: Effects of physical effort. Biol. Psychol. 2009, 82, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Johnsen, B.H.; Thayer, J.F. Relationship between heart rate variability and cognitive function during threat of shock. Anxiety Stress Coping 2009, 22, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, L.; Wolf, O.T. Stress modulates the engagement of multiple memory systems in classification learning. J. Neurosci. 2012, 32, 11042–11049. [Google Scholar] [CrossRef]
- Foote, S.L.; Bloom, F.E.; Aston-Jones, G. Nucleus locus ceruleus: New evidence of anatomical and physiological specificity. Physiol. Rev. 1983, 63, 844–914. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Schwabe, L. Tell me what to do: Stress facilitates stimulus-response learning by instruction. Neurobiol. Learn. Mem. 2018, 151, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Montano, N.; Porta, A.; Cogliati, C.; Costantino, G.; Tobaldini, E.; Casali, K.R.; Iellamo, F. Heart rate variability explored in the frequency domain: A tool to investigate the link between heart and behavior. Neurosci. Biobehav. Rev. 2009, 33, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Reyes del Paso, G.A.; Langewitz, W.; Mulder, L.J.; Van Roon, A.; Duschek, S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology 2013, 50, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Friedman, B.H. An autonomic flexibility–neurovisceral integration model of anxiety and cardiac vagal tone. Biol. Psychol. 2007, 74, 185–199. [Google Scholar] [CrossRef] [PubMed]
