Influence of PM2.5 Exposure Level on the Association between Alzheimer’s Disease and Allergic Rhinitis: A National Population-Based Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Collections and Concentrations of PM2.5
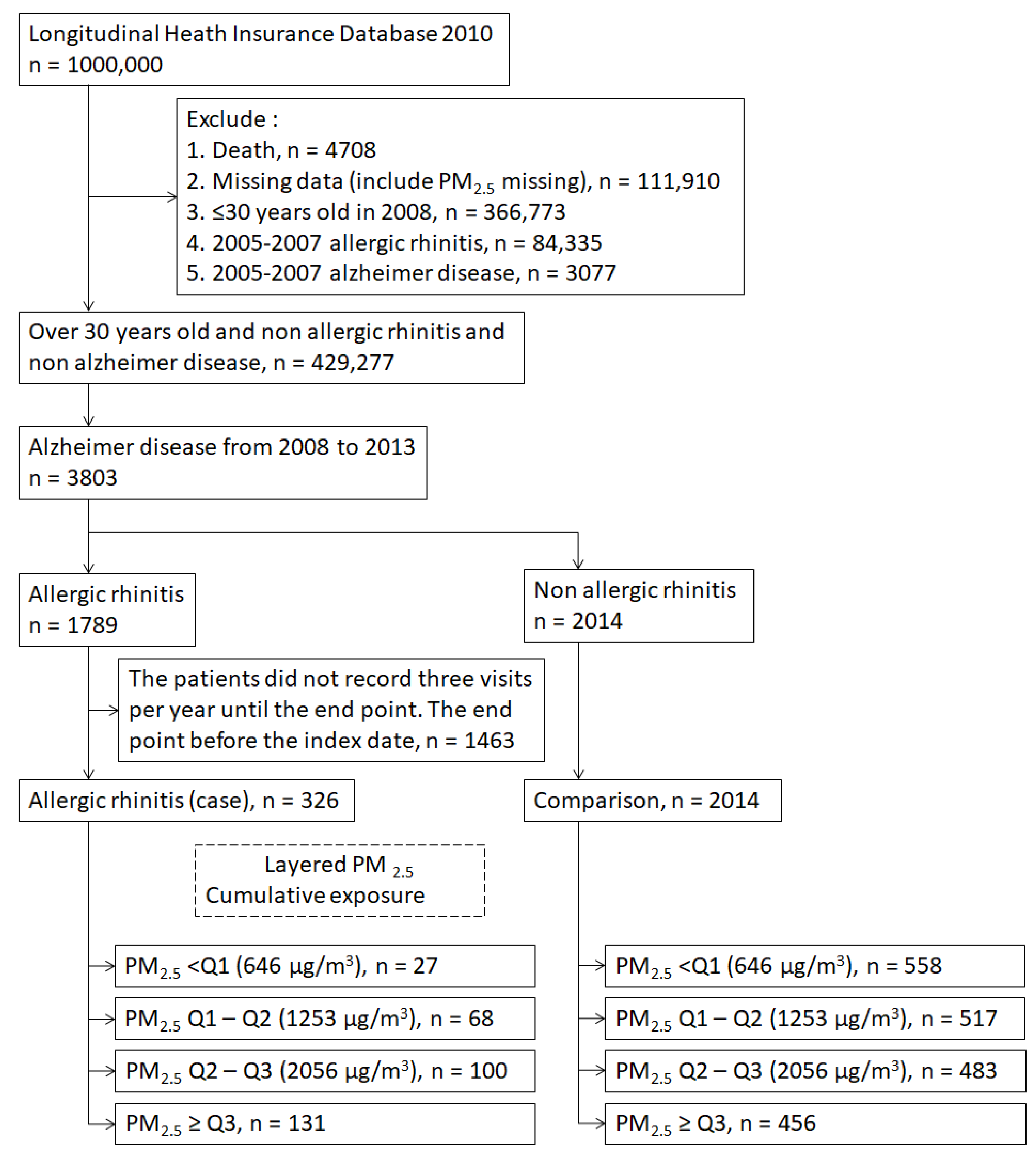
2.3. Study Population
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
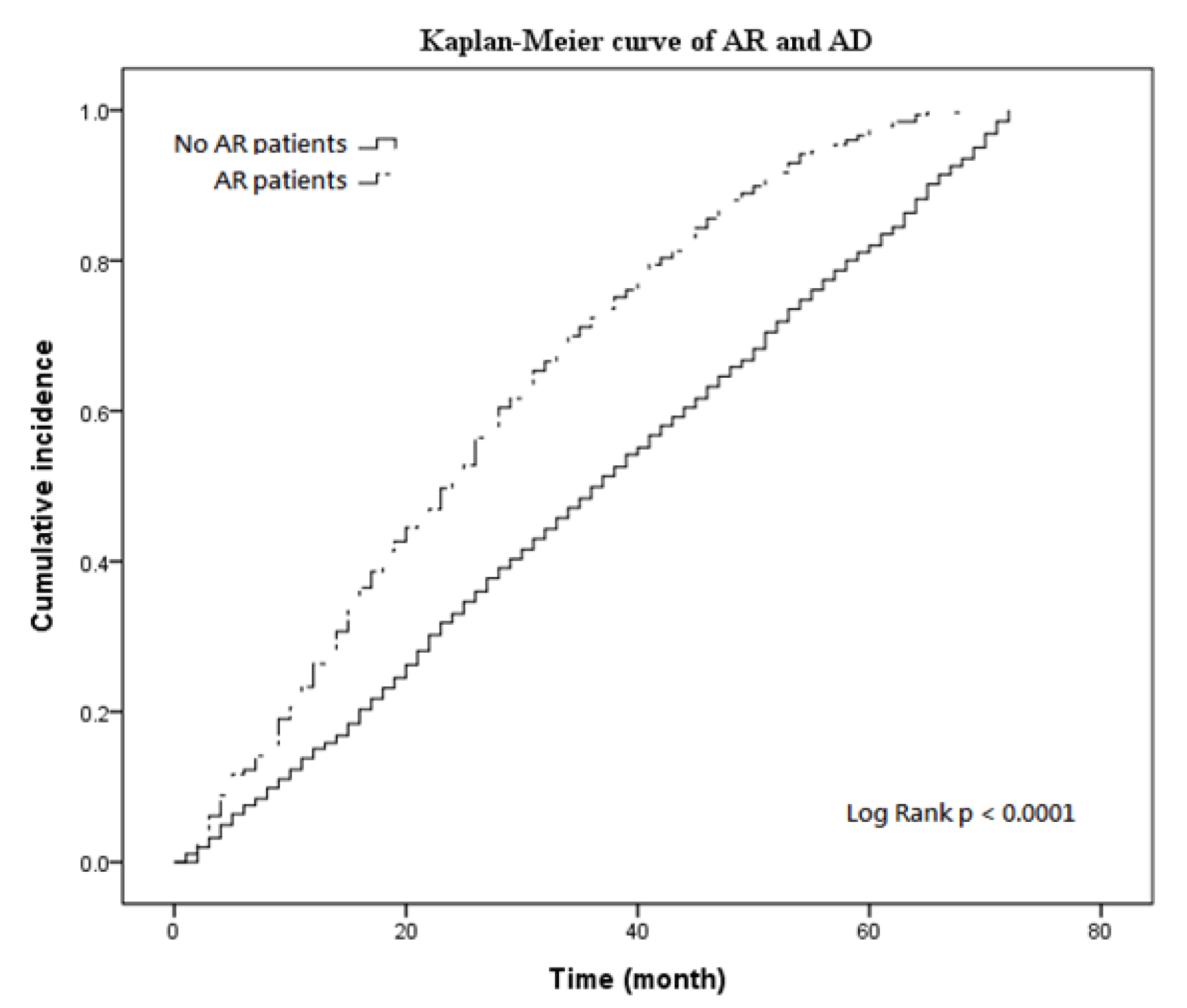
3.2. Kaplan–Meier Curves and Cox Regression
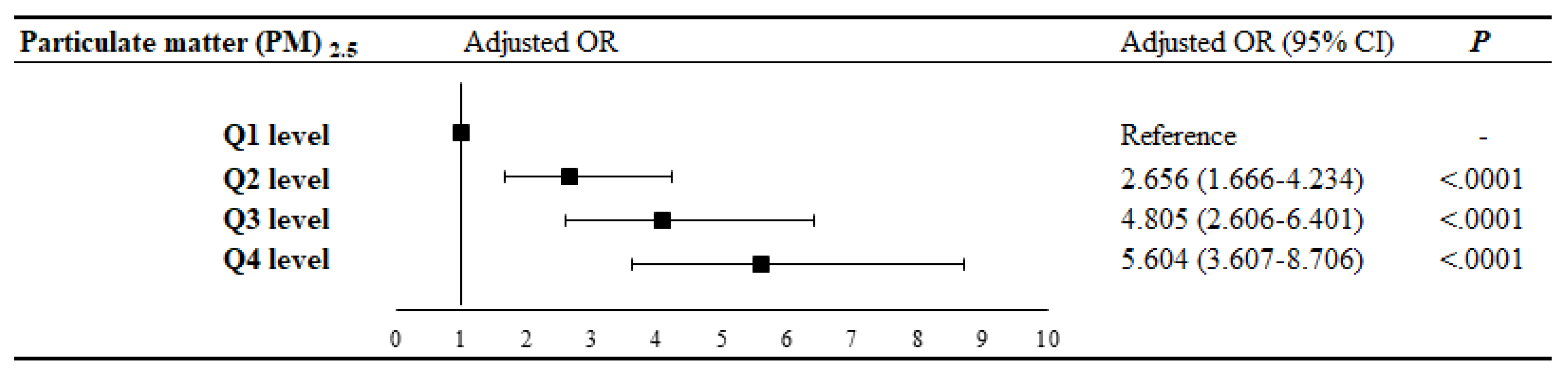
3.3. Logistic Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qiu, C.; Kivipelto, M.; von Strauss, E. Epidemiology of Alzheimer’s disease: Occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci. 2009, 11, 111–128. [Google Scholar] [PubMed]
- Swerdlow, R.H. Brain aging, Alzheimer’s disease, and mitochondria. Biochim. Biophys. Acta 2011, 1812, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Stibich, M. Alzheimer’s Disease and Dementia Life Expectancy. 2018. Available online: https://www.verywellhealth.com/life-expectancy-in-alzheimers-disease-and-dementia-2223967 (accessed on 11 September 2018).
- Alzheimer’s Disease International. World Alzheimer Report; Alzheimer’s Disease International: London, UK, 2018; pp. 6–8. [Google Scholar]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2017, 13, 325–373. [Google Scholar]
- The Lancet Neurology. Alzheimer’s disease: A time for cautious optimism. Lancet Neurol. 2015, 14, 779. [Google Scholar] [CrossRef]
