Hydrochemistry and Dissolved Inorganic Carbon (DIC) Cycling in a Tropical Agricultural River, Mun River Basin, Northeast Thailand
Abstract
1. Introduction
2. Materials and Methods
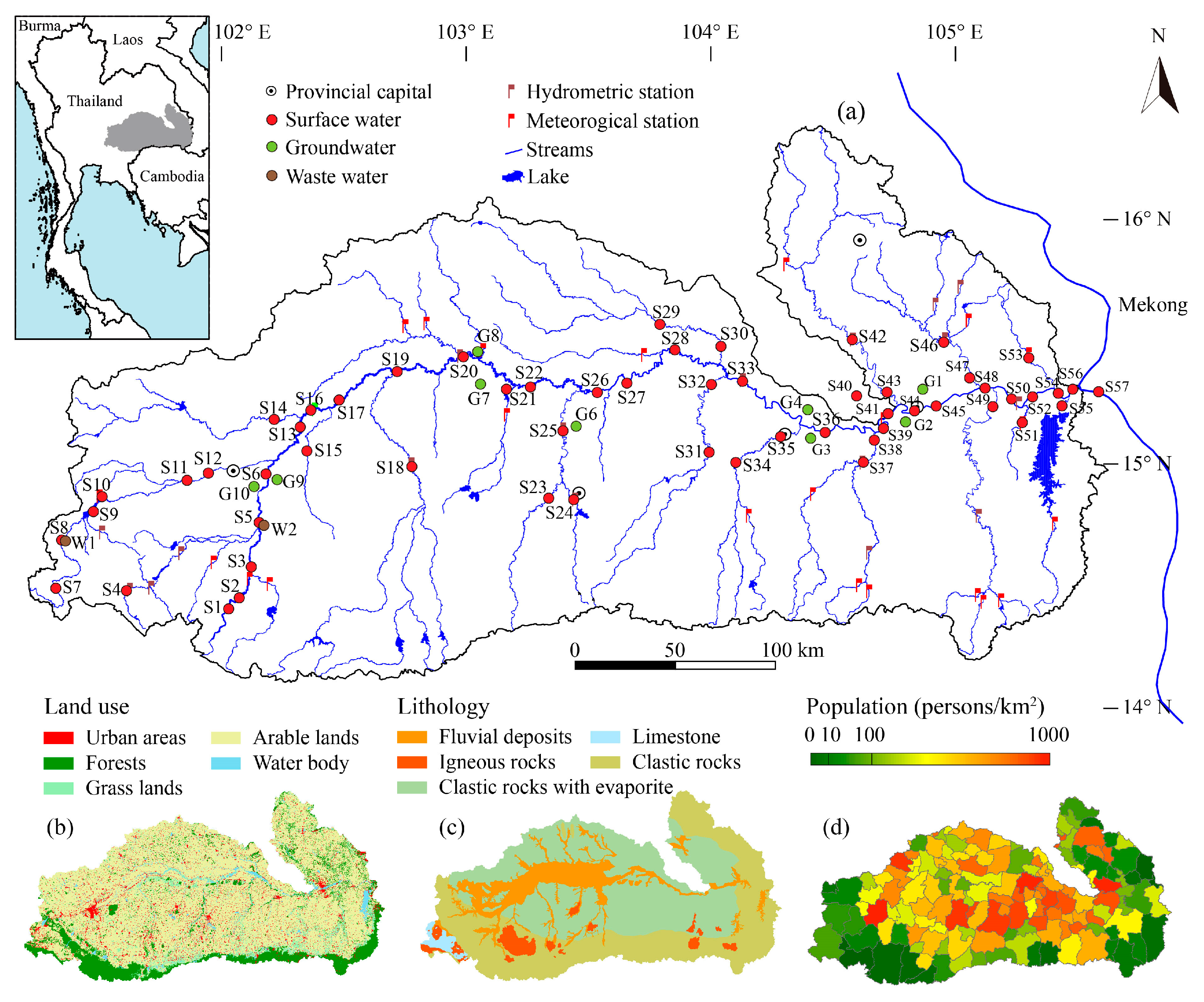
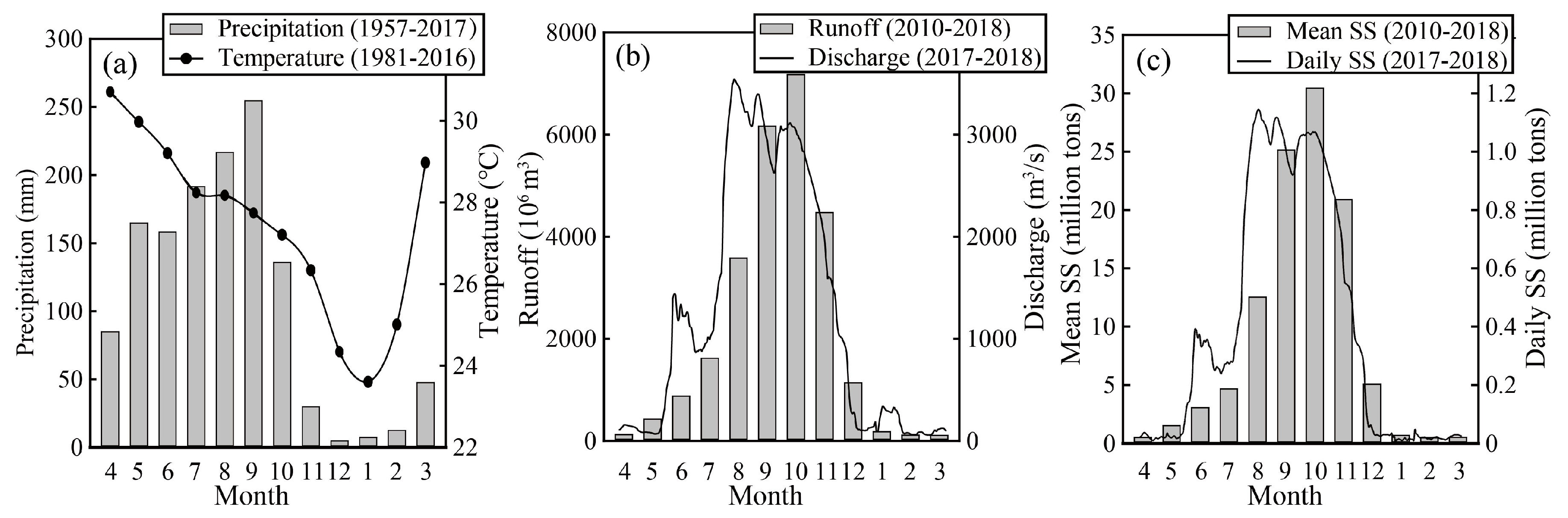
2.1. Study Area
2.2. Water Sampling and Analysis
2.3. Data Processing
3. Results
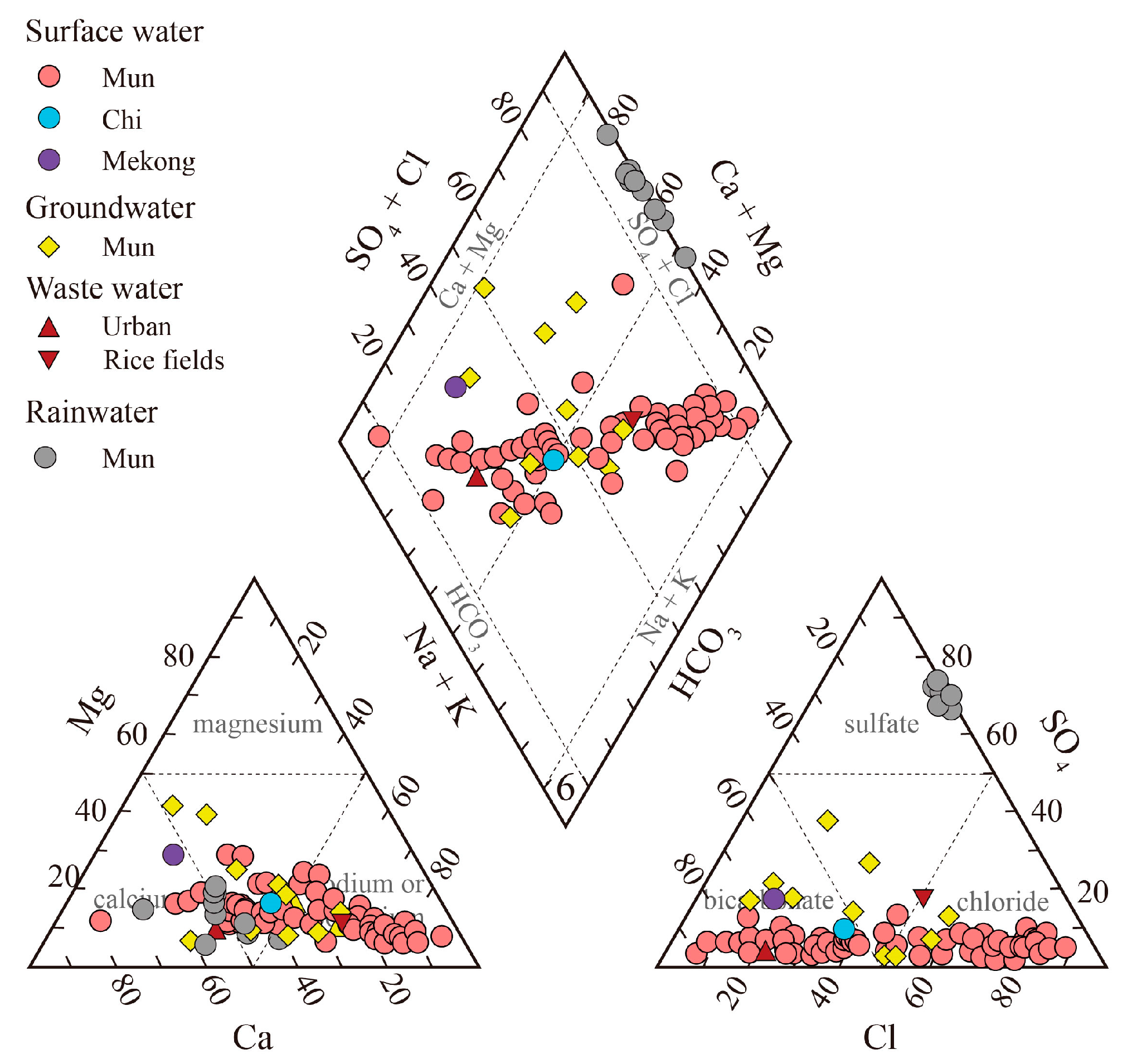
3.1. Geochemistry of the Mun River Water
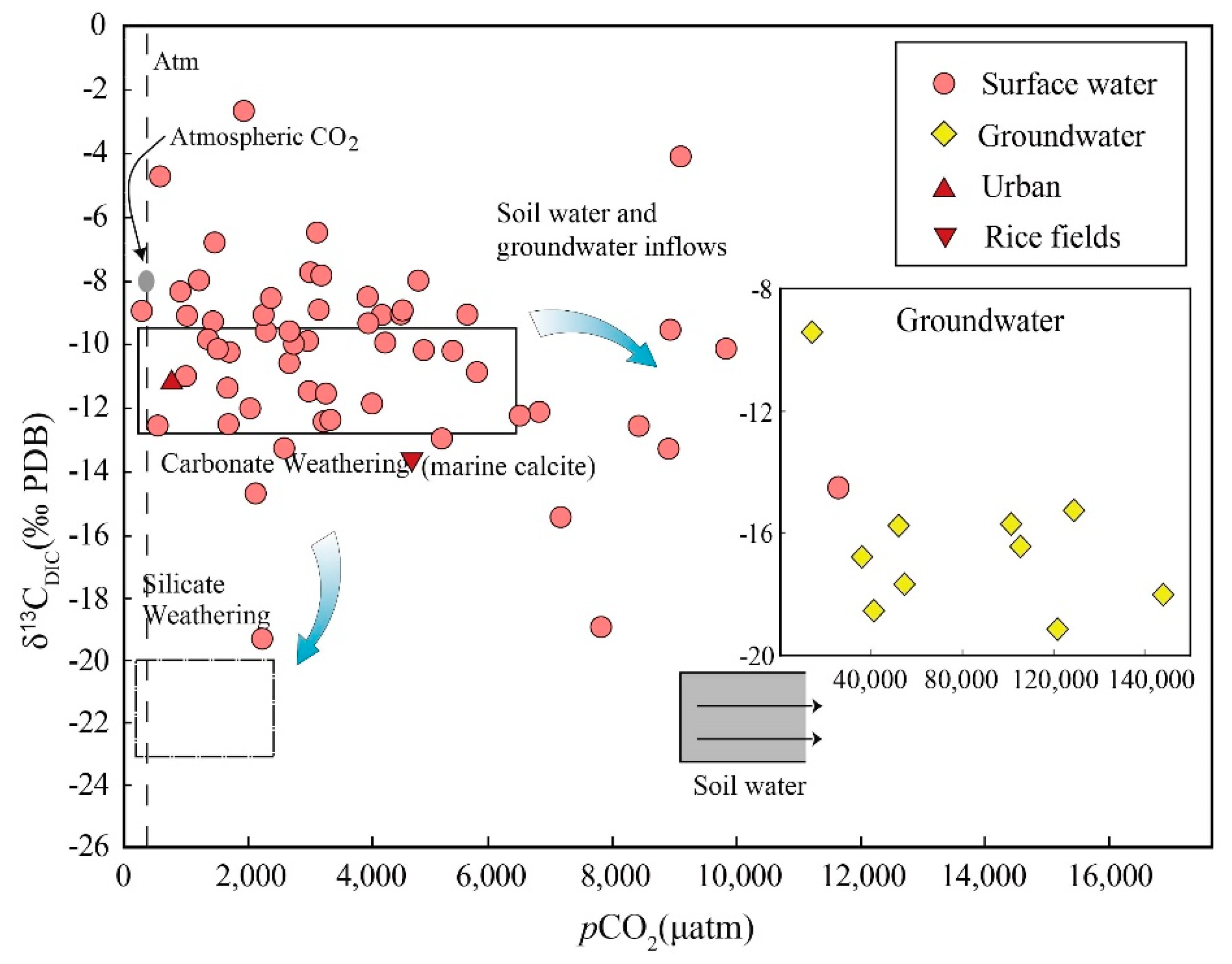
3.2. DIC System and δ13CDIC Values
4. Discussion
4.1. Sources of Groundwater DIC
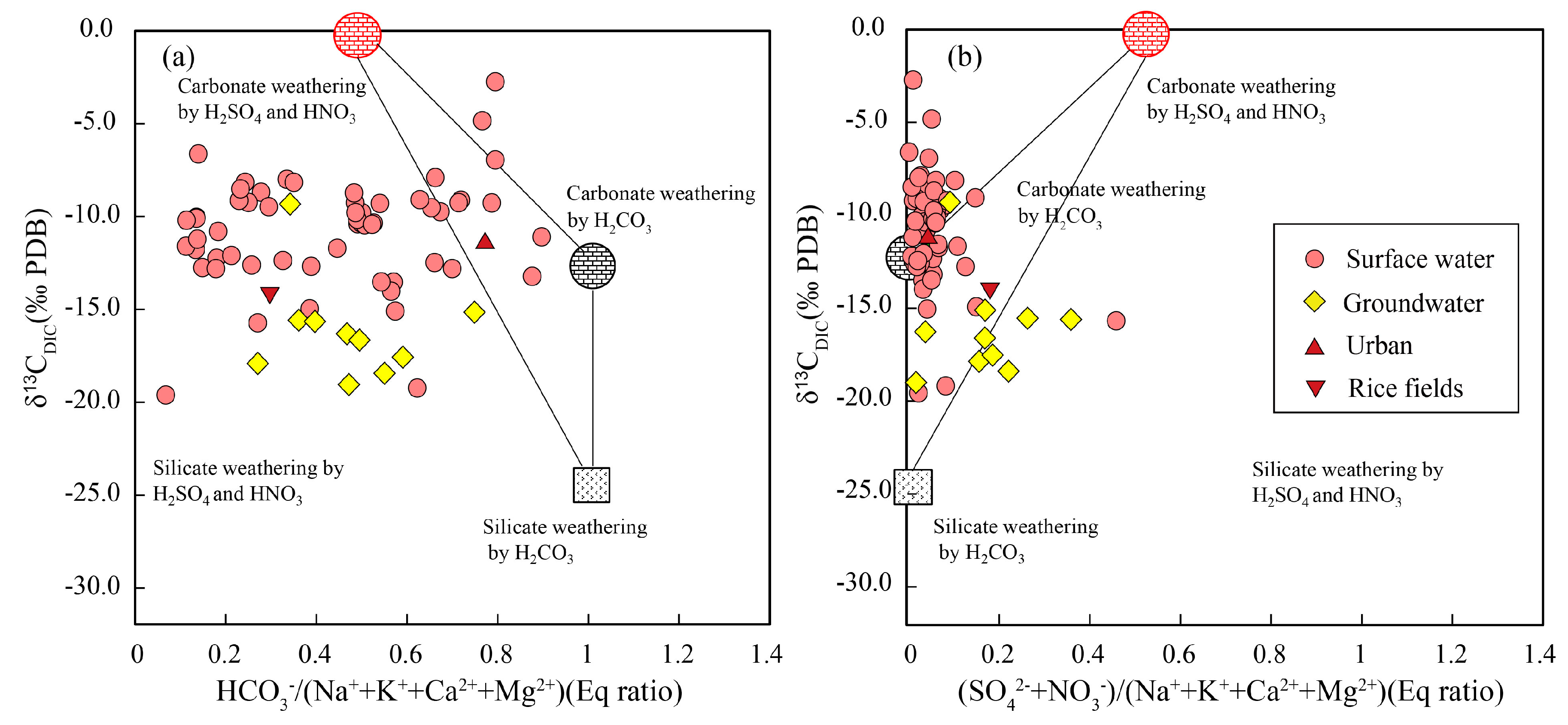
4.2. Rock Weathering Versus the δ13CDIC Values
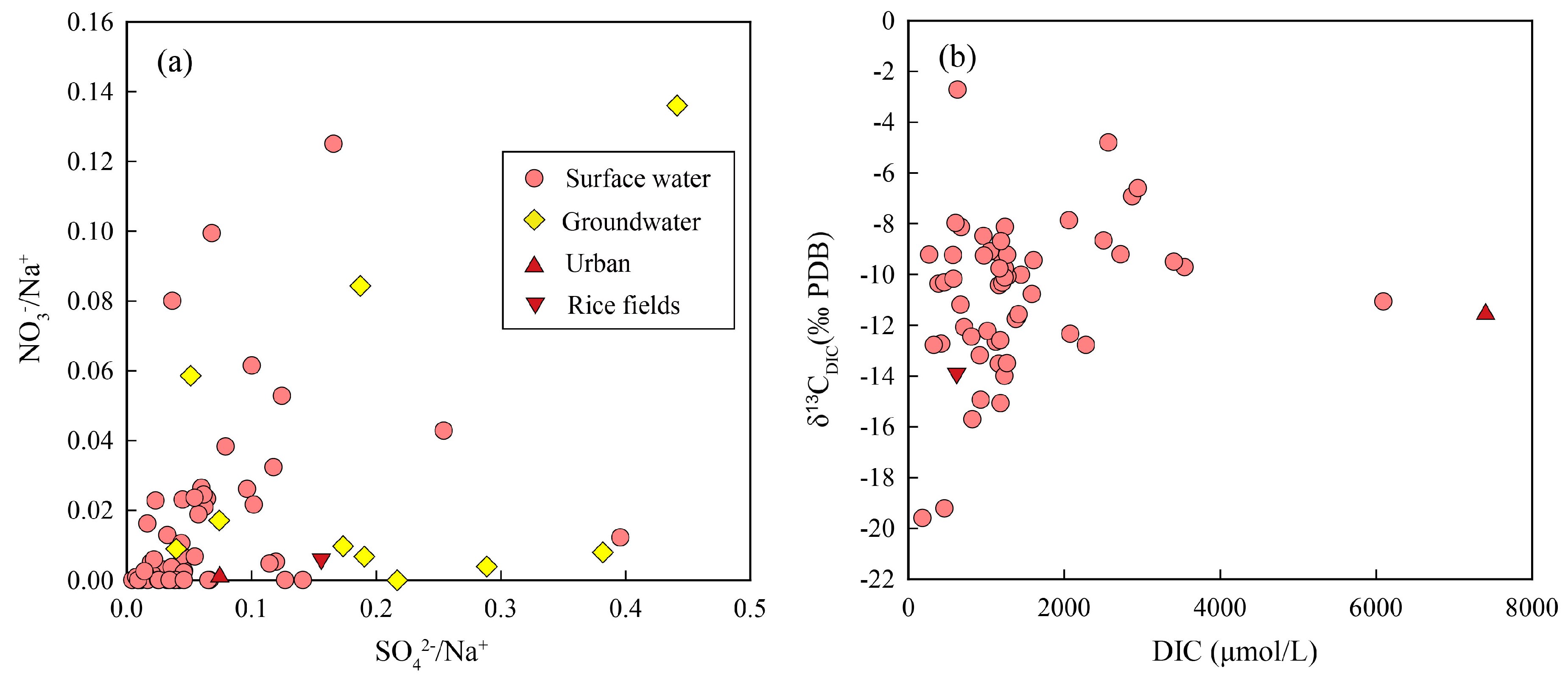
4.3. Carbon Isotopic Composition of Anthropogenic DIC
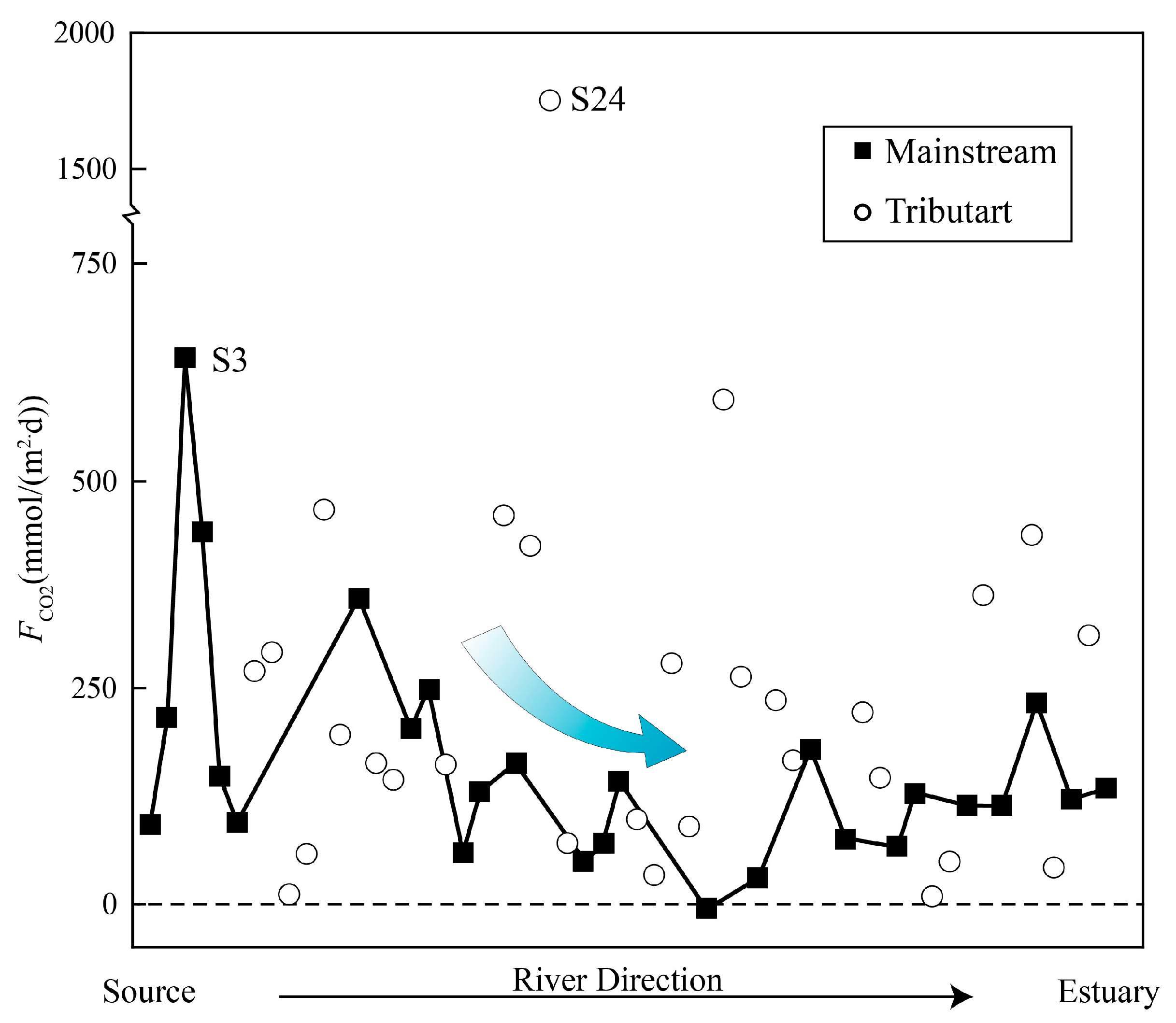
4.4. DIC Evasion from the River System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raymond, P.A.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Humborg, C.; et al. Global carbon dioxide emissions from inland waters. Nature 2013, 503, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Dubois, K.D.; Lee, D.; Veizer, J. Isotopic constraints on alkalinity, dissolved organic carbon, and atmospheric carbon dioxide fluxes in the Mississippi River. J. Geophys. Res. Biogeosci. 2010, 115, 1–11. [Google Scholar] [CrossRef]
- Duvert, C.; Butman, D.E.; Marx, A.; Ribolzi, O.; Hutley, L.B. CO2 evasion along streams driven by groundwater inputs and geomorphic controls. Nat. Geosci. 2018, 11, 813–818. [Google Scholar] [CrossRef]
- Campeau, A.; Bishop, K.; Nilsson, M.B.; Klemedtsson, L.; Laudon, H.; Leith, F.I.; Öquist, M.; Wallin, M.B. Stable Carbon Isotopes Reveal Soil-Stream DIC Linkages in Contrasting Headwater Catchments. J. Geophys. Res. Biogeosci. 2018, 123, 149–167. [Google Scholar] [CrossRef]
