Abstract
Risk prediction and response measures may differ in tuberculosis (TB) patients with low sputum smear positivity for acid-fast bacillus (AFB) compared to those who are smear negative. However, previous studies using the tuberculin skin test (TST) did not show that differences in measures are important. This study compared results of interferon-gamma release assays (IGRA) between contacts of pulmonary TB patients with AFB smear positivity and those with smear negativity using QuantiFERON®-TB Gold In-Tube (QFT) assays. Close contacts of TB patients with culture-confirmed infections between April 2010 and December 2012 in Ibaraki, Japan, were enrolled, and 439 Japanese contacts of 129 index TB patients were examined. Adjusted odds ratios of QFT in contacts were 0.68 (95% confidence interval: 0.17–2.8) for AFB scanty patients, 1.12 (0.45–2.8) for AFB 1+, 1.20 (0.48–3.0) for AFB 2+, and 4.96 (1.9–12.9) for AFB 3+, compared to those who were smear negative. Differences in IGRA positivity were not significant between close contacts of TB patients with low positive and negative smears.
1. Introduction
Tuberculosis (TB) is a major health concern, causing an estimated 1.3 million deaths in 2017 [1]. Contact exposure to Mycobacterium tuberculosis (Mtb) from TB patients increases the risk of contracting TB infections [2]. Infection risk resulting from exposure to pulmonary TB patients is influenced by host, bacterial, and environmental factors.
Sputum smear acid-fast bacillus (AFB) microscopy in index TB patients is used as an important predictive factor for pulmonary TB infection risk among contacts [2,3], as well as for risk-stratification of Mtb exposure. Therefore, sputum smear positivity or negativity for AFB of the index patient occasionally affects response measures of TB. The World Health Organization (WHO) formerly recommended that in low- and middle-income countries, contact investigations must be conducted for households and close contacts when the index case has sputum smear positive pulmonary tuberculosis [4]. In Japan, if a sputum smear positive pulmonary TB patient refuses admission, the local government by law mandates compulsive hospitalization in a designated hospital.
In detail, smears are graded as negative, scanty, 1+, 2+, or 3+ for AFB. The grades of smear positivity are used for risk stratification to implement response measures; for example, to delineate responses between patients with sputum smear negativity and low sputum smear positivity. Therefore, it is important to validate the degree to which low smear positivity for AFB can predict transmission risk to contacts as compared to negative smears.
TB infections remain latent in most infected contact cases. [5] Tuberculin skin tests (TSTs) and interferon-gamma release assays (IGRA) are used for identification of latent tuberculosis infection (LTBI). IGRA detects interferon-gamma release from lymphocytes after in vitro incubation of whole blood with Mtb antigens. According to a review by the WHO, the pooled risk ratio estimate for TST was 1.49 (95% confidence interval: 0.79–2.80), and that for IGRA was 2.03 (1.18–3.50). Although the estimate for IGRA was slightly higher than that for TST, the 95% confidence intervals for the estimates for TST and IGRA overlapped and were imprecise. The WHO guidelines recommend that either TST or IGRA be used to test for LTBI and that the availability and affordability of the tests will determine which will be chosen by clinicians and program managers [5].
A few studies using TSTs compared LTBI in contacts of patients with low smear positivity versus smear negativity [6,7,8,9]. However, these studies did not show any differences between close contacts of patients with low sputum smear positivity versus those with negative smears. Studies that used IGRAs for comparing contacts of patients positive for low grade sputum smear with contacts of patients with negative smears have not been reported.
This study used IGRAs to evaluate infection status among contacts in order to examine the differences in infections between close contacts of pulmonary TB patients with low sputum smear positivity for AFB and those of patients with smear negative results.
2. Materials and Methods
2.1. Study Design and Setting
This study used a cross-sectional design and recruited patients from hospitals in the Ibaraki prefecture of Japan. The Ibaraki prefecture neighbors metropolitan Tokyo and in 2012 had an annual tuberculosis incidence rate of 14.0 cases per 100,000 population [10].