- Pawankar, R.; Bunnag, C.; Khaltaev, N.; Bousquet, J. Allergic Rhinitis and Its Impact on Asthma in Asia Pacific and the ARIA Update 2008. World Allergy Organ J. 2012, 5, S212–S217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidman, M.D.; Gurgel, R.K.; Lin, S.Y.; Schwartz, S.R.; Baroody, F.M.; Bonner, J.R.; Dawson, D.E.; Dykewicz, M.S.; Hackell, J.M.; Han, J.K.; et al. Guideline Otolaryngology Development Group. AAO-HNSF. Clinical practice guideline: Allergic rhinitis. Otolaryngol. Head Neck Surg. 2015, 152, S1–S43. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, Z.Q.; Yang, P.C. Treatment of allergic rhinitis with probiotics: An alternative approach. N. Am. J. Med. Sci. 2013, 5, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Bjerg, A.; Ekerljung, L.; Middelveld, R.; Dahlén, S.E.; Forsberg, B.; Franklin, K.; Larsson, K.; Lötvall, J.; Olafsdóttir, I.S.; Torén, K.; et al. Increased prevalence of symptoms of rhinitis but not of asthma between 1990 and 2008 in Swedish adults: Comparisons of the ECRHS and GA²LEN surveys. PLoS ONE 2011, 6, e16082. [Google Scholar] [CrossRef] [PubMed]
- De Marco, R.; Cappa, V.; Accordini, S.; Rava, M.; Antonicelli, L.; Bortolami, O.; Braggion, M.; Bugiani, M.; Casali, L.; Cazzoletti, L.; et al. GEIRD Study Group. Trends in the prevalence of asthma and allergic rhinitis in Italy between 1991 and 