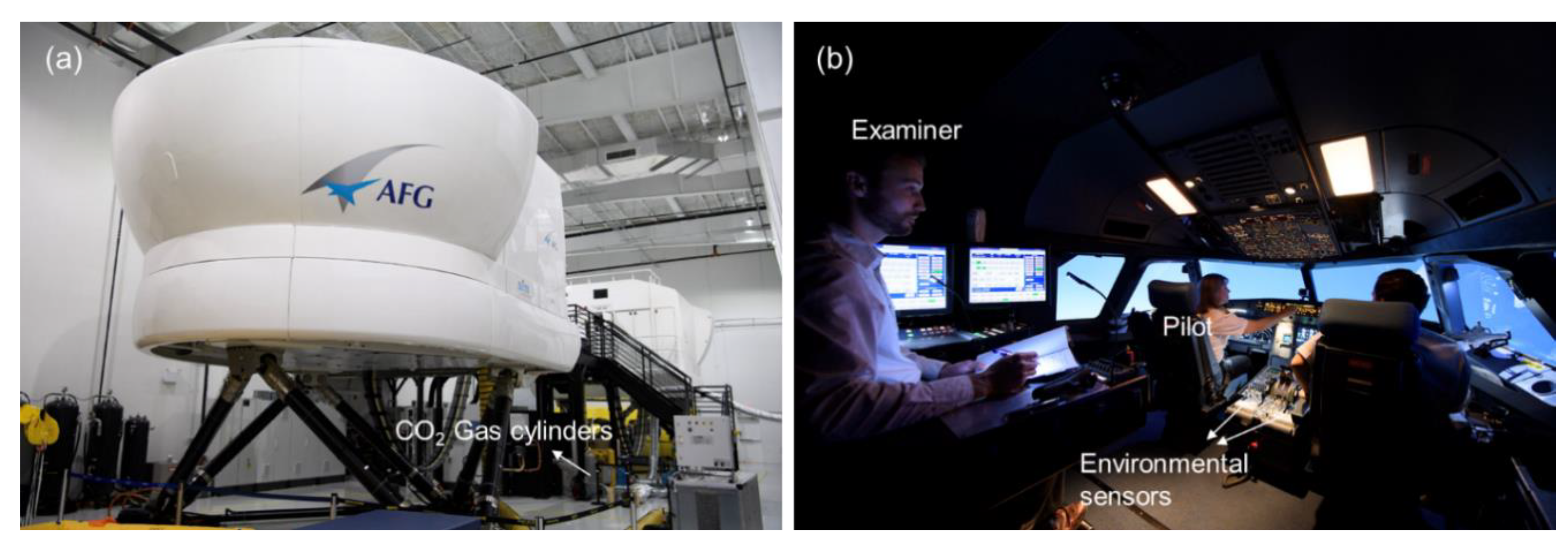
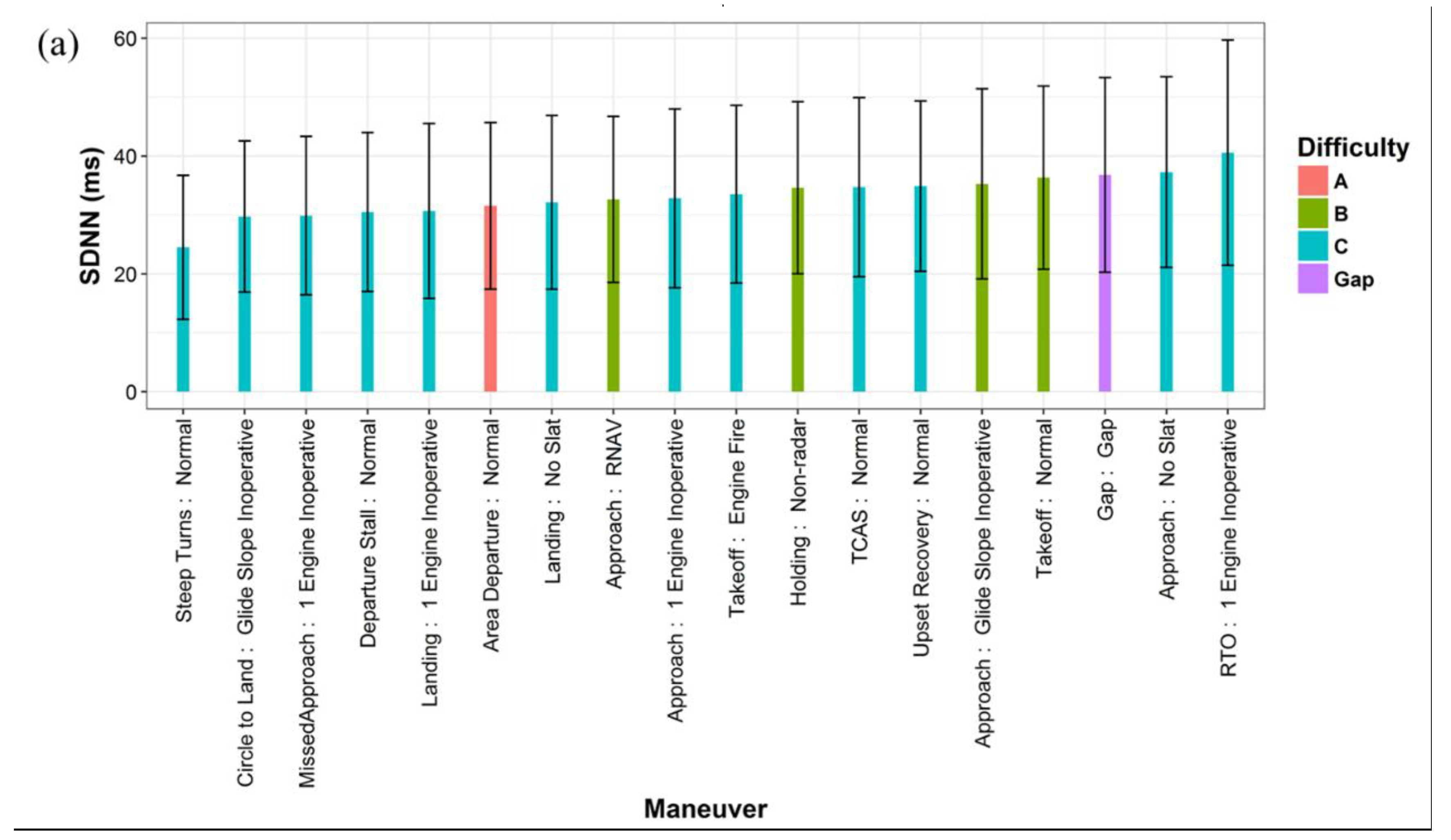
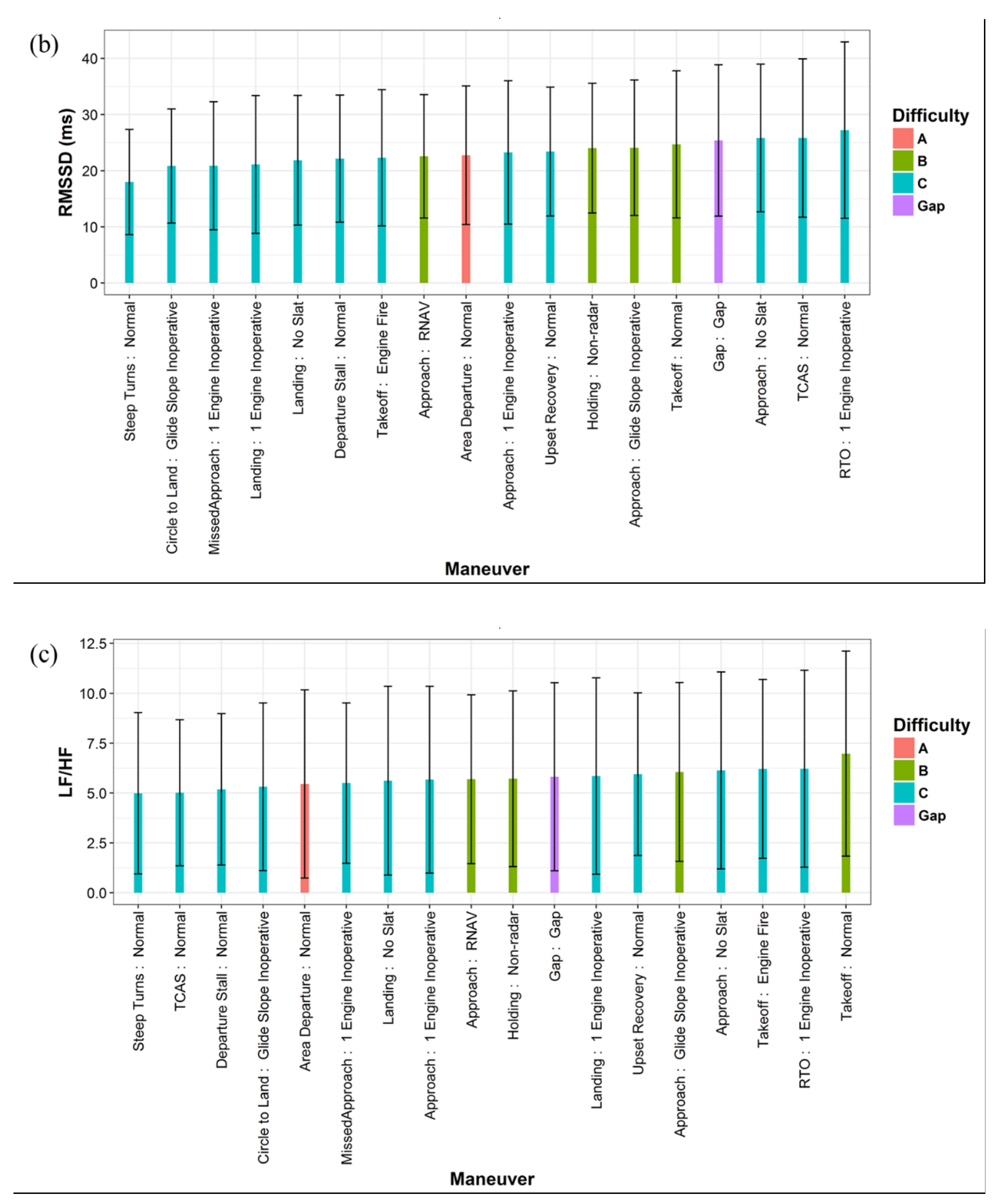
| Category | Percentage | |
|---|---|---|
| Gender | Male | 100% |
| Female | 0% | |
| Age | 30–40 | 37% |
| 41–50 | 27% | |
| >50 | 36% | |
| BMI (kg/m2) | 20–25 | 40% |
| 25–30 | 37% | |
| >30 | 23% | |
| Ethnicity | White/Caucasian | 20% |
| Latino | 74% | |
| Black or African American | 3% | |
| Multiracial | 3% | |
| Flight experience | Regularly fly 65+ hours/month as a pilot | 70% |
| Regularly fly 65+ hours/month in simulation | 30% | |
| HRV Index | Category | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|---|
| SDNN (ms) | Pilots (during simulations) | 34.1 | 12.7 | 32.1 | 12.3 | 59.3 |
| Pilots (before simulations) | 40.0 | 11.7 | 40.9 | 19.3 | 58.0 | |
| Normative values | 50.0 | 16.0 | 51.0 | 32.0 | 93.0 | |
| RMSSD (ms) | Pilots (during simulations) | 23.8 | 10.2 | 22.6 | 6.2 | 48.0 |
| Pilots (before simulations) | 26.7 | 9.4 | 26.7 | 12.8 | 48.4 | |
| Normative values | 42.0 | 15.0 | 42.0 | 19.0 | 75.0 | |
| LF/HF | Pilots (during simulations) | 5.7 | 2.8 | 5.5 | 1.5 | 14.1 |
| Pilots (before simulations) | 5.5 | 2.4 | 5.8 | 2.8 | 12.0 | |
| Normative values | 2.8 | 2.6 | 2.1 | 1.1 | 11.6 |