- Li, S.; Lu, X.X.; Bush, R.T. CO2 partial pressure and CO2 emission in the Lower Mekong River. J. Hydrol. 2013, 504, 40–56. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupre, B.; Louvat, P.; Allegre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Han, G.; Liu, C.-Q. Water geochemistry controlled by carbonate dissolution: A study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chem. Geol. 2004, 204, 1–21. [Google Scholar] [CrossRef]
- Regnier, P.; Friedlingstein, P.; Ciais, P.; Mackenzie, F.T.; Gruber, N.; Janssens, I.A.; Laruelle, G.G.; Lauerwald, R.; Luyssaert, S.; Andersson, A.J.; et al. Anthropogenic perturbation of the carbon fluxes from land to ocean. Nat. Geosci. 2013, 6, 597–607. [Google Scholar] [CrossRef]
- Chetelat, B.; Liu, C.Q.; Zhao, Z.Q.; Wang, Q.L.; Li, S.L.; Li, J.; Wang, B.L. Geochemistry of the dissolved load of the Changjiang Basin rivers: Anthropogenic impacts and chemical weathering. Geochim. Cosmochim. Acta 2008, 72, 4254–4277. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Xu, Y.; Zhang, Y.; Guo, L.; Zheng, Q.; Tang, L. Quantitative Study on the Changes of Karst Groundwater Level and Hydrochemistry in Jinci Spring Catchment, Shanxi, China. Expo. Health 2019, 11, 1–13. [Google Scholar] [CrossRef]
- Deirmendjian, L.; Anschutz, P.; Morel, C.; Mollier, A.; Augusto, L.; Loustau, D.; Cotovicz, L.C., Jr.; Buquet, D.; Lajaunie, K.; Chaillou, G.; et al. Importance of the vegetation-groundwater-stream continuum to understand transformation of biogenic carbon in aquatic systems—A case study based on a pine-maize comparison in a lowland sandy watershed (Landes de Gascogne, SW France). Sci. Total Environ. 2019, 661, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Marescaux, A.; Thieu, V.; Borges, A.V.; Garnier, J. Seasonal and spatial variability of the partial pressure of carbon dioxide in the human-impacted Seine River in France. Sci. Rep. 2018, 8, 13961. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Li, J.; Khadka, M.B.; Martin, J.B.; Zhang, T.; Yu, S.; Yuan, D. In-stream metabolism and atmospheric carbon sequestration in a groundwater-fed karst stream. Sci. Total Environ. 