Synergy of Photocatalysis and Adsorption for Simultaneous Removal of Hexavalent Chromium and Methylene Blue by g-C3N4/BiFeO3/Carbon Nanotubes Ternary Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Functionalized Ternary Magnetic Composite CNBT
2.2. Characterization
2.3. Photoelectrochemical Measurements
2.4. Photocatalytic Activity Testing
2.5. Adsorption Studies
3. Results and Discussion
3.1. Sample Characterization
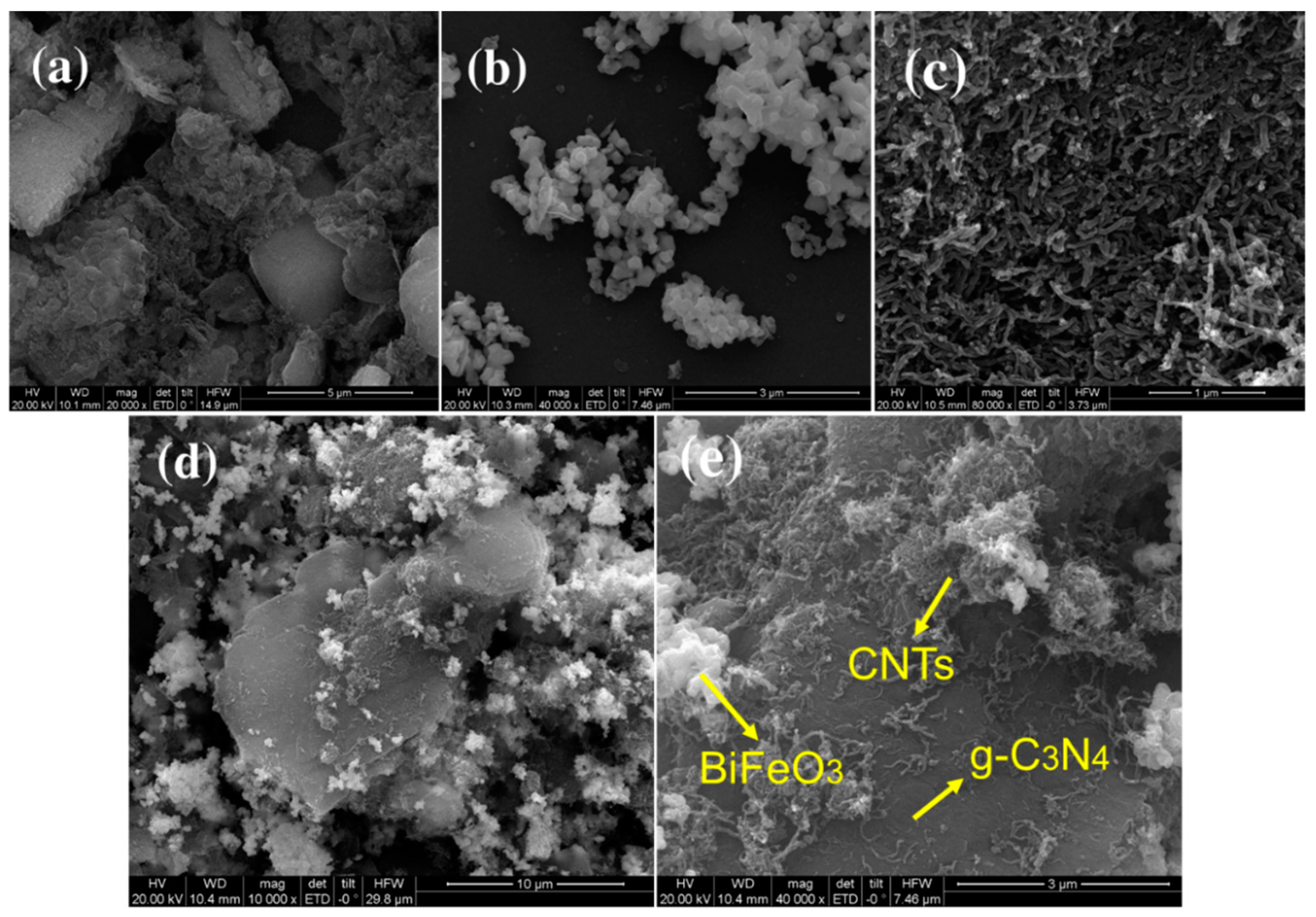
3.1.1. Electron Microscopy
3.1.2. FTIR
3.1.3. TG-DSC
3.1.4. VSM Measurements
3.1.5. ESR
3.1.6. XRD
3.1.7. XPS
3.2. Investigation on Charge Separation and Optical Properties
3.3. Adsorption Studies
3.3.1. Adsorption Properties of Different Materials
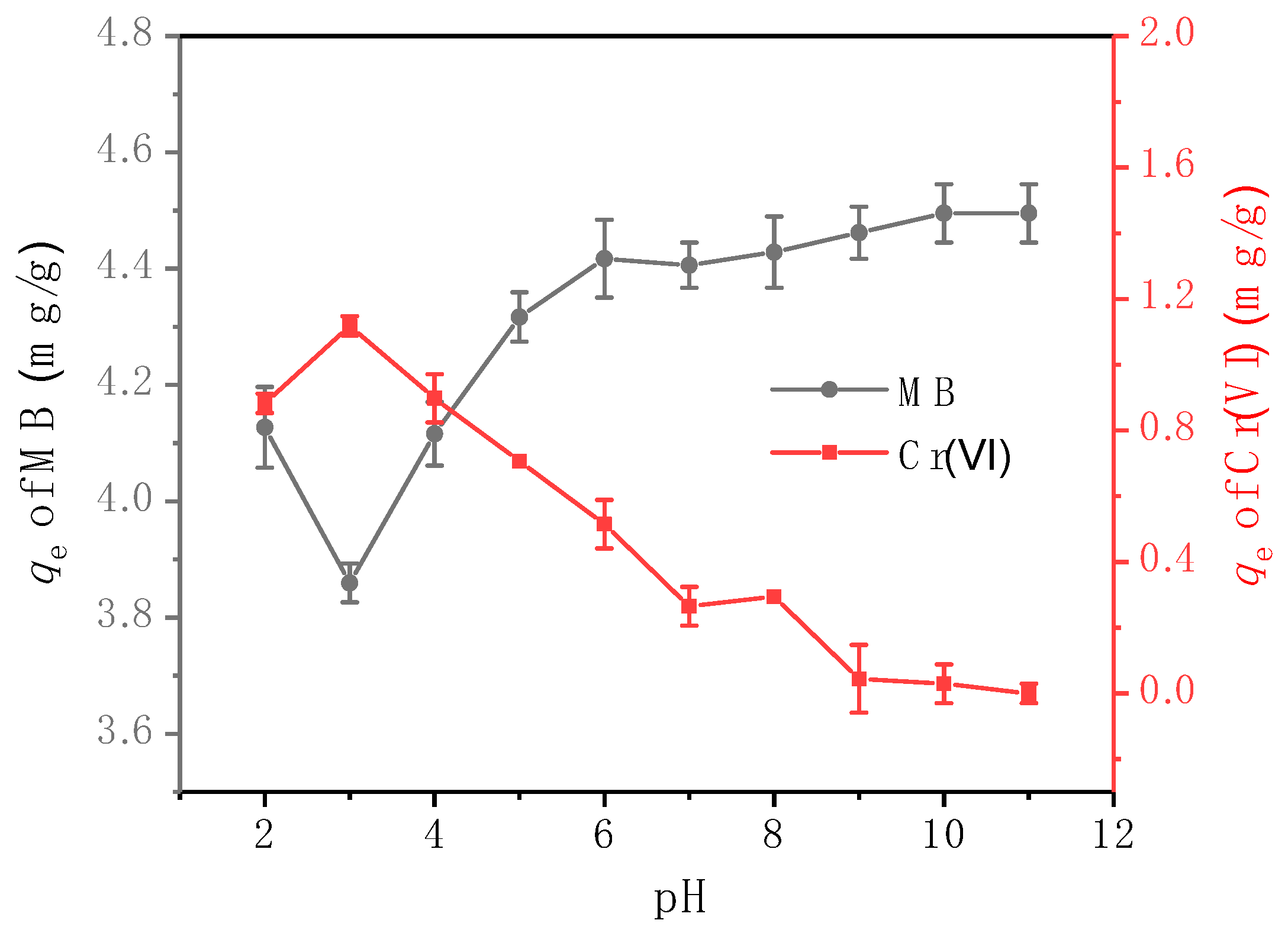
3.3.2. Adsorption Efficiencies at Different pH
3.4. Photocatalytic Experiments
3.4.1. Photocatalytic Properties of Different Materials
3.4.2. The Relationship between the Two Pollutants
3.4.3. Possible Photocatalytic Mechanism in Reaction System
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Han, D.; Currell, M.J.; Cao, G. Deep challenges for China’s war on water pollution. Environ. Pollut. 2016, 218, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, S.; Xia, L.; Wang, Z.; Suo, N.; Chen, H.; Long, Y.; Zhou, B.; Yu, Y. In-situ ion exchange electrocatalysis biological coupling (i-IEEBC) for simultaneously enhanced degradation of organic pollutants and heavy metals in electroplating wastewater. J. Hazard. Mater. 2019, 364, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; He, J.; Zhang, J.; Yang, B.; Zhao, X. Simultaneous Cr(VI) removal and bisphenol A degradation in a solar-driven photocatalytic fuel cell with dopamine modified carbon felt cathode. Appl. Surf. Sci. 2019, 471, 912–920. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Mitoraj, D.; Lamdab, U.; Kangwansupamonkon, W.; Pacia, M.; Macyk, W.; Wetchakun, N.; Beranek, R. Revisiting the problem of using methylene blue as a model pollutant in photocatalysis: The case of InVO4/BiVO4 composites. J. Photochem. Photobiol. A Chem. 2018, 366, 103–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Li, H.; Ge, H.; Bian, Z. The enhanced photoreduction of Cr(VI) to Cr(III) using carbon dots coupled TiO2 mesocrystals. Appl. Catal. B Environ. 2018, 226, 213–219. [Google Scholar] [CrossRef]
