Antibiotic Resistance of Airborne Viable Bacteria and Size Distribution in Neonatal Intensive Care Units
Abstract
1. Introduction
2. Materials and Methods
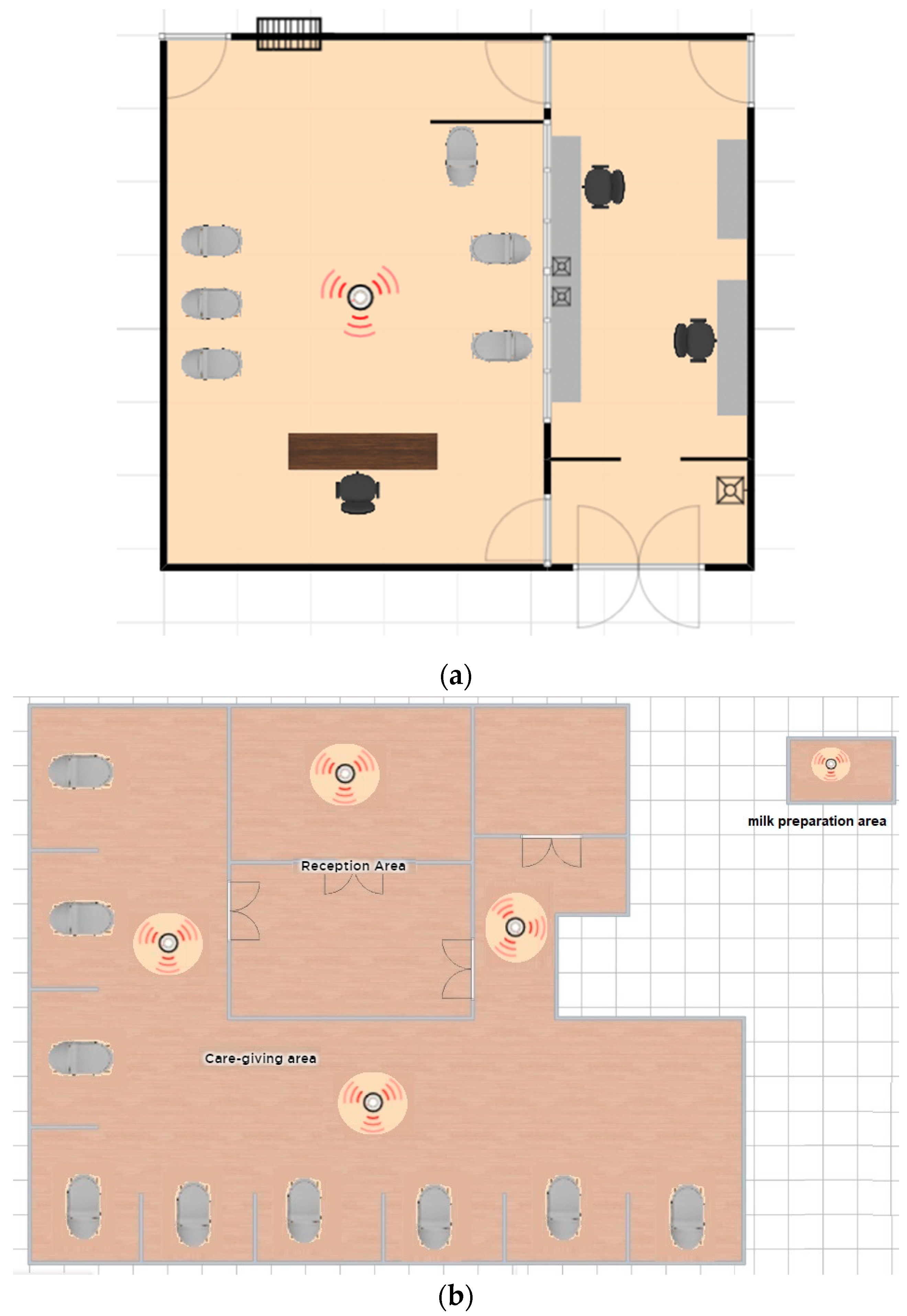
2.1. Neonatal Intensive Care Units (NICU)
2.2. Airborne Bacteria and Particle Number Samples Collection
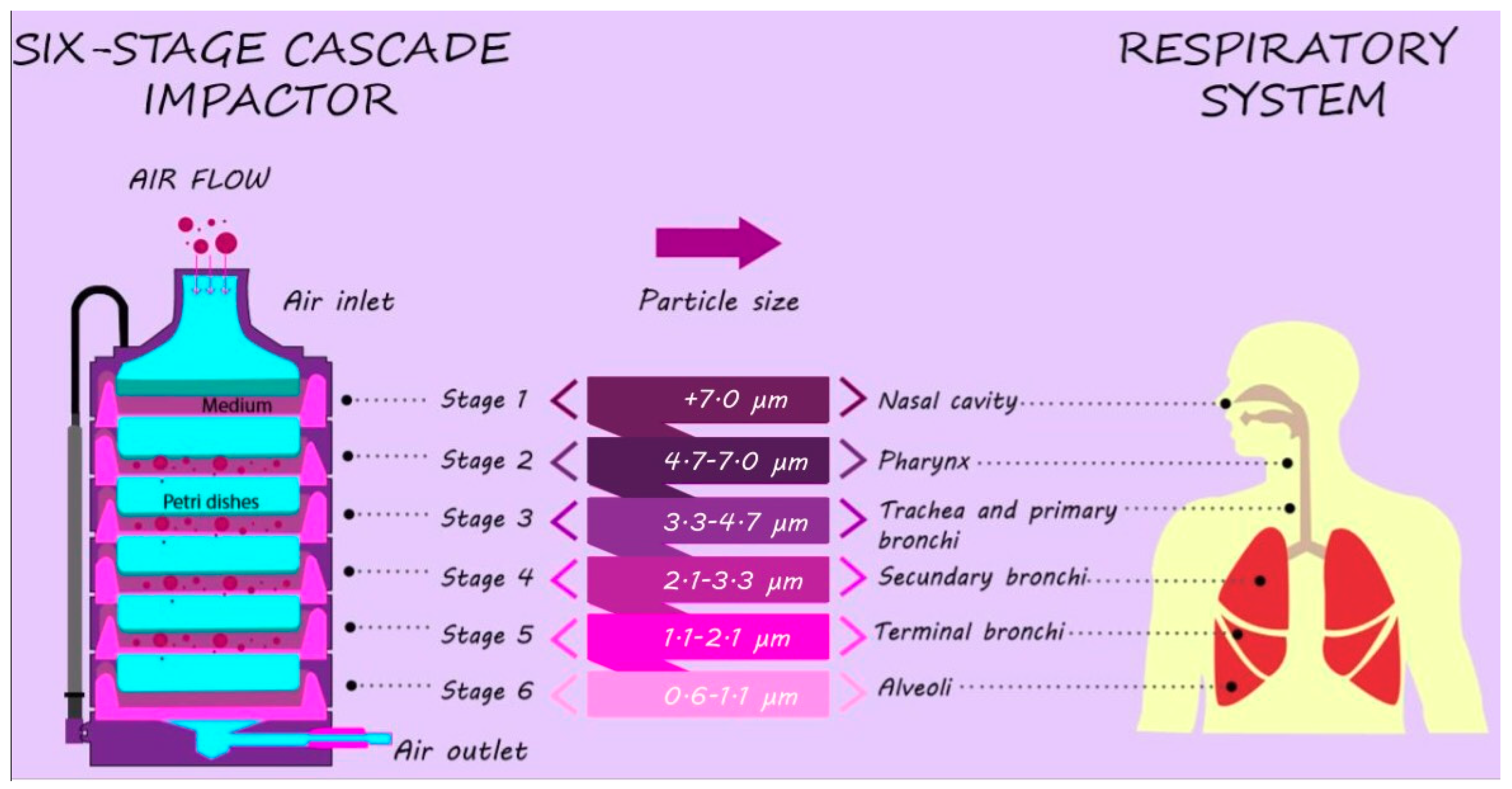
2.3. Bioaerosol Cultivation and Concentration Assessment
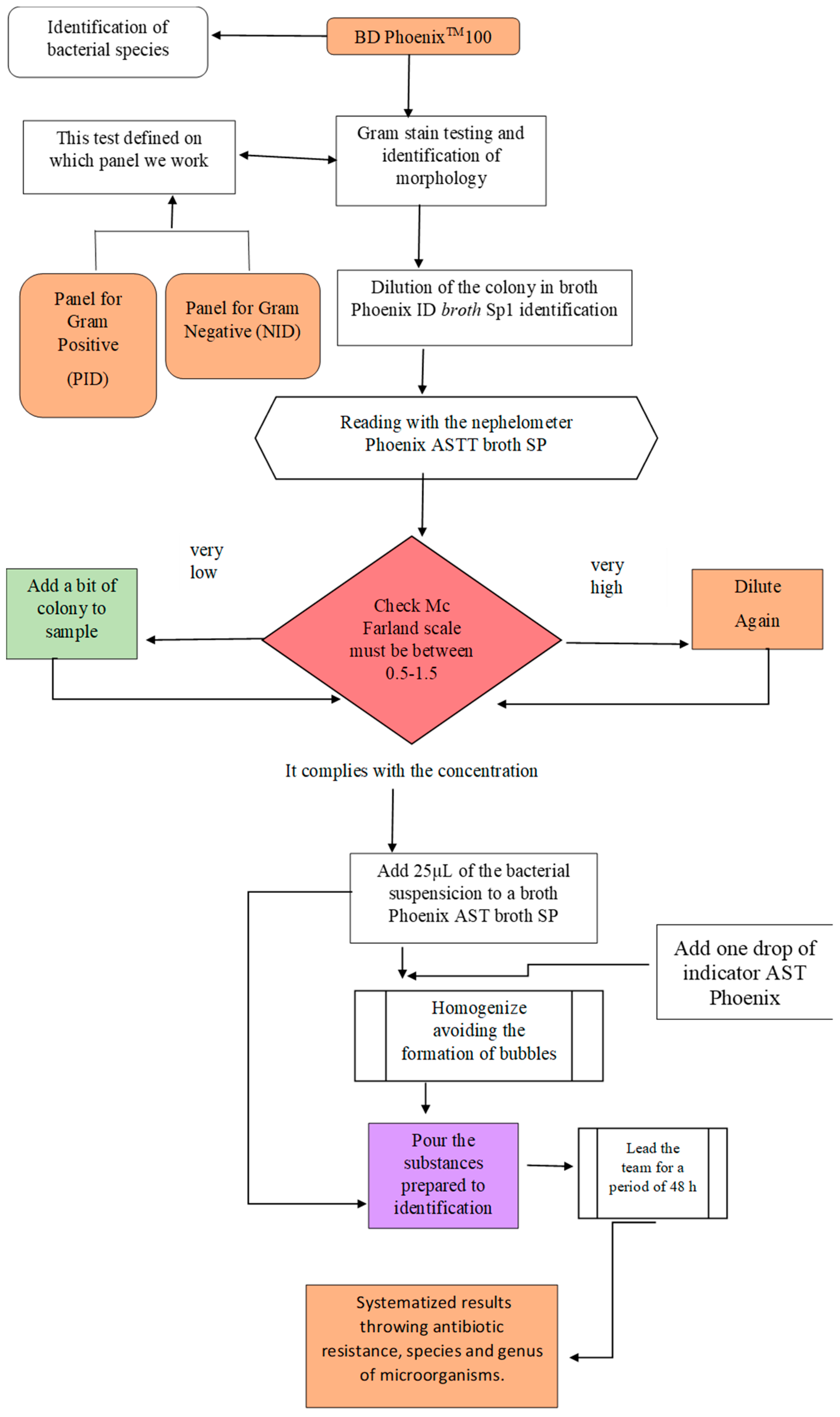
2.4. Bacteria Identification and Antibiotic Resistance
2.5. Data Analysis
3. Results
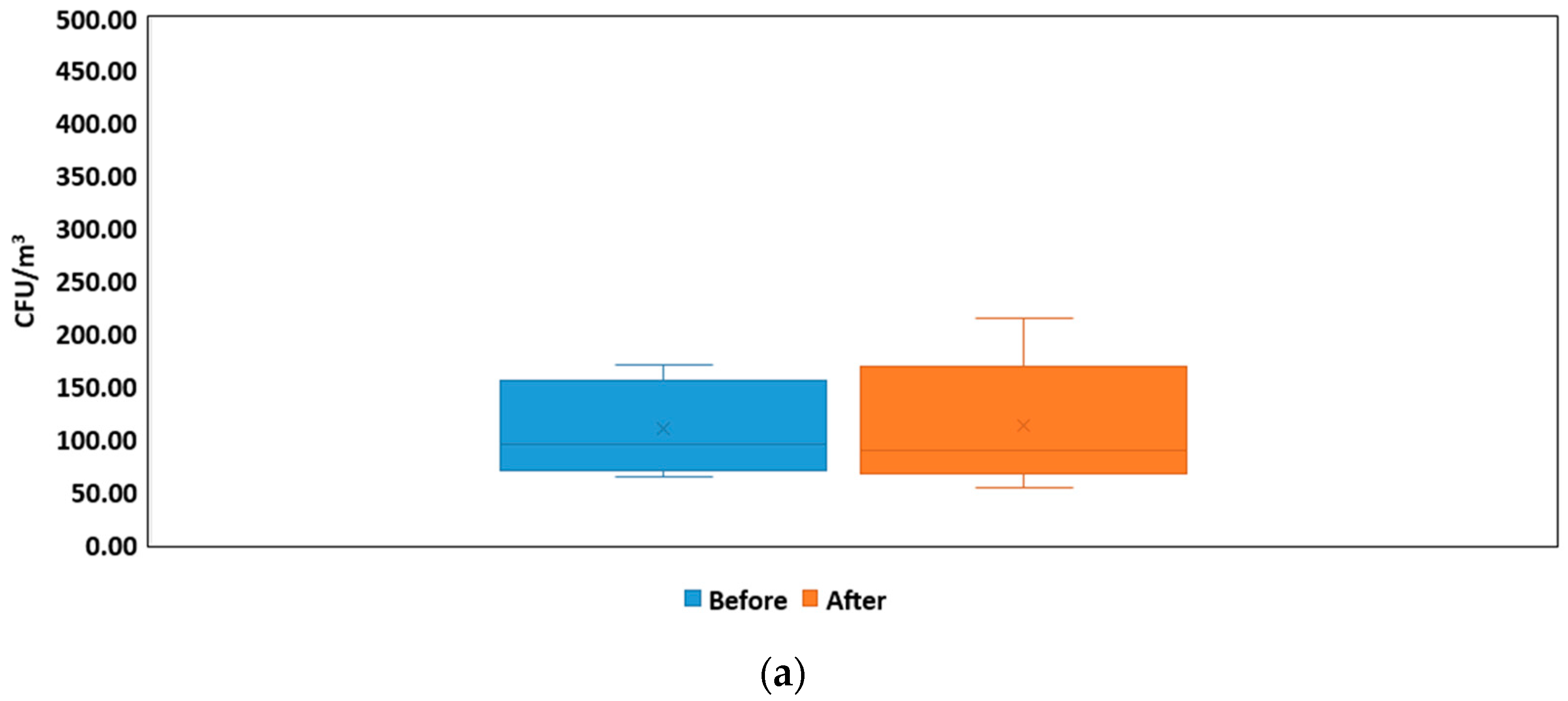
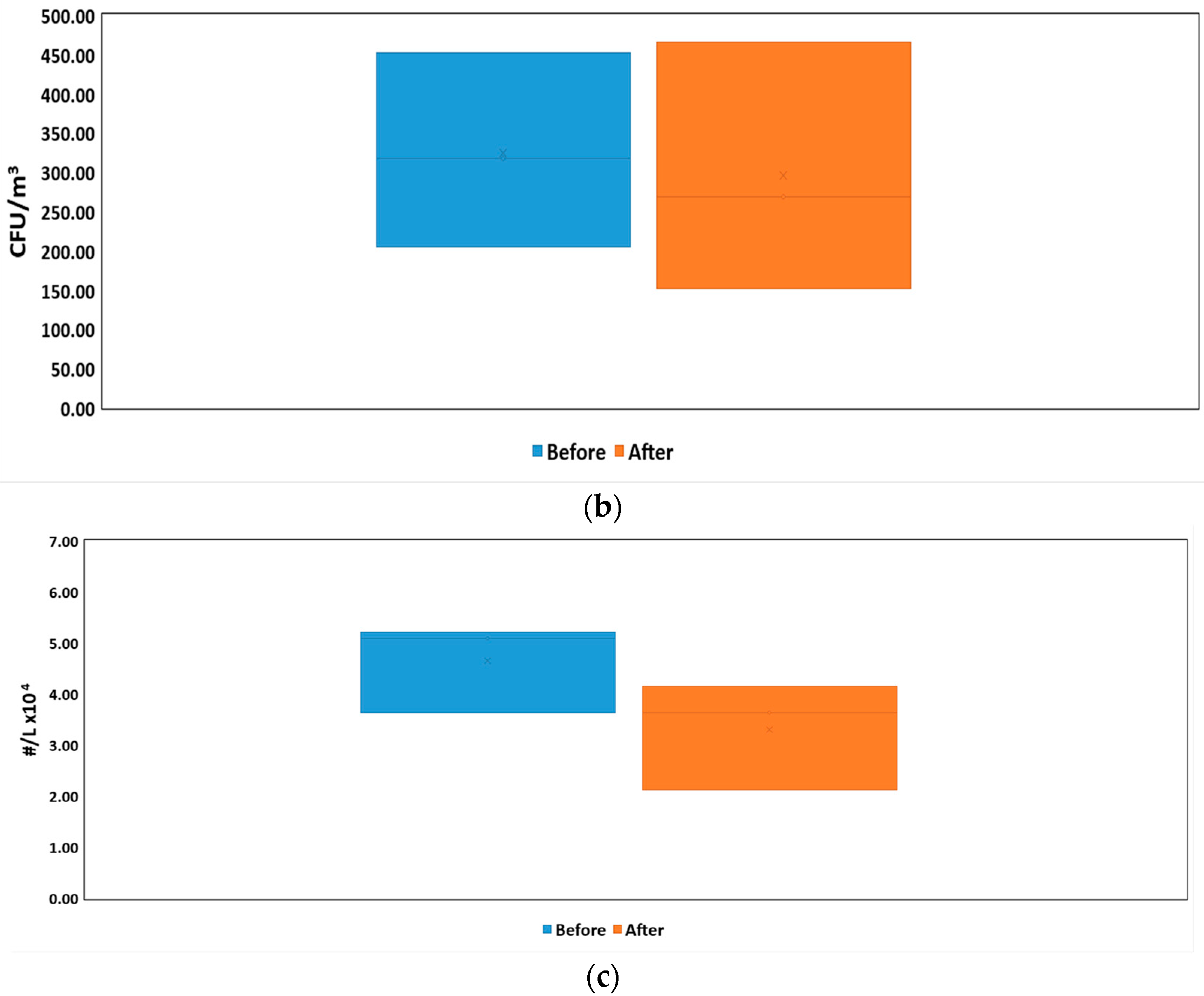
3.1. Size Distribution and Bacteria Bioaerosols Concentration
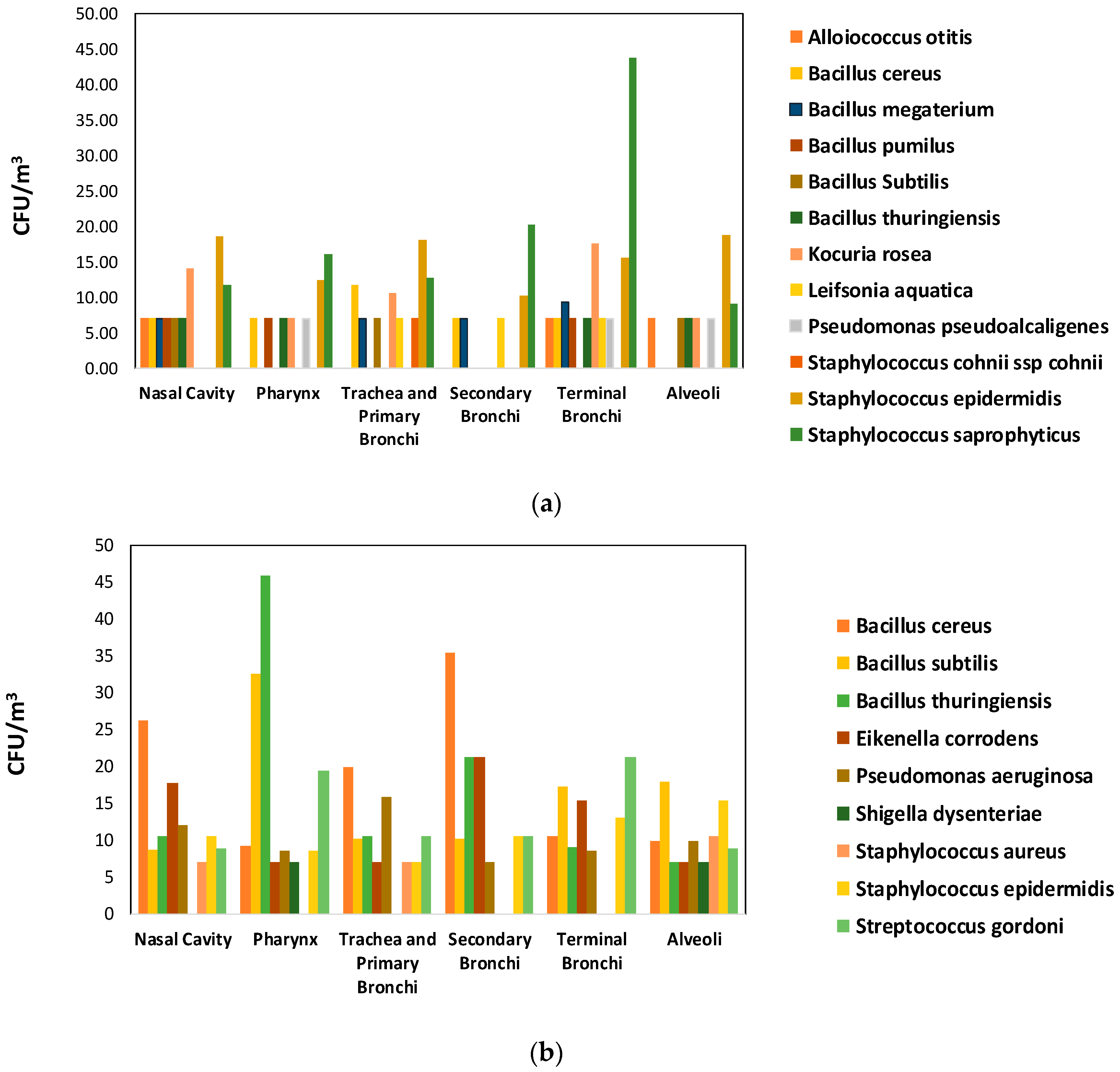
3.2. Species Identification and Mean Concentration in Air
3.3. Antibiotic Resistance of Airborne Bacteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, M.; Yan, X.; Qiu, T.; Han, M.; Wang, X. Variation of correlations between factors and culturable airborne bacteria and fungi. Atmos. Environ. 2016, 128, 10–19. [Google Scholar] [CrossRef]
- Jara Hernández, E.L.; Piraquive Mórtola, J.S. Determinación de la Calidad de Aire Intrmural en la Clínica Veterinaria, Universidad de la Salle; Universidad de la Salle: Bogotá, Colombia, 2016. [Google Scholar]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Airborne bioaerosols and their impact on human health. J. Environ. Sci. (China) 2018, 67, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Colbeck, I.; Lazaridis, M.; Liu, K.F.; Hung, M.; Shen, Y.; Reijula, K.; Tringe, S.G.; Zhang, T.; Liu, X.; et al. Bioaerosol: A bridge and opportunity for many scientific research fields. J. Aerosol Sci. 2018, 115, 108–112. [Google Scholar] [CrossRef]
- Smets, W.; Moretti, S.; Denys, S.; Lebeer, S. Airborne bacteria in the atmosphere: Presence, purpose, and potential. Atmos. Environ. 2016, 139, 214–221. [Google Scholar] [CrossRef]
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W.; et al. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef]
- Walser, S.M.; Gerstner, D.G.; Brenner, B.; Bünger, J.; Eikmann, T.; Janssen, B.; Kolb, S.; Kolk, A.; Nowak, D.; Raulf, M.; et al. Evaluation of exposure-response relationships for health effects of microbial bioaerosols - A systematic review. Int. J. Hyg. Environ. Health 2015, 218, 577–589. [Google Scholar] [CrossRef]
- Edith, G.; Espinoza, C. Determinación de Organismos Mesófilos Aerobios en el Ambiente de Cd. Obregón, Sonora; Mediante el uso del Monitor aéreo Microbiológico y Método de Cuenta en Placa Abierta. Available online: https://docplayer.es/42154583-Tesis-que-para-obtener-el-titulo-de-ingeniero-biotecnologo-presenta-gloria-edith-camacho-espinoza.html (accessed on 17 May 2019).
- Pelczar, M.J.; Chan, E.C.S.; Krieg, N.R. Microbiology, 5th ed.; Tata Mc Graw Hill: New York, NY, USA, 1993. [Google Scholar]