2017, 579, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Campeau, A.; Wallin, M.B.; Giesler, R.; Löfgren, S.; Mörth, C.-M.; Schiff, S.; Venkiteswaran, J.J.; Bishop, K. Multiple sources and sinks of dissolved inorganic carbon across Swedish streams, refocusing the lens of stable C isotopes. Sci. Rep. 2017, 7, 9158. [Google Scholar] [CrossRef]
- Richey, J.E.; Melack, J.M.; Aufdenkampe, A.K.; Ballester, V.M.; Hess, L.L. Outgassing from Amazonian rivers and wetlands as a large tropical source of atmospheric CO2. Nature 2002, 416, 617–620. [Google Scholar] [CrossRef]
- Shin, W.J.; Chung, G.S.; Lee, D.; Lee, K.S. Dissolved inorganic carbon export from carbonate and silicate catchments estimated from carbonate chemistry and δ13CDIC. Hydrol. Earth Syst. Sci. 2011, 15, 2551–2560. [Google Scholar] [CrossRef]
- Telmer, K.; Veizer, J. Carbon fluxes, pCO2 and substrate weathering in a large northern river basin, Canada: Carbon isotope perspectives. Chem. Geol. 1999, 159, 61–86. [Google Scholar] [CrossRef]
- Raymond, P.A.; Caraco, N.F.; Cole, J.J. Carbon dioxide concentration and atmospheric flux in the Hudson River. Estuaries 1997, 20, 381–390. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Zhang, J.; Xu, H.; Wei, X. Human impact on the historical change of CO2 degassing flux in River Changjiang. Geochem. Trans. 2007, 8, 7. [Google Scholar] [CrossRef]
- Cartwright, I. The origins and behavior of carbon in a major semi-arid river, the Murray River, Australia, as constrained by carbon isotopes and hydrochemistry. Appl. Geochem. 2010, 25, 1734–1745. [Google Scholar] [CrossRef]
- Wachniew, P. Isotopic composition of dissolved inorganic carbon in a large polluted river: The Vistula, Poland. Chem. Geol. 2006, 233, 293–308. [Google Scholar] [CrossRef]
- Liang, B.; Han, G.; Liu, M.; Li, X.; Song, C.; Zhang, Q.; Yang, K. Spatial and Temporal Variation of Dissolved Heavy Metals in the Mun River, Northeast Thailand. Water 2019, 11, 380. [Google Scholar] [CrossRef]