- Ning, J.; Wang, M.; Luo, X.; Hu, Q.; Hou, R.; Chen, W.; Chen, D.; Wang, J.; Liu, J. SiO2 Stabilized Magnetic Nanoparticles as a Highly Effective Catalyst for the Degradation of Basic Fuchsin in Industrial Dye Wastewaters. Molecules 2018, 23, 2573. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Zhou, F.; Owens, G.; Chen, Z. Burkholderia cepacia immobilized on eucalyptus leaves used to simultaneously remove malachite green (MG) and Cr(VI). Colloids Surf. B Biointerfaces 2018, 172, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ok, Y.S.; Mohan, D.; Pittman, C.U.; Dou, X. Carbamazepine removal from water by carbon dot-modified magnetic carbon nanotubes. Environ. Res. 2019, 169, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Wei, Z.; Miao, H.; Yao, W.; Li, H.; Zhu, Y. Enhanced organic pollutant photodegradation via adsorption/photocatalysis synergy using a 3D g-C3N4/TiO2 free-separation photocatalyst. Chem. Eng. J. 2019, 370, 287–294. [Google Scholar] [CrossRef]
- Chen, F.; An, W.; Liu, L.; Liang, Y.; Cui, W. Highly efficient removal of bisphenol A by a three-dimensional graphene hydrogel-AgBr@rGO exhibiting adsorption/photocatalysis synergy. Appl. Catal. B Environ. 2017, 217, 65–80. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, Y.; Cui, W.; Liang, Y.; Zhu, Y. Removal of bisphenol A over a separation free 3D Ag3PO4 -graphene hydrogel via an adsorption-photocatalysis synergy. Appl. Catal. B Environ. 2017, 212, 41–49. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Mu, X.; Fan, X.; Li, R.; Zhang, Y.; Song, P.; Ma, F.; Sun, M. Site-selected N vacancy of g-C3N4 for photocatalysis and physical mechanism. Appl. Mater. Today 2018, 13, 329–338. [Google Scholar] [CrossRef]
- Cai, X.; He, J.; Chen, L.; Chen, K.; Li, Y.; Zhang, K.; Jin, Z.; Liu, J.; Wang, C.; Wang, X.; et al. A 2D-g-C3N4 nanosheet as an eco-friendly adsorbent for various environmental pollutants in water. Chemosphere 2017, 171, 192–201. [Google Scholar] [CrossRef]
- Tan, J.Z.Y.; Nursam, N.M.; Xia, F.; Sani, M.-A.; Li, W.; Wang, X.; Caruso, R.A. High-Performance Coral Reef-like Carbon Nitrides: Synthesis and Application in Photocatalysis and Heavy Metal Ion Adsorption. ACS Appl. Mater. Interfaces 2017, 9, 4540–4547. [Google Scholar] [CrossRef]