2010. Eur. Respir. J. 2012, 39, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Teng, B.; Zhang, X.; Yi, C.; Zhang, Y.; Ye, S.; Wang, Y.; Tong, D.Q.; Lu, B. The Association between Ambient Air Pollution and Allergic Rhinitis: Further Epidemiological Evidence from Changchun, Northeastern China. Int. J. Environ. Res. Public Health 2017, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, E.; Standl, M.; Cyrys, J.; Berdel, D.; von Berg, A.; Bauer, C.P.; Krämer, U.; Sugiri, D.; Lehmann, I.; Koletzko, S.; et al. A longitudinal analysis of associations between traffic-related air pollution with asthma, allergies and sensitization in the GINIplus and LISAplus birth cohorts. Peer J. 2013, 1, e193. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Guo, X.; Cheung, F.M.H.; Yung, K.K.L. The association between PM2.5 exposure and neurological disorders: A systematic review and meta-analysis. Sci. Total Environ. 2019, 655, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Sarlus, H.; Höglund, C.O.; Karshikoff, B.; Wang, X.; Lekander, M.; Schultzberg, M.; Oprica, M. Allergy influences the inflammatory status of the brain and enhances tau-phosphorylation. J. Cell. Mol. Med. 2012, 16, 2401–2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taiwan Air Quality Monitoring Network. 2019; TAQMN Site. Available online: http://taqm.epa.gov.tw/taqm/en/b0101.aspx (accessed on 11 August 2019).