2.2. Index TB Patients and Exposure
Index TB cases eligible for inclusion in this study involved patients with pulmonary TB diagnosed by designated hospitals with confirmed Mtb culture growth that were registered at any government public health center in Ibaraki from April 2010 to December 2012. We randomly enrolled participants from among index patients who had at least one close contact. The public health centers investigated the patients and collected information regarding cough, maximum smear grading for AFB of three consecutive sputum samples, and chest radiograph results. The exposure measured in this study was the AFB grading of three sputum smears in index patients. Low smear positivity was defined as a grade of either scanty or 1+.
2.3. Subject Contacts
Subject contacts were considered close contacts of enrolled index TB patients. Per the Japan national TB control program regulations and 2010 guidelines [11], contacts of index TB patients were visited and interviewed by public health nurses. Close contacts included in this study were those who had lived or worked in the same room as the index TB patient. Since the prevalence of tuberculosis and LTBI was shown to be higher in populations from foreign countries or those aged over 60 years old [12,13], contacts that met these criteria were excluded from the study.
2.4. Outcome
Since nearly all Japanese residents have received mandatory Bacillus Calmette-Guerin (BCG) vaccines during childhood, the IGRA was more suited for determining LTBI [14]. Furthermore, published guidelines recommend that the IGRA, QuantiFERON®-TB Gold In-Tube (QFT) assay (Cellestis Limited, Carnegie, Australia), be preferentially used in health screenings, except among children, two or three months after exposure to TB [11]. The QFT antigens included a mixture of peptides representing ESAT-6, CFP-10, and TB7.7 proteins. In this study, blood samples were collected from contacts by public health nurses and transported with appropriate temperature control to the Ibaraki Health Service Association laboratory. The whole blood was collected in three tubes (specific antigen, positive control, and negative control) at the temperature of 22 ± 5 ℃. It stimulated the antigenic mixture in the tube. The tubes were incubated at 37 ℃ for 16 to 24 h, and centrifugated. INF-γ release was measured by ELISA. QFT assays were performed following the manufacturer’s recommended protocol. The assay was evaluated by the difference of readouts between the antigenic mixture and the negative control.
The main study outcome measured was QFT assay positivity. Cut-off value of 0.35 IU/mL was used in the analysis according to the guidelines [11].
2.5. Statistical Analysis
Contact characteristics were summarized. The QFT positivity rates of contacts were calculated and compared across index patients or contact risk factors. Logistic regression analyses were used to calculate adjusted odds ratios. Data were presented as counts with percentages or odds ratios with 95% confidence intervals (95% CI). Statistical analyses were performed using R (version 2.4-0; The R Foundation for Statistical Computing, Vienna, Austria).
2.6. Ethical Approval
The study protocol was approved on 19 November 2012 by the Ibaraki Prefecture Epidemiological Research Joint Ethics Review Committee (protocol number: H24-01). The study was conducted in accordance with the Declaration of Helsinki. Per protocol, we collected data from original databases established by individual public health centers following data anonymization. Per protocol, instead of written informed consent, the study plan and means to opt out of the study were advertised on the Ibaraki prefecture website homepage.
3. Results
A total of 129 index TB patients registered at public health centers in the Ibaraki prefecture during the study period were randomly selected, and a total of 515 close contacts of these patients screened were included in this study. Of these, 43 foreign contacts and 33 Japanese contacts aged 60 years and over were excluded and the remaining 439 close contacts were included in the analysis (Figure 1). The mean and medium (range) of number of contacts per TB case were, respectively, 4.8 and 2 (1–43).
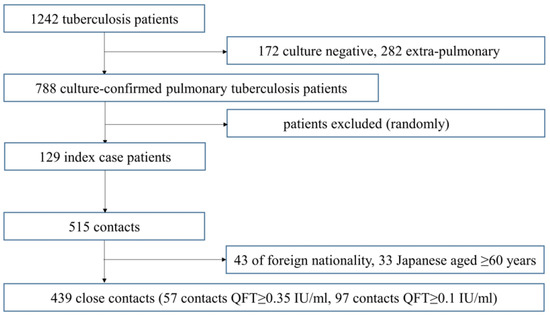
Figure 1.