- Cabo Verde, S.; Almeida, S.M.; Matos, J.; Guerreiro, D.; Meneses, M.; Faria, T.; Botelho, D.; Santos, M.; Viegas, C. Microbiological assessment of indoor air quality at different hospital sites. Res. Microbiol. 2015, 166, 557–563. [Google Scholar] [CrossRef]
- Abdel-Wahab, F.; Ghoneim, M.; Khashaba, M.; El-Gilany, A.-H.; Abdel-Hady, D. Nosocomial infection surveillance in an Egyptian neonatal intensive care unit. J. Hosp. Infect. 2013, 83, 196–199. [Google Scholar] [CrossRef]
- Youn, K.; Shin, Y. Daekeun Distribution characteristics of airborne bacteria and fungi in the general hospitals of Korea. Ind. Health 2010, 48, 236–243. [Google Scholar]
- Jaffal, A.A.; Nsanze, H.; Bener, A.; Ameen, A.S.; Banat, I.M.; Mogheth, A.A. El Hospital airborne microbial pollution in a desert country. Environ. Int. 1997, 23, 167–172. [Google Scholar] [CrossRef]
- Hernandez, M.; León, S. Determinación de la Calidad del Aire Extramural E Intramural en la Sala de Cirugía del Hospital el Tunal de la Cuidad De Bogotá Para el Desarrollo de Mecanismos de Control y Minimización De Riesgo Causado por Microorganismos Potencialmente Nosocomiales. Available online: http://repository.lasalle.edu.co/bitstream/handle/10185/14040/T41.08%20H43d.pdf?sequence=1&isAllowed=y (accessed on 17 May 2019).
- Leung, M.; Chan, A.H.S. Control and management of hospital indoor air quality. Med. Sci. Monit. 2006, 12, SR17–SR23. [Google Scholar]