| Variable | SDNN (ms) (R-Squared = 0.393) | RMSSD (ms) (R-Squared = 0.443) | LF/HF (R-Squared = 0.189) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | p-Value | Estimate | Std. Error | p-Value | Estimate | Std. Error | p-Value | |
| Intercept | 19.21 | 1.54 | <0.001 | 12.89 | 1.20 | <0.001 | 5.96 | 0.49 | <0.001 |
| Age > 50 | 0.00 (Reference) | ||||||||
| 41 < Age < 50 | 11.62 | 0.86 | <0.001 | 4.13 | 0.68 | <0.001 | 1.33 | 0.27 | <0.001 |
| 30 < Age < 40 | 18.11 | 0.78 | <0.001 | 16.56 | 0.61 | <0.001 | −3.28 | 0.25 | <0.001 |
| BMI > 30 | 0.00 (Reference) | ||||||||
| 25 < BMI < 30 | 4.20 | 0.91 | <0.001 | 5.18 | 0.71 | <0.001 | −0.65 | 0.29 | 0.026 |
| 20 < BMI < 25 | 3.54 | 0.85 | <0.001 | 4.37 | 0.67 | <0.001 | −0.28 | 0.27 | 0.305 |
| Regularly fly 65+ hours/month in simulation | 0.00 (Reference) | ||||||||
| Regularly fly 65+ hours /month as a pilot | 6.16 | 0.82 | <0.001 | 0.43 | 0.64 | 0.507 | 1.36 | 0.26 | <0.001 |
| High CO2 | 0.00 (Reference) | ||||||||
| Medium CO2 | 0.64 | 0.73 | 0.384 | −0.15 | 0.57 | 0.797 | 0.64 | 0.23 | 0.006 |
| Low CO2 | 0.10 | 0.75 | 0.895 | −0.78 | 0.59 | 0.182 | 0.78 | 0.24 | 0.001 |
| Gap time | 0.00 (Reference) | ||||||||
| Difficulty A | −5.53 | 1.74 | 0.002 | −2.86 | 1.36 | 0.036 | −0.36 | 0.55 | 0.509 |
| Difficulty B | −2.03 | 1.37 | 0.138 | −1.40 | 1.07 | 0.191 | 0.22 | 0.43 | 0.605 |
| Difficulty C | −4.13 | 1.27 | 0.001 | −2.66 | 1.00 | 0.008 | −0.24 | 0.40 | 0.558 |