Flow chart of study outline.
Table 1 describes the characteristics of contacts based on the index patient’s AFB smear status, with 103, 41, 115, 132, and 48 contacts who had negative, scanty, 1+, 2+, and 3+ grades, respectively.

Table 1.
Characteristics of close contacts.
A total of 57 (13%) contacts tested positive using QFT analysis. Four contacts with QFT-positive results contracted TB. Crude odds ratios of contacts showed higher QFT-positive yield in contacts of patients with cough, and high sputum smear grading (trend p-value = 0.0016). However, they did not show higher yield in contacts of patients with cavity or older age. In the multivariate analysis, the adjusted odds ratios of contact QFT based on the index patient’s smear status compared to that of smear negative patients were 0.68 (95% CI: 0.17–2.8) for scanty AFB smears, 1.12 (95% CI: 0.45–2.8) for AFB 1+ smears, 1.20 (95% CI: 0.48–3.0) for AFB 2+ smears, and 4.96 (95% CI: 1.9–12.9) for AFB 3+ smears (Table 2).

Table 2.
QuantiFERON®-TB Gold In-Tube (QFT) positivity of index tuberculosis (TB) patient close contacts.
4. Discussion
The study findings showed that close contacts of patients with sputum smears graded AFB 3+ had higher IGRA positivity than those of patients with smear negative results. We did not find significant differences in IGRA positivity between close contacts of patients with low and negative sputum smear AFB grades. As a result, this study did not provide any evidence indicating differences in transmission risk between contacts of patients with low sputum smear positivity and smear negativity.
In previous studies that did not classify patients based on sputum smear grading, contacts of patients with sputum smear positivity showed higher LTBI positivity rates than those of patients with smear negativity [2,15,16,17,18,19,20]. However, since sputum smears were not graded, these studies were unable to directly compare LTBI positivity between contacts of patients with sputum smear AFB 1+ and those of patients with smear negativity. Other studies conducted in high-incidence countries reported that contacts of patients with high sputum smear positivity had higher TST or IGRA yields than those of patients with low smear positivity. However, these studies did not include contacts of patients with negative sputum smears [21,22,23,24,25].
Several studies compared infection status among close contacts of patients with sputum smear gradings for AFB, including patients with smear negativity, using TST [6,7,8,9]. In these studies, there was a significant trend for increases in the number of infections in contacts as the degree of smear positivity in index TB patients increased. However, these studies failed to show the differences in infectivity between contacts of patients with low grade sputum smear positivity and sputum smear negativity. In addition, previous studies have not used IGRA measures to compare infections in contacts of patients with low grades and negative AFB smears. Our results and the lack of direct findings in other studies indicate that there is insufficient evidence to suggest differences in transmission risk or recommended response measures between low sputum smear positivity and smear negativity.
We used evaluations of IGRA outcomes in this study. Nienhaus et al. reported that agreement of IGRA and TST is excellent with little potential that TST is more likely to detect old infections than IGRA [10]. A systematic review and meta-analysis found that commercial IGRAs had a higher positive and negative predictive value for progression from latent infection to active disease [26]. Both TST and IGRA are widely available and have been used for LTBI testing, and the advantages and disadvantages of these tests have been previously described [27]. Japanese guidelines recommend that IGRA should be preferably used for health screening among adults because of higher specificity in BCG-vaccinated individuals [11,14]. However, the WHO guidelines note that BCG vaccination should not be a determining factor when selecting a test [5]. The present study used IGRA testing and did not show evidence warranting the implementation of different infection prevention measures for close contacts of patients with low sputum smear positivity and those of patients with smear negative results.