- Coronell, W.; Rojas, J.; Escamilla, M.; Manotas, M.; Sánchez, M. Infección Nosocomial En Unidades de Cuidados Intensivos Neonatales. Precop. Scp. 2010, 9, 30–39. [Google Scholar]
- Morgado Gamero, W.B.; Ramírez, M.C.; Parody, A.; Viloria, A.; López, M.H.A.; Kamatkar, S.J. Concentrations and Size Distributions of Fungal Bioaerosols in a Municipal Landfill. In Data Mining and Big Data; Tan, Y., Shi, Y., Tang, Q., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 244–253. [Google Scholar]
- Zhu, H.; Phelan, P.; Duan, T.; Raupp, G.; Fernando, H.J.S. Characterizations and relationships between outdoor and indoor bioaerosols in an office building. China Particuology 2003, 1, 119–123. [Google Scholar] [CrossRef]
- Wang, W.; Ma, Y.; Ma, X.; Wu, F.; Ma, X.; An, L.; Feng, H. Diversity and seasonal dynamics of airborne bacteria in the Mogao Grottoes, Dunhuang, China. Aerobiologia 2012, 28, 27–38. [Google Scholar] [CrossRef]
- Claudia Andrea, C.T. Evaluación del Comportamiento de Aerobacterias en el Corregimiento de Cuatro Bocas. Tubará, Atlántico. Available online: https://www.researchgate.net/profile/Wendy_Morgado_Gamero/publication/325478384_EVALUATION_OF_THE_BEHAVIOR_OF_AIRBORNE_BACTERIA_IN_THE_VILLAGE_OF_CUATRO_BOCAS_TUBARA_ATLANTICO/links/5b106cdea6fdcc4611d977e4/EVALUATION-OF-THE-BEHAVIOR-OF-AIRBORNE-BACTERIA-IN-THE-VILLAGE-OF-CUATRO-BOCAS-TUBARA-ATLANTICO.pdf?origin=publication_detail (accessed on 17 May 2019).