- Liu, J.; Han, G.; Liu, X.; Liu, M.; Song, C.; Zhang, Q.; Yang, K.; Li, X. Impacts of Anthropogenic Changes on the Mun River Water: Insight from Spatio-Distributions and Relationship of C and N Species in Northeast Thailand. Int. J. Environ. Res. Public Health 2019, 16, 659. [Google Scholar] [CrossRef]
- Akter, A.; Babel, M.S. Hydrological modeling of the Mun River basin in Thailand. J. Hydrol. 2012, 452, 232–246. [Google Scholar] [CrossRef]
- Nimnate, P.; Choowong, M.; Thitimakorn, T.; Hisada, K. Geomorphic criteria for distinguishing and locating abandoned channels from upstream part of Mun River, Khorat Plateau, northeastern Thailand. Environ. Earth Sci. 2017, 76, 331. [Google Scholar] [CrossRef]
- Prabnakorn, S.; Maskey, S.; Suryadi, F.X.; de Fraiture, C. Rice yield in response to climate trends and drought index in the Mun River Basin, Thailand. Sci. Total Environ. 2018, 621, 108–119. [Google Scholar] [CrossRef]
- Bhattarai, R.; Dutta, D. Estimation of Soil Erosion and Sediment Yield Using GIS at Catchment Scale. Water Resour. Manag. 2006, 21, 1635–1647. [Google Scholar] [CrossRef]
- Wu, Q.; Han, G. δ13CDIC tracing of dissolved inorganic carbon sources at Three Gorges Reservoir, China. Water Sci. Technol. 2017, 77, 555–564. [Google Scholar] [CrossRef]
- Atekwana, E.A.; Krishnamurthy, R.V. Seasonal variations of dissolved inorganic carbon and δ13C of surface waters: Application of a modified gas evolution technique. J. Hydrol. 1998, 205, 265–278. [Google Scholar] [CrossRef]
- Doctor, D.H.; Kendall, C.; Sebestyen, S.D.; Shanley, J.B.; Ohte, N.; Boyer, E.W. Carbon isotope fractionation of dissolved inorganic carbon (DIC) due to outgassing of carbon dioxide from a headwater stream. Hydrol. Process. 2008, 22, 2410–2423. [Google Scholar] [CrossRef]
- Yang, W.; Amundson, R.; Trumbore, S. A model for soil 14CO2 and its implications for using 14C to date pedogenic carbonate. Geochim. Cosmochim. Acta 1994, 58, 393–399. [Google Scholar] [CrossRef]