The 2012 and 2013 WHO recommendations stated that contact investigations should always be done when the index case has sputum smear positive pulmonary TB [4,28], whereas 2015 recommendations targeting high- or upper middle-income countries and the 2018 guidelines do not mention smear positivity results for initiation of contact investigations [5,29]. In the United States, the Centers for Disease Control and Prevention noted that index patients with positive AFB sputum smear results or pulmonary cavities should receive the highest priority for examination, and sputum smear positivity has long been incorporated into contact investigation screening algorithms [23,30]. However, we do not have enough evidence for the same risk prediction and response measures between contacts of patient with low sputum smear positivity and smear negativity.
Japanese legislation mandates admission and hospitalization of smear positive pulmonary TB patients until three follow-up smear AFB tests are negative following treatment. However, international standards note that the exclusive use of health facility-based directly observed treatment (DOT) may be associated with disadvantages [31].
This study had several limitations. First, the relatively small sample size may have prevented the detection of statistical significance. Second, we were unable to evaluate the demographics or cough frequency in index patients, and we did not have access to associated environmental factors. Environmental factors that would have been useful for analysis include individual contact duration time, information on each patient’s living situation including the level of crowding, and proximity to the index patient, as these may have affected risk of infection. Third, IGRA readouts suffer from within-subject variability, which may have affected the study findings [27]. Lastly, HIV infection status was not described; however, since HIV prevalence is very low, 0.8 cases per 100,000 in the Ibaraki prefecture, the overall effect on this dataset would be limited.
Given the limitations described above, further studies need to be implemented to assess the differences in IGRA positivity between contacts of TB patients and low sputum smear positivity and smear negativity. These studies would include larger sample sizes and evaluate environmental factors present in various settings. Relative risk as well as statistical significance based on differences in positive contact results between groups should be considered for TB response decisions.
5. Conclusions
This study did not find significant differences in the number of IGRA-positive contacts of index TB patients with low sputum smear positivity and those of patients with negative smears. Future studies with larger sample sizes and evaluation of environmental factors are necessary to conclusively evaluate the relationship of low smear positivity and smear negativity of TB patients with infection risk of contacts.
Author Contributions
Conceptualization, T.O. and R.U.; investigation, T.O. and N.N.; formal analysis, T.O. and R.U.; validation, K.I.; writing, T.O. and N.N.
Funding
This research received no external funding.
Acknowledgments
We are grateful to Ms. Naomi Takano from the Hokota Public Health Center of the Ibaraki prefectural government and Ms. Shizue Kobayashi of the Shimozuma Health Center of the Shimozuma city government for their contributions to data collecting and processing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Global Tuberculosis 2018 Report; WHO/CDS/TB: Paris, France, 2018. [Google Scholar]
- Capewell, S.; Leitch, A.G. The value of contact procedures for tuberculosis in Edinburgh. Br. J. Dis. Chest 1984, 78, 317–329. [Google Scholar] [CrossRef]