| Variable | Estimate | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|
| Intercept | 2.02 | -- | 0.004 |
| SDNN | 0.014 | 1.37 (0.93, 2.02) 1 | 0.111 |
| High CO2 | 1.00 (Reference) | ||
| Medium CO2 | 0.50 | 1.65 (1.06, 2.59) | 0.028 |
| Low CO2 | 0.63 | 1.87 (1.17, 3.01) | 0.009 |
| Difficulty A | 1.00 (Reference) | ||
| Difficulty B | −0.46 | 0.63 (0.17, 2.27) | 0.478 |
| Difficulty C | −1.43 | 0.24 (0.07, 0.79) | 0.020 |
| Variable | Estimate | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|
| Intercept | 2.19 | -- | 0.001 |
| RMSSD | 0.013 | 1.22 (0.87, 1.73) 1 | 0.251 |
| High CO2 | 1.00 (Reference) | ||
| Medium CO2 | 0.52 | 1.68 (1.07, 2.63) | 0.024 |
| Low CO2 | 0.63 | 1.88 (1.17, 3.02) | 0.009 |
| Difficulty A | 1.00 (Reference) | ||
| Difficulty B | −0.45 | 0.64 (0.18, 2.30) | 0.478 |
| Difficulty C | −1.44 | 0.24 (0.07, 0.79) | 0.020 |
| Variable | Estimate | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|
| Intercept | 2.67 | -- | <0.001 |
| LF/HF | −0.038 | 1.20 (0.94, 1.51) 1 | 0.137 |
| High CO2 | 1.00 (Reference) | ||
| Medium CO2 | 0.56 | 1.76 (1.12, 2.75) | 0.014 |
| Low CO2 | 0.65 | 1.92 (1.19, 3.08) | 0.007 |
| Difficulty A | 1.00 (Reference) | ||
| Difficulty B | −0.44 | 0.64 (0.18, 2.31) | 0.497 |