- Ortiz, J.; Préndez Bolívar, M.M.; Solezzi, S. Utilizacion y manejo de un impactador de cascada ANDERSEN en el estudio de los aerosoles atmosfericos. Medio Ambiente 1986, 8, 3–8. [Google Scholar]
- Heo, Y.; Park, J.; Lim, S.; Hur, H.; Kim, D.; Park, K. Size-resolved culturable airborne bacteria sampled in rice field, sanitary landfill, and waste incineration sites. J. Environ. Monit. 2010, 12, 1619. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Hou, P.A. Bioaerosol characteristics in hospital clean rooms. Sci. Total Environ. 2003, 305, 169–176. [Google Scholar] [CrossRef]
- Morgado Gamero, W.B.; Agudelo-Castañeda, D.; Ramirez, M.C.; Hernandez, M.M.; Mendoza, H.P.; Parody, A.; Viloria, A. Hospital Admission and Risk Assessment Associated to Exposure of Fungal Bioaerosols at a Municipal Landfill Using Statistical Models. In Intelligent Data Engineering and Automated Learning—IDEAL 2018; Yin, H., Camacho, D., Novais, P., Tallón-Ballesteros, A.J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 210–218. [Google Scholar]
- Ghosh, B.; Lal, H.; Srivastava, A. Review of bioaerosols in indoor environment with special reference to sampling, analysis and control mechanisms. Environ. Int. 2015, 85, 254–272. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, C.N. Airborne microbiological characteristics in public buildings of Korea. Build. Environ. 2007, 42, 2188–2196. [Google Scholar] [CrossRef]
- Awad, A.H.; Saeed, Y.; Hassan, Y.; Fawzy, Y.; Osman, M. Air microbial quality in certain public buildings, Egypt: A comparative study. Atmos. Pollut. Res. 2018, 9, 617–626. [Google Scholar] [CrossRef]
- He, C.; Mackay, I.M.; Ramsay, K.; Liang, Z.; Kidd, T.; Knibbs, L.D.; Johnson, G.; McNeale, D.; Stockwell, R.; Coulthard, M.G.; et al. Particle and bioaerosol characteristics in a paediatric intensive care unit. Environ. Int. 2017, 107, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Licina, D.; Bhangar, S.; Brooks, B.; Baker, R.; Firek, B.; Tang, X.; Morowitz, M.J.; Banfield, J.F.; Nazaroff, W.W. Concentrations and sources of airborne particles in a neonatal intensive care unit. PLoS ONE 2016, 11, e0154991. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.; Sanz, J.L. Cuestiones en Microbiología; Editorial Hélice: Madrid, Spain, 2004. [Google Scholar]
- Maldonado-Vega, M.; Peña-Cabriales, J.J.; De los Santos, S.; Castellanos-Arévalo, A.P.; Camarena-Pozos, D.; Arévalo-Rivas, B.; Valdés-Santiago, L.; Hernández-Valadez, L.J.; Guzmán De Peña, D.L. Bioaerosoles y evaluación de la calidad del aire en dos centros hospitalarios ubicados en León, Guanaguato, México (Bioaerosols and air quality assessment in two hospitals located in León, Guanajuato, Mexico). Rev. Int. Contam. Ambient 2014, 30, 351–363. [Google Scholar]
- SAMPSP (Sociedad Andaluza de Medicina Preventiva y Salud Pública). Recomendaciones Para la Monitorización de la Calidad Microbiológica del Aire (Bioseguridad Ambiental) en Zonas Hospitalarias de Riesgo; SAMPSP: Andalucía, Spain, 2016; pp. 1–35. [Google Scholar]
- Colombia. Ministerio de Salud y Protección Social Resolución 002003 de 2014. Available online: https://www.minsalud.gov.co/Normatividad_Nuevo/Resoluci%C3%B3n%202003%20de%202014.pdf (accessed on 17 May 2019).
- Macher, J.M.; Chatigny, M.A.; Burge, H.A. Sampling Airborne Microorganisms and Aeroallergens. In Air Sampling Instruments for Evaluation of Atmospheric Contaminan; ACGIH: Cincinnati, OH, USA, 1995; pp. 589–617. [Google Scholar]
- Rao, C.Y.; Burge, H.A.; Chang, J.C. Review of quantitative standards and guidelines for fungi in indoor air. J. Air Waste Manag. Assoc. 1996, 46, 899–908. [Google Scholar] [CrossRef]
- IAQA (Indoor Air Quality Association). Indoor Air Quality Standard #95-1 Recommended for Florida; IAQA: Longwood, FL, USA, 1995. [Google Scholar]
- Heida, H.; Bartman, F.; van der Zee, S.C. Occupational Exposure and Indoor Air Quality Monitoring in a Composting Facility. Am. Ind. Hyg. Assoc. J. 1995, 56, 39–43. [Google Scholar] [CrossRef]
- OSHA (Occupational Safety and Health Administration). Indoor Air Quality; OSHA: Washington, DC, USA, 1994; pp. 15968–16039. [Google Scholar]
- Aryanna Kelly Pinheiro, S. Microbiota Fúngica do Ambiente da uti Neonatal e de Amostras Clínicas dos Recém-Nascidos Internados no Hospital Universitário de Maceió. Master’s Thesis, Universidade Federal de Alagoas, Maceio, Brazil, 2009. [Google Scholar]
- Viegas, C.; Faria, T.; dos Santos, M.; Carolino, E.; Gomes, A.Q.; Sabino, R.; Viegas, S. Fungal burden in waste industry: An occupational risk to be solved. Environ. Monit. Assess. 2015, 187, 199. [Google Scholar] [CrossRef]
- Yamamoto, N.; Schmechel, D.; Chen, B.T.; Lindsley, W.G.; Peccia, J. Comparison of quantitative airborne fungi measurements by active and passive sampling methods. J. Aerosol Sci. 2011, 42, 499–507. [Google Scholar] [CrossRef]
- Sadyś, M.; Kennedy, R.; West, J.S. Potential impact of climate change on fungal distributions: Analysis of 2 years of contrasting weather in the UK. Aerobiologia 2016, 32, 127–137. [Google Scholar] [CrossRef]
- Dungan, R.S. Board-invited review: Fate and transport of bioaerosols associated with livestock operations and manures. J. Anim. Sci. 2010, 88, 3693–3706. [Google Scholar] [CrossRef]
- Pahari, A.K.; Dasgupta, D.; Patil, R.S.; Mukherji, S. Emission of bacterial bioaerosols from a composting facility in Maharashtra, India. Waste Manag. 2016, 53, 22–31. [Google Scholar] [CrossRef]