- Lao, X.Q.; Guo, C.; Chang, L.Y.; Bo, Y.; Zhang, Z.; Chuang, Y.C.; Jiang, W.K.; Lin, C.; Tam, T.; Lau, A.K.H.; et al. Long-term exposure to ambient fine particulate matter (PM2.5) and incident type 2 diabetes: A longitudinal cohort study. Diabetologia 2019, 62, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Hsiao, C.Y.; Lee, J.F.; Chen, Y.C.; Nfor, O.N.; Huang, J.Y.; Wang, L.; Ho, C.C.; Liaw, Y.P. Relationship between Schizophrenia and Low-Income Based on Age and Sex: Results from a Nation-wide Population-Based Longitudinal Study. Neuropsychiatry 2018, 8, 1000–1006. [Google Scholar]
- Lin, Y.J.; Tian, W.H.; Chen, C.C. Urbanization and the utilization of outpatient services under National Health Insurance in Taiwan. Health Policy 2011, 103, 236–243. [Google Scholar] [CrossRef]
- Kwak, C.; Clayton-Matthews, A. Multinomial logistic regression. Nurs Res. 2002, 51, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Stephen, T.J.; Mark, E.L. Pollen dispersal models in Quaternary plant ecology: Assumptions, parameters, and prescriptions. Bot. Rev. 1999, 65, 39–75. [Google Scholar]
- Diaz-Quijano, F.A. A simple method for estimating relative risk using logistic regression. BMC Med. Res. Methodol. 2012, 15, 12–14. [Google Scholar] [CrossRef]
- Eriksson, U.K.; Gatz, M.; Dickman, P.W.; Fratiglioni, L.; Pedersen, N.L. Asthma, eczema, rhinitis and the risk for dementia. Dement. Geriatr. Cogn. Disord. 2008, 25, 148–156. [Google Scholar] [CrossRef]
- Bożek, A.; Bednarski, P.; Jarzab, J. Allergic rhinitis, bronchial asthma and other allergies in patients with Alzheimer’s disease: Unnoticed issue. Postep. Dermatol. Alergol. 2016, 33, 353–358. [Google Scholar] [CrossRef]
- Yu, J.T.; Tan, L.; Song, J.H.; Sun, Y.P.; Chen, W.; Miao, D.; Tian, Y. Interleukin-18 promoter polymorphisms and risk of late onset Alzheimer’s disease. Brain Res. 2009, 1253, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Sarlus, H.; Eyjolfsdottir, H.; Eriksdotter, M.; Oprica, M.; Schultzberg, M. Influence of Allergy on Immunoglobulins and Amyloid-β in the Cerebrospinal Fluid of Patients with Alzheimer’s Disease. J. Alzheimers. Dis. 2015, 48, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Gardener, S.L.; Rainey-Smith, S.R.; Martins, R.N. Diet and Inflammation in Alzheimer’s Disease and Related Chronic Diseases: A Review. J. Alzheimers. Dis. 2016, 50, 301–334. [Google Scholar] [CrossRef] [PubMed]
- Sakallioglu, O.; Polat, C.; Akyigit, A.; Cetiner, H.; Duzer, S. Allergic rhinitis and arterial blood pressure: A population-based study. J Laryngol. Otol. 2018, 132, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Trakaki, A.; Sturm, G.J.; Pregartner, G.; Scharnagl, H.; Eichmann, T.O.; Trieb, M.; Knuplez, E.; Holzer, M.; Stadler, J.T.; Heinemann, A.; et al. Allergic rhinitis is associated with complex alterations in high-density lipoprotein composition and function. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2019, 1864, 1280–1292. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.J.; Lee, W.S.; Jo, H.Y.; Kim, C.H.; Eom, J.S.; Mok, J.H.; Kim, M.H.; Lee, K.; Kim, K.U.; Lee, M.K.; et al. Effects of particulate matter on respiratory disease and the impact of meteorological factors in Busan, Korea. Respir. Med. 2017, 124, 79–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, H.; Xin, J.; Yuan, Q.; Wang, M.; Cheng, L.; Zhang, Z.; Lu, M. The effects of particulate matters on allergic rhinitis in Nanjing, China. Environ. Sci. Pollut. Res. Int. 2019, 26, 11452–11457. [Google Scholar] [CrossRef]