- Wu, X.; Wang, X.; Wang, F.; Yu, H. Soluble g-C3N4 nanosheets: Facile synthesis and application in photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 247, 70–77. [Google Scholar] [CrossRef]
- Carvalho, K.T.G.; Nogueira, A.E.; Lopes, O.F.; Byzynski, G.; Ribeiro, C. Synthesis of g-C3N4/Nb2O5 heterostructures and their application in the removal of organic pollutants under visible and ultraviolet irradiation. Ceram. Int. 2017, 43, 3521–3530. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, H.; Quan, X.; Chen, S.; Zhang, Y.; Zhao, H.; Wang, H. Fabrication of atomic single layer graphitic-C3N4 and its high performance of photocatalytic disinfection under visible light irradiation. Appl. Catal. B Environ. 2014, 152, 46–50. [Google Scholar] [CrossRef]
- Wang, H.-H.; Zhang, B.; Li, X.-H.; Antonietti, M.; Chen, J.-S. Activating Pd nanoparticles on sol-gel prepared porous g-C3N4/SiO2 via enlarging the Schottky barrier for efficient dehydrogenation of formic acid. Inorg. Chem. Front. 2016, 3, 1124–1129. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Zhou, D.; Wang, Z.; Jin, M. Chemical co-precipitation synthesis and properties of pure-phase BiFeO3. Chem. Phys. Lett. 2018, 713, 185–188. [Google Scholar] [CrossRef]
- Gao, F.; Chen, X.Y.; Yin, K.B.; Dong, S.; Ren, Z.F.; Yuan, F.; Yu, T.; Zou, Z.G.; Liu, J.M. Visible-Light Photocatalytic Properties of Weak Magnetic BiFeO3 Nanoparticles. Adv. Mater. 2007, 19, 2889–2892. [Google Scholar] [CrossRef]
- Wang, X.; Mao, W.; Zhang, J.; Han, Y.; Quan, C.; Zhang, Q.; Yang, T.; Yang, J.; Li, X.; Huang, W. Facile fabrication of highly efficient g-C3N4/BiFeO3 nanocomposites with enhanced visible light photocatalytic activities. J. Colloid Interface Sci. 2015, 448, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhu, L.; Wang, N.; Tang, H.; Cao, M.; She, Y. Efficient Removal of Organic Pollutants with Magnetic Nanoscaled BiFeO3 as a Reusable Heterogeneous Fenton-Like Catalyst. Environ. Sci. Technol. 2010, 44, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, N.; Wu, H.; Li, W.; Fang, Z.; Xu, Z.; Qian, X. Flexible design of carbon nanotubes grown on carbon nanofibers by PECVD for enhanced Cr(VI) adsorption capacity. Sep. Purif. Technol. 2018, 207, 406–415. [Google Scholar] [CrossRef]