- Adams, R.I.; Miletto, M.; Lindow, S.E.; Taylor, J.W.; Bruns, T.D. Airborne bacterial communities in residences: Similarities and differences with fungi. PLoS ONE 2014, 9, e91283. [Google Scholar] [CrossRef] [PubMed]
- Prussin, A.J.; Marr, L.C. Sources of airborne microorganisms in the built environment. Microbiome 2015, 3, 78. [Google Scholar] [CrossRef] [PubMed]
- Wai Tham, K. Indoor air quality and its effects on humans—A review of challenges and developments in the last 30 years. Energy Build. 2016, 130, 637–650. [Google Scholar] [CrossRef]
- Uhrbrand, K.; Schultz, A.C.; Koivisto, A.J.; Nielsen, U.; Madsen, A.M. Assessment of airborne bacteria and noroviruses in air emission from a new highly-advanced hospital wastewater treatment plant. Water Res. 2017, 112, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Raynor, P.C.; Davies, P.R.; Torremorell, M. Concentration, size distribution, and infectivity of airborne particles carrying swine viruses. PLoS ONE 2015, 10, e0135675. [Google Scholar] [CrossRef] [PubMed]
- Pastuszka, J.S.; Kyaw Tha Paw, U.; Lis, D.O.; Wlazło, A.; Ulfig, K. Bacterial and fungal aerosol in indoor environment in Upper Silesia, Poland. Atmos. Environ. 2000, 34, 3833–3842. [Google Scholar] [CrossRef]
- Asif, A.; Zeeshan, M.; Hashmi, I.; Zahid, U.; Bhatti, M.F. Microbial quality assessment of indoor air in a large hospital building during winter and spring seasons. Build. Environ. 2018, 135, 68–73. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Y.; Yu, J.; Ai, Q.; Li, J.; Peng, N.; Song, S.; He, Y.; Wang, Z. Synergy of ambroxol with vancomycin in elimination of catheter-related Staphylococcus epidermidis biofilm in vitro and in vivo. J. Infect. Chemother. 2015, 21, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Hardjawinata, K.; Setiawati, R.; Dewi, W. Bactericidal efficacy of ultraviolet irradiation on Staphylococcus aureus. Asian J. Oral Maxillofac. Surg. 2005, 17, 157–161. [Google Scholar] [CrossRef]
- Sohn, K.M.; Baek, J.Y.; Kim, S.H.; Cheon, S.; Kim, Y.S. Catheter-related bacteremia caused by kocuria salsicia: The first case. J. Infect. Chemother. 2015, 21, 305–307. [Google Scholar] [CrossRef]
- Uekotter, A.; Konig, J.; Peters, G.; Becker, K. Port-Infection Due to Kocuria Rhizophila in an 8 Year Old Child with Methylmalonic Aciduria. In Proceedings of the 58th Annual Conference of the German Society of Hygiene and Microbiology, Wurzburg, Germany, 1–4 October 2006. [Google Scholar]
- Gholami, M.; Etemadifar, Z.; Bouzari, M. Isolation a new strain of Kocuria rosea capable of tolerating extreme conditions. J. Environ. Radioact. 2015, 144, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Xaplanteri, P.; Chondroleou, A.; Kolonitsiou, F.; Skintzi, A.; Anastassiou, E.D.; Marangos, M.; Spiliopoulou, I. Postpartum bacteraemia outbreak due to Bacillus cereus in the delivery room. New Microbes New Infect. 2019, 29, 100510. [Google Scholar] [CrossRef] [PubMed]
- De La Rosa, M.C.; Mosso, M.A.; Ullán, C. El aire: Hábitat y medio de transmisión de microorganismos. Obs. Medioambient. 2002, 5, 28. [Google Scholar]
- Shivamurthy, V.M.; Gantt, S.; Reilly, C.; Tilley, P.; Guzman, J.; Tucker, L. Bacillus pumilus Septic Arthritis in a Healthy Child. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 3265037. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, Y.; Gao, M. Genomic analysis of a ginger pathogen Bacillus pumilus providing the understanding to the pathogenesis and the novel control strategy. Sci. Rep. 2015, 5, 10259. [Google Scholar] [CrossRef] [PubMed]
- Kimouli, M.; Vrioni, G.; Papadopoulou, M.; Koumaki, V.; Petropoulou, D.; Gounaris, A.; Friedrich, A.W.; Tsakris, A. Two cases of severe sepsis caused by Bacillus pumilus in neonatal infants. J. Med. Microbiol. 2012, 61, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Celandroni, F.; Salvetti, S.; Gueye, S.A.; Mazzantini, D.; Lupetti, A.; Senesi, S.; Ghelardi, E. Identification and pathogenic potential of clinical Bacillus and Paenibacillus isolates. PLoS ONE 2016, 11, e0152831. [Google Scholar] [CrossRef] [PubMed]
- Camacho, R.; Aguilar, E.M.; Quezada, H.; Medina, Ó.; Patiño, G.; Cárdenas, H.M.; Ramos, R. Characterization of Cry toxins from autochthonous Bacillus thuringiensis isolates from Mexico. Bol. Med. Hosp. Infant. Mex. 2017, 74, 193–199. [Google Scholar]
- Oggioni, M.R.; Pozzi, G.; Valensin, P.E.; Galieni, P.; Bigazzi, C. Recurrent septicemia in an immunocompromised patient due to probiotic strains of Bacillus subtilis. J. Clin. Microbiol. 1998, 36, 325–326. [Google Scholar]
- Sheikh, A.F.; Saki, N.; Roointan, M.; Ranjbar, R.; Yadyad, M.J.; Kaydani, A.; Aslani, S.; Babaei, M.; Goodarzi, H. Identification of Alloiococcus otitidis, Streptococcus pneumoniae, Moraxella catarrhalis and Haemophilus influenzae in children with otitis media with effusion. Jundishapur J. Microbiol. 2015, 8, e17985. [Google Scholar]
- Tano, K.; Von Essen, R.; Eriksson, P.O.; Sjöstedt, A. Alloiococcus otitidis—Otitis media pathogen or normal bacterial flora? APMIS 2008, 116, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Harimaya, A.; Takada, R.; Somekawa, Y.; Fujii, N.; Himi, T. High frequency of Alloiococcus otitidis in the nasopharynx and in the middle ear cavity of otitis-prone children. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Hage, J.E.; Schoch, P.E.; Cunha, B.A. Pseudomonas pseudoalcaligenes peritoneal dialysis-associated peritonitis. Perit. Dial. Int. 2013, 33, 223–224. [Google Scholar] [CrossRef]