| Difficulty C | −1.48 | 0.23 (0.07, 0.76) | 0.016 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; MacNaughton, P.; Cadet, L.R.; Cedeno-Laurent, J.G.; Flanigan, S.; Vallarino, J.; Donnelly-McLay, D.; Christiani, D.C.; Spengler, J.D.; Allen, J.G. Heart Rate Variability and Performance of Commercial Airline Pilots during Flight Simulations. Int. J. Environ. Res. Public Health 2019, 16, 237. https://doi.org/10.3390/ijerph16020237
Cao X, MacNaughton P, Cadet LR, Cedeno-Laurent JG, Flanigan S, Vallarino J, Donnelly-McLay D, Christiani DC, Spengler JD, Allen JG. Heart Rate Variability and Performance of Commercial Airline Pilots during Flight Simulations. International Journal of Environmental Research and Public Health. 2019; 16(2):237. https://doi.org/10.3390/ijerph16020237
Chicago/Turabian StyleCao, Xiaodong, Piers MacNaughton, Leslie R. Cadet, Jose Guillermo Cedeno-Laurent, Skye Flanigan, Jose Vallarino, Deborah Donnelly-McLay, David C. Christiani, John D. Spengler, and Joseph G. Allen. 2019. "Heart Rate Variability and Performance of Commercial Airline Pilots during Flight Simulations" International Journal of Environmental Research and Public Health 16, no. 2: 237. https://doi.org/10.3390/ijerph16020237
APA StyleCao, X., MacNaughton, P., Cadet, L. R., Cedeno-Laurent, J. G., Flanigan, S., Vallarino, J., Donnelly-McLay, D., Christiani, D. C., Spengler, J. D., & Allen, J. G. (2019). Heart Rate Variability and Performance of Commercial Airline Pilots during Flight Simulations. International Journal of Environmental Research and Public Health, 16(2), 237. https://doi.org/10.3390/ijerph16020237