- Wang, Y.L.; Gao, W.; Li, Y.; Wang, Y.F. Concentration-dependent effects of PM2.5 mass on expressions of adhesion molecules and inflammatory cytokines in nasal mucosa of rats with allergic rhinitis. Eur. Arch. Otorhinolaryngol. 2017, 274, 3221–3229. [Google Scholar] [CrossRef]
- Mady, L.J.; Schwarzbach, H.L.; Moore, J.A.; Boudreau, R.M.; Kaffenberger, T.M.; Willson, T.J.; Lee, S.E. The association of air pollutants and allergic and nonallergic rhinitis in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2018, 8, 369–376. [Google Scholar] [CrossRef]
- Guss, J.; Doghramji, L.; Reger, C.; Chiu, A.G. Olfactory dysfunction in allergic rhinitis. ORL J. Otorhinolaryngol. Relat. Spec. 2009, 71, 268–272. [Google Scholar] [CrossRef]
- Napeñas, J.J.; Zakrzewska, J.M. Diagnosis and management of trigeminal neuropathic pains. Pain Manag. 2011, 1, 353–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cingi, C.; Gevaert, P.; Mösges, R.; Rondon, C.; Hox, V.; Rudenko, M.; Muluk, N.B.; Scadding, G.; Manole, F.; Hupin, C.; et al. Multi-morbidities of allergic rhinitis in adults: European Academy of Allergy and Clinical Immunology Task Force Report. Clin. Transl. Allergy 2017, 7, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doerfler, H.; Hummel, T.; Klimek, L.; Kobal, G. Intranasal trigeminal sensitivity in subjects with allergic rhinitis. Eur. Arch. Otorhinolaryngol. 2006, 263, 86–90. [Google Scholar] [CrossRef] [PubMed]
| Variable | Comparison | AR | Chi-Square | p-Value | ||
|---|---|---|---|---|---|---|
| (n = 2014) | (n = 370) | |||||
| Gender | ||||||
| Female | 1088 | (54.02%) | 158 | (48.47%) | 3.4787 | 0.0622 |
| Male | 926 | (45.98%) | 168 | (51.53%) | ||
| Age | ||||||
| 30–44 | 89 | (4.42%) | 18 | (5.52%) | 6.0638 | 0.1085 |
| 45–59 | 241 | (11.97%) | 50 | (15.34%) | ||
| 60–74 | 766 | (38.03%) | 130 | (39.88%) | ||
| >75 | 918 | (45.58%) | 128 | (39.26%) | ||
| Low income | ||||||
| Yes | 1234 | (61.27%) | 207 | (63.50%) | 0.5876 | 0.4434 |
| No | 780 | (38.73%) | 119 | (36.50%) | ||
| Urbanization level | ||||||
| Highly urbanized | 474 | (23.54%) | 79 | (24.23%) | 3.5588 | 0.7818 |
| Moderate urbanization | 545 | (27.06%) | 96 | (29.45%) | ||
| Emerging town | 291 | (14.45%) | 44 | (13.50%) | ||
| General town | 370 | (18.37%) | 61 | (18.71%) | ||
| Aged Township | 92 | (4.57%) | 9 | (2.76%) | ||
| Agricultural town | 152 | (7.55%) | 22 | (6.75%) | ||
| Remote township | 90 | (4.47%) | 15 | (4.60%) | ||
| Particulate matter (PM2.5) cumulative exposure | ||||||
| Q1 level | 558 | (27.71%) | 27 | (7.3%) | 84.4076 | <0.0001 |
| Q2 level | 517 | (25.67%) | 68 | (18.38%) | ||
| Q3 level | 483 | (23.98%) | 100 | (27.03%) | ||
| Q4 level | 456 | (22.64%) | 131 | (35.41%) | ||
| Variable | Alzheimer Disease | |
|---|---|---|
| Adjusted HR (95% CI) | p-Value | |
| Allergic rhinitis infection (reference: general population) | ||