- Sulpher, J.; Desjardins, M.; Lee, B.C. Central venous catheter-associated Leifsonia aquatica bacteremia in a hemodialysis-dependent patient. Diagn. Microbiol. Infect. Dis. 2008, 61, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Kolata, J.; Bode, L.; Holtfreter, S.; Steil, L.; Kusch, H.; Holtfreter, B.; Hecker, M.; Engelmann, S.; van Belkum, A.; Völker, U.; et al. The Human Antibody Response to Staphylococcus Aureus Bacteremia. In Proceedings of the 63rd Annual meeting of the German Society for Hygiene and Microbiology, Essen, Germany, 25–28 September 2011. [Google Scholar]
- Orden-Martínez, B.; Martínez-Ruiz, R.; Millán-Pérez, R. ¿Qué estamos aprendiendo de Staphylococcus saprophyticus? Enferm. Infecc. Microbiol. Clin. 2008, 26, 495–499. [Google Scholar] [CrossRef]
- Ziebuhr, W.; Hennig, S.; Eckart, M.; Kränzler, H.; Batzilla, C.; Kozitskaya, S. Nosocomial infections by Staphylococcus epidermidis: How a commensal bacterium turns into a pathogen. Int. J. Antimicrob. Agents 2006, 28, 14–20. [Google Scholar] [CrossRef]
- Sabaté Brescó, M.; Harris, L.G.; Thompson, K.; Stanic, B.; Morgenstern, M.; O’Mahony, L.; Richards, R.G.; Moriarty, T.F. Pathogenic Mechanisms and Host Interactions in Staphylococcus epidermidis Device-Related Infection. Front. Microbiol. 2017, 8, 1401. [Google Scholar] [CrossRef]
- Dong, Y.; Speer, C.P. The role of Staphylococcus epidermidis in neonatal sepsis: Guarding angel or pathogenic devil? Int. J. Med. Microbiol. 2014, 304, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.A.; Bennett, A.M.; Walker, J.T. Aerosol survival of Staphylococcus epidermidis. J. Hosp. Infect. 2011, 78, 216–220. [Google Scholar] [CrossRef]
- Borrego, S.C.; Perdomo, I.; De la Paz, J.; Gómez de Saravia, S.; Guiamet, P. Relevamiento microbiológico del aire y de materiales almacenados en el Archivo Histórico del Museo de La Plata, Argentina y en el Archivo Nacional de la República de Cuba. Rev. Del Mus. La Plata 2011, 18, 1–18. [Google Scholar]
- Jadhav, A.R.; Belfort, M.A.; Dildy, G.A. Eikenella corrodens chorioamnionitis: Modes of infection? Am. J. Obstet. Gynecol. 2009, 200, e4–e5. [Google Scholar] [CrossRef] [PubMed]
- Sweidan, A.; Chollet-Krugler, M.; Sauvager, A.; van de Weghe, P.; Chokr, A.; Bonnaure-Mallet, M.; Tomasi, S.; Bousarghin, L. Antibacterial activities of natural lichen compounds against Streptococcus gordonii and Porphyromonas gingivalis. Fitoterapia 2017, 121, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Nayar, G.; Darley, E.S.R.; Hammond, F.; Matthews, S.; Turton, J.; Wach, R. Does screening neonates in the neonatal intensive care unit for Pseudomonas aeruginosa colonization help prevent infection? J. Hosp. Infect. 2018, 100, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Macedo, M.; Blanco, J. Infecciones hospitalarias. Control 2008, 245–254. [Google Scholar]
- Soldera, J.; Nedel, W.L.; Cardoso, P.R.; d’Azevedo, P.A. Bacteremia due to Staphylococcus cohnii ssp. urealyticus caused by infected pressure ulcer: Case report and review of the literature. Sao Paulo Med. J. 2013, 131, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, S.; Garcia, J.; Morfín, R.; Villarreal, L.; Camacho, A.; Rodríguez, E.; Bocanegra, P.; Maldonado, H.; Dowd, S.; Garza, E. Draft genome sequences of two opportunistic pathogenic strains of Staphylococcus cohnii isolated from human patients. Stand. Genomic Sci. 2017, 12. [Google Scholar]
- Shahandeh, Z.; Shafi, H.; Sadighian, F. Association of staphylococcus cohnii subspecies urealyticum infection with recurrence of renal staghorn stone. J. Intern. Med. 2015, 6, 40–42. [Google Scholar]
- Paul, M.; Gupta, R.; Khush, S.; Thakur, R. Kocuria rosea: An emerging pathogen in acute bacterial meningitis- Case report. J. Microbiol. Antimicrob. Agents 2015, 1, 4–7. [Google Scholar]
- Moreira, J.S.; Riccetto, A.G.L.; da Silva, M.T.N.; dos Santos Vilela, M.M. Endocarditis by Kocuria rosea in an immunocompetent child. Braz. J. Infect. Dis. 2015, 19, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Corti, M.; Villafañe, M.F.; Soto, I.; Palmieri, O.; Callejo, R. Bacteriemia por Kocuria rosea en un paciente con SIDA. Rev. Chil. Infectología 2012, 29, 355–356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hilliard, N.J.; Schelonka, R.L.; Waites, K.B. Bacillus cereus bacteremia in a preterm neonate. J. Clin. Microbiol. 2003, 41, 3441–3444. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.; Silva, A.; Magalhães, M.J.; Sá, C.; Abreu, E. Severe Bacillus cereus infection in a neonatal intensive care unit. Case Rep. Perinat. Med 2014, 3, 159–162. [Google Scholar] [CrossRef][Green Version]
- Puvabanditsin, S.; Zaafran, A.; Garrow, E.; Diwan, R.; Mehta, D.; Phattraprayoon, N. Bacillus cereus meningoencephalitis in a neonate. Hong Kong J. Paediatr. 2007, 12, 293–296. [Google Scholar]
- Venkatesh, M.P.; Placencia, F.; Weisman, L.E. Coagulase-Negative Staphylococcal Infections in the Neonate and Child: An Update. Semin. Pediatr. Infect. Dis. 2006, 17, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Yusef, D.; Shalakhti, T.; Awad, S.; Algharaibeh, H.; Khasawneh, W. Clinical characteristics and epidemiology of sepsis in the neonatal intensive care unit in the era of multi-drug resistant organisms: A retrospective review. Pediatr. Neonatol. 2018, 59, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Puzari, M.; Sharma, M.; Chetia, P. Emergence of antibiotic resistant Shigella species: A matter of concern. J. Infect. Public Health 2018, 11, 451–454. [Google Scholar] [PubMed]
- Boletín epidemiológico de resistencia bacteriana—SIVIBAC año 2014. Available online: http://www.saludcapital.gov.co/DSP/Boletines%20temticos/IAAS/2014/Bolet%C3%ADn%20Resistencia%20Bacteriana%202014.pdf (accessed on 17 May 2019).