- Hu, X.; Wang, W.; Xie, G.; Wang, H.; Tan, X.; Jin, Q.; Zhou, D.; Zhao, Y. Ternary assembly of g-C3N4/graphene oxide sheets /BiFeO3 heterojunction with enhanced photoreduction of Cr(VI) under visible-light irradiation. Chemosphere 2019, 216, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Noimark, S.; Weiner, J.; Noor, N.; Allan, E.; Williams, C.K.; Shaffer, M.S.P.; Parkin, I.P. Dual-Mechanism Antimicrobial Polymer-ZnO Nanoparticle and Crystal Violet-Encapsulated Silicone. Adv. Funct. Mater. 2015, 25, 1367–1373. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Zeng, G.; Feng, C.; Dong, H.; Wang, J.; Feng, H.; Liu, Y.; Zhou, Y.; Pang, Y. Plasmonic resonance excited dual Z-scheme BiVO4/Ag/Cu2O nanocomposite: Synthesis and mechanism for enhanced photocatalytic performance in recalcitrant antibiotic degradation. Environ. Sci. Nano 2017, 4, 1494–1511. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Shainy, F.; Christa, J. Synthesis and characterization of polyacrylic acid- grafted-carboxylic graphene/titanium nanotube composite for the effective removal of enrofloxacin from aqueous solutions: Adsorption and photocatalytic degradation studies. J. Hazard. Mater. 2017, 324, 117–130. [Google Scholar] [CrossRef]
- Tseng, W.J.; Lin, R.D. BiFeO3/alpha-Fe2O3 core/shell composite particles for fast and selective removal of methyl orange dye in water. J. Colloid Interface Sci. 2014, 428, 95–100. [Google Scholar] [CrossRef]
- Sankar Ganesh, R.; Sharma, S.K.; Sankar, S.; Divyapriya, B.; Durgadevi, E.; Raji, P.; Ponnusamy, S.; Muthamizhchelvan, C.; Hayakawa, Y.; Kim, D.Y. Microstructure, structural, optical and piezoelectric properties of BiFeO3 nanopowder synthesized from sol-gel. Curr. Appl. Phys. 2017, 17, 409–416. [Google Scholar] [CrossRef]
- Kang, H.W.; Lim, S.N.; Song, D.; Park, S.B. Organic-inorganic composite of g-C3N4-SrTiO3:Rh photocatalyst for improved H2 evolution under visible light irradiation. Int. J. Hydrog. Energy 2012, 37, 11602–11610. [Google Scholar] [CrossRef]