| Allergic rhinitis infection | 2.008 (1.780–2.266) | <0.0001 |
| Age in 2008 (per year) | 1.002 (0.998–1.005) | 0.3928 |
| Gender (reference: female) | ||
| Male | 0.900 (0.829–0.978) | 0.0130 |
| Low income (reference: no) | ||
| Yes | 1.129 (1.028–1.240) | 0.0113 |
| Urbanization level (reference: Moderate urbanization) | ||
| Highly urbanized | 0.968 (0.863–1.085) | 0.5759 |
| Emerging town | 1.066 (0.933–1.219) | 0.3464 |
| General town | 0.958 (0.842–1.089) | 0.5089 |
| Aged Township | 1.051 (0.845–1.309) | 0.6537 |
| Agricultural town | 0.969 (0.814–1.154) | 0.7230 |
| Remote township | 1.268 (1.026–1.567) | 0.0283 |
| Comorbidity (ref: without) | ||
| Hypertension | 0.771 (0.703–0.845) | <0.0001 |
| Hyperlipidemia | 0.752 (0.685–0.826) | <0.0001 |
| Diabetes | 0.982 (0.894–1.078) | 0.6995 |
| Huntington’s Disease | 4.024 (0.559–28.99) | 0.1670 |
| Parkinson’s Disease | 0.980 (0.860–1.116) | 0.7566 |
| Extrapyramidal signs | 0.773 (0.644–0.926) | 0.0053 |
| Sleep Apnea | 1.001 (0.656–1.529) | 0.9955 |
| Nutrition Deficiencies | 0.888 (0.604–1.305) | 0.5444 |
| Cox regression | Particulate Matter (PM) 2.5 Cumulative Exposure | |||||||
|---|---|---|---|---|---|---|---|---|
| Q1 Level | Q2 Level | Q3 Level | Q4 Level | |||||
| Allergic Rhinitis Infection | Allergic Rhinitis Infection | Allergic Rhinitis Infection | Allergic Rhinitis Infection | |||||
| (Reference: General Population) | (Reference: General Population) | (Reference: General Population) | (Reference: General Population) | |||||
| p | p | p | p | |||||
| Crude | 1.454 (0.986–2.142) | 0.0586 | 1.663 (1.290–2.143) | <0.0001 | 3.128 (2.510–3.898) | <0.0001 | 8.315 (6.657–10.385) | <0.0001 |
| HR (95% CI) | ||||||||
| Adjusted | 1.533 (1.023–2.297) | 0.0385 | 1.812 (1.396–2.353) | <0.0001 | 3.331 (2.653–4.183) | <0.0001 | 8.981 (7.100–11.360) | <0.0001 |
| HR (95% CI) | ||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.-L.; Ho, Y.-C.; Luo, C.-W.; Lee, S.-S.; Kuan, Y.-H. Influence of PM2.5 Exposure Level on the Association between Alzheimer’s Disease and Allergic Rhinitis: A National Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 3357. https://doi.org/10.3390/ijerph16183357
Li R-L, Ho Y-C, Luo C-W, Lee S-S, Kuan Y-H. Influence of PM2.5 Exposure Level on the Association between Alzheimer’s Disease and Allergic Rhinitis: A National Population-Based Cohort Study. International Journal of Environmental Research and Public Health. 2019; 16(18):3357. https://doi.org/10.3390/ijerph16183357
Chicago/Turabian StyleLi, Ruo-Ling, Yung-Chyuan Ho, Ci-Wen Luo, Shiuan-Shinn Lee, and Yu-Hsiang Kuan. 2019. "Influence of PM2.5 Exposure Level on the Association between Alzheimer’s Disease and Allergic Rhinitis: A National Population-Based Cohort Study" International Journal of Environmental Research and Public Health 16, no. 18: 3357. https://doi.org/10.3390/ijerph16183357
APA StyleLi, R.-L., Ho, Y.-C., Luo, C.-W., Lee, S.-S., & Kuan, Y.-H. (2019). Influence of PM2.5 Exposure Level on the Association between Alzheimer’s Disease and Allergic Rhinitis: A National Population-Based Cohort Study. International Journal of Environmental Research and Public Health, 16(18), 3357. https://doi.org/10.3390/ijerph16183357