- Monteil, H.; Harf-Monteil, C. Aerobic gram-negative bacilli: Newer nosocomial pathogens. Int. J. Antimicrob. Agents 1997, 8, 217–231. [Google Scholar] [CrossRef]
- Klingenberg, C.; Rønnestad, A.; Anderson, A.S.; Abrahamsen, T.G.; Zorman, J.; Villaruz, A.; Flaegstad, T.; Otto, M.; Sollid, J.E. Persistent strains of coagulase-negative staphylococci in a neonatal intensive care unit: Virulence factors and invasiveness. Clin. Microbiol. Infect. 2007, 13, 1100–1111. [Google Scholar] [CrossRef]
- Trubiano, J.A.; Padiglione, A.A. Nosocomial infections in the intensive care unit. Anaesth. Intensive Care Med. 2015, 16, 598–602. [Google Scholar] [CrossRef]
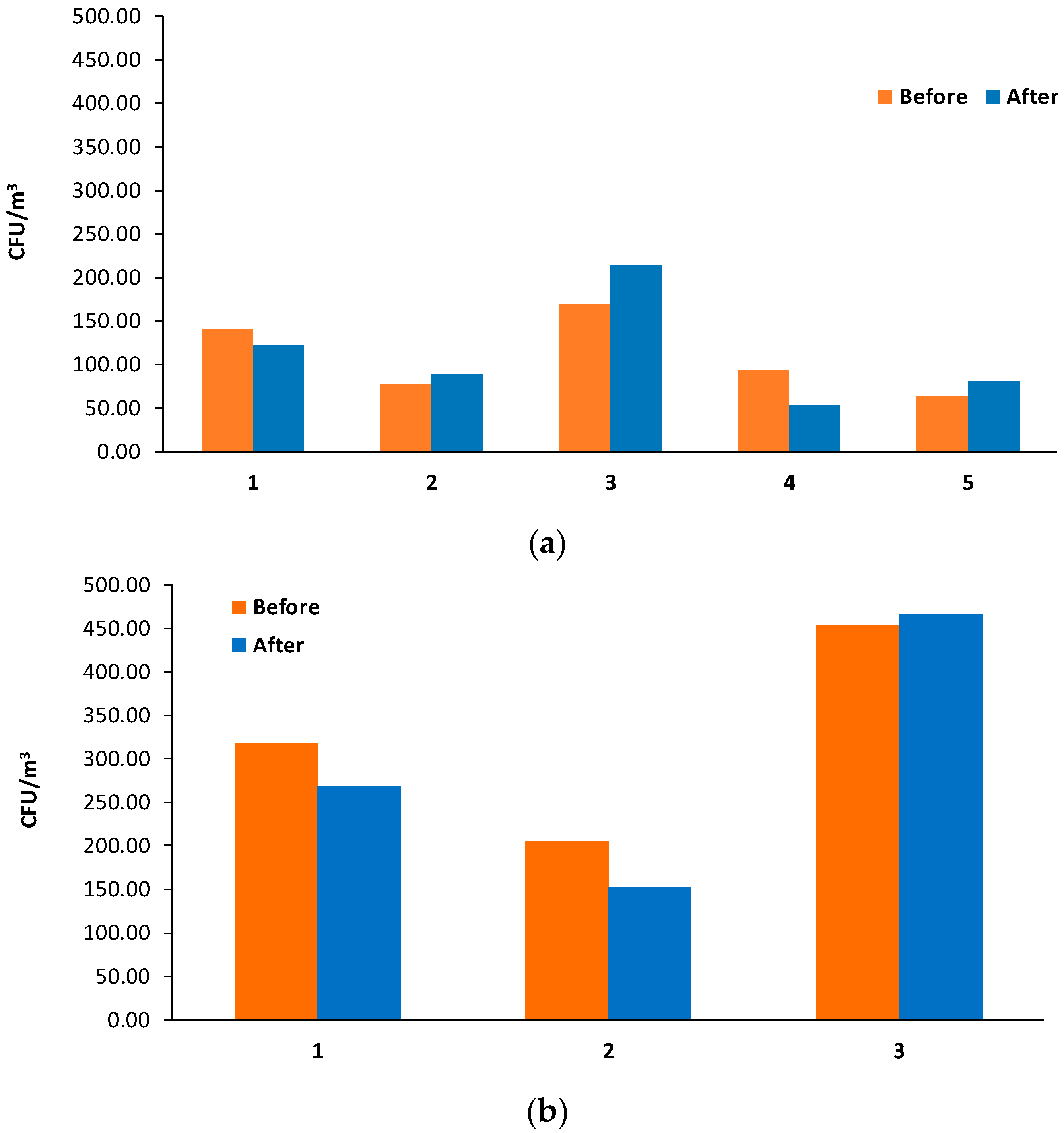

| Organization | Value | Reference | |
|---|---|---|---|
| Concentration of bacteria in air (mean and range) | 110.13 CFU/m3 (53.00–214.37 CFU/m3) | - | This study—NICU#1 |
| 310.37 CFU/m3 (151.94–466.43 CFU/m3) | - | This study—NICU#2 | |
| American Conference of Governmental Industrial Hygienists (ACGIH) | <100 CFU/m3 | Low | [34] |
| 100–1000 CFU/m3 | Intermediate | ||
| >1000 CFU/m3 | High. | ||
| Healthy Buildings International | <750 CFU/m3 | The total of bacteria and fungi in the air are fine if the species are not infectious or allergenic. | [35] |
| Indoor Air Quality Association (IAQ) | <150 CFU/m3 | If it is a mixture of species it is fine. | [36] |
| The Netherlands/research methods in biological indoor air pollution | >104 CFU/m3 | The total fungus and bacteria are a threat to health. | [37] |
| >500 CFU/m3 | A species of potentially pathogenic nature is a threat to health. | ||
| Occupational Safety and Health Administration (OSHA) | >1000 CFU/m3 | Indicates contamination | [38] |
| >106 Fungus/Bacteria/dust | Indicates contamination |
| NICU#1—Airborne Bacteria | |||||
|---|---|---|---|---|---|
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
| Between groups | 58.82 | 1 | 58.82 | 0.22 | 0.639 |
| Intra groups | 5.18 × 104 | 194 | 267.20 | - | - |
| Total (Corrected.) | 5.19 × 104 | 195 | - | - | - |
| NICU#2—airborne bacteria | |||||
| Between ** groups | 1300.64 | 1 | 1300.64 | 0.06 | 0.81 |
| Intra groups | 81,217.98 | 4 | 20,304.49 | - | - |
| Total (Corrected.) | 82,518.61 | 5 | - | - | - |
| NICU#2—particle number | |||||
| Between ** groups | 15,116,795.93 | 1.00 | 15,116,795.93 | 0.08 | 0.79 |
| Intra groups | 1,971,755,605.47 | 10.00 | 197,175,560.55 | - | - |
| Total (Corrected.) | 1,986,872,401 | 11 | - | - | - |
| Microorganism | Before (CFU/m3) | After (CFU/m3) | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | ||
| Alloiococcus otitidis | 7 | 7 | 7 | 7 | |||||||
| Bacillus cereus | 7 | 7 | 21 | 7 | 7 | 7 | 7 | 7 | 8 | ||
| Bacillus megaterium | 7 | 7 | 7 | 7 | 7 | 9 | 8 | ||||
| Bacillus pumilus | 7 | 7 | 7 | 7 | 7 | ||||||
| Bacillus subtilis | 7 | 7 | 7 | 7 | 7 | ||||||
| Bacillus thuringiensis | 7 | 7 | 7 | 7 | 7 | ||||||
| Kocuria. Rosea | 7 | 9 | 7 | 7 | 28 | 28 | 12 | ||||
| Leifsonia aquatica | 7 | 7 | 7 | 7 | |||||||
| Pseudomona pseudoalcaligenes | 7 | 7 | 7 | 7 | |||||||
| S. cohnii ssp. | 7 | 7 | |||||||||
| S. epidermidis | 11 | 13 | 27 | 18 | 11 | 8 | 15 | 20 | 16 | 19 | 16 |
| S. saprophyticus | 33 | 7 | 21 | 7 | 18 | 9 | 30 | 7 | 21 | ||
| Microorganism | Before (CFU/m3) | After (CFU/m3) | Total | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | ||
| Bacillus cereus | 13 | 25 | 38 | ||||
| Bacillus subtilis | 10 | 7 | 24 | 12 | 22 | 77 | |
| Bacillus thuringiensis | 14 | 13 | 27 | ||||
| Eikenella corrodens | 12 | 16 | 28 | ||||
| Pseudomonas aeruginosa | 10 | 11 | 20 | ||||
| Staphylococcus aureus | 7 | 12 | 19 | ||||
| Staphylococcus epidermidis | 10 | 15 | 25 | ||||
| Streptococcus gordoni | 13 | 12 | 26 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgado-Gamero, W.B.; Mendoza Hernandez, M.; Castillo Ramirez, M.; Medina-Altahona, J.; De La Hoz, S.; Posso Mendoza, H.; Parody, A.; Teixeira, E.C.; Agudelo-Castañeda, D.M. Antibiotic Resistance of Airborne Viable Bacteria and Size Distribution in Neonatal Intensive Care Units. Int. J. Environ. Res. Public Health 2019, 16, 3340. https://doi.org/10.3390/ijerph16183340
Morgado-Gamero WB, Mendoza Hernandez M, Castillo Ramirez M, Medina-Altahona J, De La Hoz S, Posso Mendoza H, Parody A, Teixeira EC, Agudelo-Castañeda DM. Antibiotic Resistance of Airborne Viable Bacteria and Size Distribution in Neonatal Intensive Care Units. International Journal of Environmental Research and Public Health. 2019; 16(18):3340. https://doi.org/10.3390/ijerph16183340
Chicago/Turabian StyleMorgado-Gamero, Wendy Beatriz, Martha Mendoza Hernandez, Margarita Castillo Ramirez, Jhorma Medina-Altahona, Stephanie De La Hoz, Heidy Posso Mendoza, Alexander Parody, Elba C. Teixeira, and Dayana Milena Agudelo-Castañeda. 2019. "Antibiotic Resistance of Airborne Viable Bacteria and Size Distribution in Neonatal Intensive Care Units" International Journal of Environmental Research and Public Health 16, no. 18: 3340. https://doi.org/10.3390/ijerph16183340
APA StyleMorgado-Gamero, W. B., Mendoza Hernandez, M., Castillo Ramirez, M., Medina-Altahona, J., De La Hoz, S., Posso Mendoza, H., Parody, A., Teixeira, E. C., & Agudelo-Castañeda, D. M. (2019). Antibiotic Resistance of Airborne Viable Bacteria and Size Distribution in Neonatal Intensive Care Units. International Journal of Environmental Research and Public Health, 16(18), 3340. https://doi.org/10.3390/ijerph16183340