- Cerling, T.E.; Solomon, D.K.; Quade, J.; Bowman, J.R. On the isotopic composition of carbon in soil carbon dioxide. Geochim. Cosmochim. Acta 1991, 55, 3403–3405. [Google Scholar] [CrossRef]
- Aucour, A.-M.; Sheppard, S.M.; Guyomar, O.; Wattelet, J. Use of δ13C to trace origin and cycling of inorganic carbon in the Rhone river syetem. Chem. Geol. 1999, 159, 87–105. [Google Scholar] [CrossRef]
- Das, A.; Krishnaswami, S.; Bhattacharya, S.K. Carbon isotope ratio of dissolved inorganic carbon (DIC) in rivers draining the Deccan Traps, India: Sources of DIC and their magnitudes. Earth Planet. Sci. Lett. 2005, 236, 419–429. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, G.; Liu, Q.; Huang, C.; Li, H. Studies on the Spatiotemporal Variability of River Water Quality and Its Relationships with Soil and Precipitation: A Case Study of the Mun River Basin in Thailand. Int. J. Environ. Res. Public Health 2018, 15, 2466. [Google Scholar] [CrossRef]
- Li, S.-L.; Liu, C.-Q.; Li, J.; Lang, Y.-C.; Ding, H.; Li, L. Geochemistry of dissolved inorganic carbon and carbonate weathering in a small typical karstic catchment of Southwest China: Isotopic and chemical constraints. Chem. Geol. 2010, 277, 301–309. [Google Scholar] [CrossRef]
- Oliva, P.; Viers, J.; Dupré, B. Chemical weathering in granitic environments. Chem. Geol. 2003, 202, 225–256. [Google Scholar] [CrossRef]
- West, A.; Galy, A.; Bickle, M. Tectonic and climatic controls on silicate weathering. Earth Planet. Sci. Lett. 2005, 235, 211–228. [Google Scholar] [CrossRef]
- Millot, R.; Gaillardet, J.; Dupré, B.; Allègre, C.J. The global control of silicate weathering rates and the coupling with physical erosion: New insights from rivers of the Canadian Shield. Earth Planet. Sci. Lett. 2002, 196, 83–98. [Google Scholar] [CrossRef]