- Marais, B.J.; Gie, R.P.; Schaaf, H.S.; Hesseling, A.C.; Obihara, C.C.; Nelson, L.J.; Enarson, D.A.; Donald, P.R.; Beyers, N. The clinical epidemiology of childhood pulmonary tuberculosis: A critical review of literature from the prechemotherapy era. Int. J. Tuberc. Lung Dis. 2004, 8, 278–285. [Google Scholar] [PubMed]
- World Health Organization. Recommendations for Investigating Contacts of Persons with Infectious Tuberculosis in Low- and Middle-Income Countries; WHO/HTM/TB: Geneva, Switzerland, 2012. [Google Scholar]
- World Health Organization. Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management; WHO/CDS/TB: Geneva, Switzerland, 2018. [Google Scholar]
- Kenyon, T.A.; Creek, T.; Laserson, K.; Makhoa, M.; Chimidza, N.; Mwasekaga, M.; Tappero, J.; Lockman, S.; Moeti, T.; Binkin, N. Risk factors for transmission of Mycobacterium tuberculosis from HIV-infected tuberculosis patients, Botswana. Int. J. Tuberc. Lung Dis. 2002, 6, 843–850. [Google Scholar] [PubMed]
- Sinfield, R.; Nyirenda, M.; Haves, S.; Molyneux, E.M.; Graham, S.M. Risk factors for TB infection and disease in young childhood contacts in Malawi. Ann. Trop. Paediatr. 2006, 26, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Aissa, K.; Madhi, F.; Ronsin, N.; Delarocque, F.; Lecuyer, A.; Decludt, B.; Remus, N.; Abel, L.; Poirier, C.; Delacourt, C.; et al. Evaluation of a model for efficient screening of tuberculosis contact subjects. Am. J. Respir. Crit. Care Med. 2008, 177, 1041–1047. [Google Scholar] [CrossRef]
- Ma, N.; Zalwango, S.; Malone, L.L.; Nsereko, M.; Wampande, E.M.; Thiel, B.A.; Okware, B.; Igo, R.P.; Joloba, M.L.; Mupere, E.; et al. Tuberculosis Research Unit (TBRU). Clinical and epidemiological characteristics of individuals resistant to M. tuberculosis infection in a longitudinal TB household contact study in Kampala, Uganda. Infect. Dis. 2014, 14, 352–362. [Google Scholar]
- Ibaraki Prefectural Government. Tuberculosis Satistics in Ibaraki Prefectural Government. Available online: http://www.pref.ibaraki.jp/hokenfukushi/yobo/kiki/yobo/kansen/idwr/information/kekkaku/documents/2012.pdf (accessed on 1 August 2019).
- Isikawa, N.; Ahiko, T.; Inuzuka, K.; Inagaki, T.; Kato, S.; Kawabe, Y.; Kobayashi, N.; Sasaki, Y.; Suzuki, K.; Takamatsu, I.; et al. Kekkaku no Sesshokusha Kenko Shindan no Tebiki (Guidelines on Tuberculosis Contact Investigation Based on the Infectious Disease Control Law); Japan Anti-Tuberculosis Association: Tokyo, Japan, 2010. [Google Scholar]
- Seto, J.; Ahiko, T. Effectiveness of interferon-gamma release assays in the tuberculosis contact investigation of elderly people. Kekkaku 2014, 89, 503–508. [Google Scholar]
- Harada, N.; Nakajima, Y.; Higuchi, K.; Sekiya, Y.; Rothel, J.; Mori, T. Screening for Tuberculosis Infection Using Whole-Blood Interferon-γ and Mantoux Testing Among Japanese Healthcare Workers. Infect. Control Hosp. Epidemiol. 2006, 27, 442–448. [Google Scholar] [CrossRef]
- Nienhaus, A.; Schablon, A.; Diel, R. Interferon-Gamma Release Assay for the Diagnosis of Latent TB Infection–Analysis of Discordant Results, when Compared to the Tuberculin Skin Test. PLoS ONE 2008, 3. [Google Scholar] [CrossRef]
- Radhakrishna, S.; Frieden, T.R.; Subramani, R.; Santha, T.; Narayanan, P.R. Indian Council of Medical Research. Additional risk of developing TB for household members with a TB case at home at intake: A 15–year study. Int. J. Tuberc. Lung Dis. 2007, 11, 282–288. [Google Scholar]