- Cheng, N.; Tian, J.; Liu, Q.; Ge, C.; Qusti, A.H.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Au-Nanoparticle-Loaded Graphitic Carbon Nitride Nanosheets: Green Photocatalytic Synthesis and Application toward the Degradation of Organic Pollutants. ACS Appl. Mater. Interfaces 2013, 5, 6815–6819. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.P.; Ren, C.L.; Qu, J.C.; Chen, X.G. Preparation and characterization of Fe3O4/graphene nanocomposite and investigation of its adsorption performance for aniline and p-chloroaniline. Appl. Surf. Sci. 2012, 261, 504–509. [Google Scholar] [CrossRef]
- Hu, X.J.; Liu, Y.G.; Zeng, G.M.; Wang, H.; Hu, X.; Chen, A.W.; Wang, Y.Q.; Guo, Y.M.; Li, T.T.; Zhou, L.; et al. Effect of aniline on cadmium adsorption by sulfanilic acid-grafted magnetic graphene oxide sheets. J. Colloid Interface Sci. 2014, 426, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lai, C.; Huang, D.; Zeng, G.; Zhang, C.; Cheng, M.; Hu, L.; Wan, J.; Xiong, W.; Wen, M.; et al. Highly porous carbon nitride by supramolecular preassembly of monomers for photocatalytic removal of sulfamethazine under visible light driven. Appl. Catal. B Environ. 2018, 220, 202–210. [Google Scholar] [CrossRef]
- Yang, M.Q.; Zhang, N.; Xu, Y.J. Synthesis of fullerene, carbon nanotube, and graphene-TiO2 nanocomposite photocatalysts for selective oxidation: A comparative study. ACS Appl. Mater. Interfaces 2013, 5, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhang, G.; Zheng, R.; Wang, P. Removing lignin model pollutants with BiFeO3/g-C3N4 compound as an efficient visible-light-heterogeneous Fenton-like catalyst. J. Environ. Sci. 2016, 48, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, M.; Ma, J.; Ning, P.; Che, L. Synthesis of K-doped g-C3N4/carbon microsphere@graphene composite with high surface area for enhanced adsorption and visible photocatalytic degradation of tetracycline. J. Taiwan Inst. Chem. Eng. 2018, 91, 609–622. [Google Scholar] [CrossRef]
- Di, J.; Li, S.; Zhao, Z.; Huang, Y.; Jia, Y.; Zheng, H. Biomimetic CNT@TiO2 composite with enhanced photocatalytic properties. Chem. Eng. J. 2015, 281, 60–68. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Ke, B.; He, Z.; Cui, Y.; Pan, Z.; Li, D.; Huang, S.; Lai, C.; Su, J. Magnetic multi-walled carbon nanotubes modified with polyaluminium chloride for removal of humic acid from aqueous solution. J. Mol. Liq. 2019, 279, 241–250. [Google Scholar] [CrossRef]