- Moosdorf, N.; Hartmann, J.; Lauerwald, R.; Hagedorn, B.; Kempe, S. Atmospheric CO2 consumption by chemical weathering in North America. Geochim. Cosmochim. Acta 2011, 75, 7829–7854. [Google Scholar] [CrossRef]
- Li, X.; Han, G.; Liu, M.; Yang, K.; Liu, J. Hydro-Geochemistry of the River Water in the Jiulongjiang River Basin, Southeast China: Implications of Anthropogenic Inputs and Chemical Weathering. Int. J. Environ. Res. Public Health 2019, 16, 440. [Google Scholar] [CrossRef]
- Dalai, T.K.; Krishnaswami, S.; Sarin, M.M. Major ion chemistry in the headwaters of the Yamuna river system: Chemical weathering, its temperature dependence and CO2 consumption in the Himalaya. Geochim. Cosmochim. Acta 2002, 66, 3397–3416. [Google Scholar] [CrossRef]
- Maher, K.; Chamberlain, C.P. Hydrologic Regulation of Chemical Weathering and the Geologic Carbon Cycle. Science 2014, 343, 1502–1504. [Google Scholar] [CrossRef]
- Liu, Z.; Macpherson, G.L.; Groves, C.; Martin, J.B.; Yuan, D.; Zeng, S. Large and active CO2 uptake by coupled carbonate weathering. Earth Sci. Rev. 2018, 182, 42–49. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, C.-Q. Chemical weathering in the upper reaches of Xijiang River draining the Yunnan–Guizhou Plateau, Southwest China. Chem. Geol. 2007, 239, 83–95. [Google Scholar] [CrossRef]
- Lerman, A.; Wu, L.; Mackenzie, F.T. CO2 and H2SO4 consumption in weathering and material transport to the ocean, and their role in the global carbon balance. Mar. Chem. 2007, 106, 326–350. [Google Scholar] [CrossRef]
- Spence, J.; Telmer, K. The role of sulfur in chemical weathering and atmospheric CO2 fluxes: Evidence from major ions, δ13CDIC, and δ34SSO4 in rivers of the Canadian Cordillera. Geochim. Cosmochim. Acta 2005, 69, 5441–5458. [Google Scholar] [CrossRef]
- Liu, W.; Xu, Z.; Sun, H.; Zhao, T.; Shi, C.; Liu, T. Geochemistry of the dissolved loads during high-flow season of rivers in the southeastern coastal region of China: Anthropogenic impact on chemical weathering and carbon sequestration. Biogeosciences 2018, 15, 4955–4971. [Google Scholar] [CrossRef]