- Triasih, R.; Robertson, C.; Duke, T.; Graham, S.M. Risk of infection and disease with Mycobacterium tuberculosis among children identified through prospective community–based contact screening in Indonesia. Trop. Med. Int. Health 2015, 20, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Pagaoa, M.A.; Royce, R.A.; Chen, M.P.; Golub, J.E.; Davidow, A.L.; Hirsch-Moverman, Y.; Marks, S.M.; Teeter, L.D.; Thickstun, P.M.; Katz, D.J. Risk factors for transmission of tuberculosis among United States-born African Americans and Whites. Int. J. Tuberc. Lung Dis. 2015, 19, 1485–1492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cavany, S.M.; Sumner, T.; Vynnycky, E.; Flach, C.; White, R.G.; Thomas, H.L.; Maguire, H.; Anderson, C. An evaluation of tuberculosis contact investigations against national standards. Thorax 2017, 72, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Shen, Y.; Mupere, E.; Kizza, A.; Hill, P.C.; Whalen, C.C. Transmission of Mycobacterium Tuberculosis in Households and the Community: A Systematic Review and Meta-Analysis. Am. J. Epidemiol. 2017, 185, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.S.; Kim, I.; Kim, W.Y.; Jo, E.J.; Mok, J.; Kim, M.H.; Lee, K.; Kim, K.U.; Park, H.K.; Lee, M.K. Household tuberculosis contact investigation in a tuberculosis-prevalent country: Are the tuberculin skin test and interferon-gamma release assay enough in elderly contacts? Medicine 2018, 97, 9681–9687. [Google Scholar] [CrossRef] [PubMed]
- Rathi, S.K.; Akhtar, S.; Rahbar, M.H.; Azam, S.I. Prevalence and risk factors associated with tuberculin skin test positivity among household contacts of smear-positive pulmonary tuberculosis cases in Umerkot, Pakistan. Int. J. Tuberc. Lung Dis. 2002, 6, 851–857. [Google Scholar] [PubMed]
- Tornee, S.; Kaewkungwa, J.; Fungladda, W.; Silachamroon, U.; Akarasewi, P.; Sunakorn, P. Risk factors for tuberculosis infection among household contacts in Bangkok, Thailand. Southeast Asian J. Trop. Med. Public Health 2004, 35, 375–383. [Google Scholar]
- Nguyen, T.H.; Odermatt, P.; Slesak, G.; Barennes, H. Risk of latent tuberculosis infection in children living in households with tuberculosis patients: A cross sectional survey in remote northern Lao People’s Democratic Republic. Infect. Dis. 2009, 9, 96–105. [Google Scholar] [CrossRef]
- Rutherford, M.E.; Hill, P.C.; Maharani, W.; Apriani, L.; Sampurno, H.; van Crevel, R.; Ruslami, R. Risk factors for Mycobacterium tuberculosis infection in Indonesian children living with a sputum smear-positive case. Int. J. Tuberc. Lung Dis. 2012, 16, 1594–1599. [Google Scholar] [CrossRef]
- Acuña-Villaorduña, C.; Schmidt-Castellani, L.G.; Marques-Rodrigues, P.; White, L.F.; Hadad, D.J.; Gaeddert, M.; Ellner, J.J.; Fennelly, K.P.; Palaci, M.; Dietze, R.; et al. Cough-aerosol cultures of Mycobacterium tuberculosis in the prediction of outcomes after exposure. A household contact study in Brazil. PLoS ONE 2018, 13, e0206384. [Google Scholar] [CrossRef]
- Diel, R.; Loddenkemper, R.; Nienhaus, A. Predictive Value of Interferon-γ Release Assays and Tuberculin Skin Testing for Progression From Latent TB Infection to Disease State. Chest 2012, 142, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Denkinger, C.M.; Kik, S.V.; Rangaka, M.X.; Zwerling, A.; Oxlade, O.; Metcalfe, J.Z.; Cattamanchi, A.; Dowdy, D.W.; Dheda, K.; et al. Gamma Interferon Release Assays for Detection of Mycobacterium tuberculosis Infection. Clin Microbiol Rev 2014, 27. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Systematic Screening for Active Tuberculosis: Principles and Recommendations; WHO/HTM/TB: Geneva, Switzerland.
- World Health Organization. Guidelines on the management of latent tuberculosis infection; WHO/HTM/TB: Geneva, Switzerland, 2015; ISBN 978 92 4 154890 8. [Google Scholar]
- Julie, L.; Dixie, E.S.; Tanja, P.; Steven, L.S.; Jay, M.B.; Maria, S.P.; Mary, L.L.; Suzanne, M.H.; Teresa, F.R.; Jeffrey, D.S.; et al. Guidelines for the Investigation of Contacts of Persons with Infectious Tuberculosis. Recomm. Rep. 2005, 54, 1–47. [Google Scholar]
- TB CARE 1. International Standards for Tuberculosis Care; TB CARE 1: The Hague, The Netherlands, 2014. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).