- Shin, W.-J.; Lee, K.-S.; Park, Y.; Lee, D.; Yu, E.-J. Tracing anthropogenic DIC in urban streams based on isotopic and geochemical tracers. Environ. Earth Sci. 2015, 74, 2707–2717. [Google Scholar] [CrossRef]
- Abril, G.; Guérin, F.; Richard, S.; Delmas, R.; Galy-Lacaux, C.; Gosse, P.; Tremblay, A.; Varfalvy, L.; Dos Santos, M.A.; Matvienko, B. Carbon dioxide and methane emissions and the carbon budget of a 10-year old tropical reservoir (Petit Saut, French Guiana). Glob. Biogeochem. Cycles 2005, 19, 1–16. [Google Scholar] [CrossRef]
- Alin, S.R.; de Fátima FL Rasera, M.; Salimon, C.I.; Richey, J.E.; Holtgrieve, G.W.; Krusche, A.V.; Snidvongs, A. Physical controls on carbon dioxide transfer velocity and flux in low-gradient river systems and implications for regional carbon budgets. J. Geophys. Res. 2011, 116, 1–17. [Google Scholar] [CrossRef]
- Zou, J. Sources and Dynamics of Inorganic Carbon within the Upper Reaches of the Xi River Basin, Southwest China. PLoS ONE 2016, 11, e0160964. [Google Scholar] [CrossRef]
- Yao, G.; Gao, Q.; Wang, Z.; Huang, X.; He, T.; Zhang, Y.; Jiao, S.; Ding, J. Dynamics of CO2 partial pressure and CO2 outgassing in the lower reaches of the Xijiang River, a subtropical monsoon river in China. Sci. Total Environ. 2007, 376, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Lu, X.X.; He, M.; Zhou, Y.; Li, L.; Ziegler, A.D. Daily CO2 partial pressure and CO2 outgassing in the upper Yangtze River basin: a case study of Longchuanjiang, China. Biogeosci. Discuss. 2011, 8, 10645–10676. [Google Scholar] [CrossRef]
| River | Location | Climate | DIC | pCO2 | k | FCO2 | References |
|---|---|---|---|---|---|---|---|
| mmol/L | μatm | cm/h | mmol/(m2·d) | ||||
| Mun | Thailand | Tropic | 1.4 | 4392 | 10 | 240 | This study |
| Lower Mekong | East Asia | Tropic | 1.6 | 1090 | 26 | 195 | [5] |
| Sinamay | French | Tropic | - | - | - | 30–461 | [50] |
| Amazon | Brazil | Tropic | - | 4350 | 10 | 189.0 | [15] |
| Amazon | Brazil | Tropic | - | 3320 | 15 | 345.2 | [51] |
| Nanpan | China | Subtropics | 2.8 | 2644 | 8 | 194 | [52] |
| Beipan | China | Subtropics | 2.6 | 1287 | 8 | 78 | [52] |
| Xijiang | China | Subtropics | 1.6 | 2600 | 8–15 | 189–356 | [53] |
| Yangtza | China | Subtropics | 1.7 | 1297 | - | 14.2 | [19] |
| Longchuan | China | Subtropics | 1.1–4.6 | 1230–2100 | - | 74–156 | [54] |
| Ottawa | Canada | Temperate | 0.05–3 | 1200 | 4 | 80.8 | [17] |
| Hudson | USA | Temperate | - | 1125 | 4 | 16–s37 | [18] |
| Mississippi | USA | Temperate | 0.5 | 1335 | - | 270 | [2] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Han, G.; Liu, M.; Song, C.; Zhang, Q.; Yang, K.; Liu, J. Hydrochemistry and Dissolved Inorganic Carbon (DIC) Cycling in a Tropical Agricultural River, Mun River Basin, Northeast Thailand. Int. J. Environ. Res. Public Health 2019, 16, 3410. https://doi.org/10.3390/ijerph16183410
Li X, Han G, Liu M, Song C, Zhang Q, Yang K, Liu J. Hydrochemistry and Dissolved Inorganic Carbon (DIC) Cycling in a Tropical Agricultural River, Mun River Basin, Northeast Thailand. International Journal of Environmental Research and Public Health. 2019; 16(18):3410. https://doi.org/10.3390/ijerph16183410
Chicago/Turabian StyleLi, Xiaoqiang, Guilin Han, Man Liu, Chao Song, Qian Zhang, Kunhua Yang, and Jinke Liu. 2019. "Hydrochemistry and Dissolved Inorganic Carbon (DIC) Cycling in a Tropical Agricultural River, Mun River Basin, Northeast Thailand" International Journal of Environmental Research and Public Health 16, no. 18: 3410. https://doi.org/10.3390/ijerph16183410
APA StyleLi, X., Han, G., Liu, M., Song, C., Zhang, Q., Yang, K., & Liu, J. (2019). Hydrochemistry and Dissolved Inorganic Carbon (DIC) Cycling in a Tropical Agricultural River, Mun River Basin, Northeast Thailand. International Journal of Environmental Research and Public Health, 16(18), 3410. https://doi.org/10.3390/ijerph16183410