- Booshehri, A.Y.; Chun-Kiat Goh, S.; Hong, J.; Jiang, R.; Xu, R. Effect of depositing silver nanoparticles on BiVO4 in enhancing visible light photocatalytic inactivation of bacteria in water. J. Mater. Chem. A 2014, 2, 6209–6217. [Google Scholar] [CrossRef]
- Bai, X.; Du, Y.; Hu, X.; He, Y.; He, C.; Liu, E.; Fan, J. Synergy removal of Cr (VI) and organic pollutants over RP-MoS2/rGO photocatalyst. Appl. Catal. B Environ. 2018, 239, 204–213. [Google Scholar] [CrossRef]
- Ning, J.; He, Q.; Luo, X.; Wang, M.; Liu, D.; Wang, J.; Li, G.; Liu, J. Determination of Uric Acid in Co-Presence of Dopamine and Ascorbic Acid Using Cuprous Oxide Nanoparticle-Functionalized Graphene Decorated Glassy Carbon Electrode. Catalysts 2018, 8, 407. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Shi, Q.; Cai, Z.; Yang, Z. Acid-treated g-C3N4 with improved photocatalytic performance in the reduction of aqueous Cr(VI) under visible-light. Sep. Purif. Technol. 2015, 142, 251–257. [Google Scholar] [CrossRef]
- Liu, W.; Cao, L.; Cheng, W.; Cao, Y.; Liu, X.; Zhang, W.; Mou, X.; Jin, L.; Zheng, X.; Che, W.; et al. Single-site active cobalt-based photocatalyst with long carriers lifetime for spontaneous overall water splitting. Angew. Chem. Int. Ed. 2017, 56, 9312–9317. (In English) [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, M.; Xu, C.; Chen, S. Facile synthesis of g-C3N4/ZnO composite with enhanced visible light photooxidation and photoreduction properties. Chem. Eng. J. 2012, 209, 386–393. [Google Scholar] [CrossRef]
- Qin, F.; Wang, R.; Li, G.; Tian, F.; Zhao, H.; Chen, R. Highly efficient photocatalytic reduction of Cr(VI) by bismuth hollow nanospheres. Catal. Commun. 2013, 42, 14–19. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wang, H.; Chen, X.; Wu, Z.; Jiang, L.; Xiong, W.; Zeng, G. Facile synthesis of Sb2S3/ultrathin g-C3N4 sheets heterostructures embedded with g-C3N4 quantum dots with enhanced NIR-light photocatalytic performance. Appl. Catal. B Environ. 2016, 193, 36–46. [Google Scholar] [CrossRef]
- Ibrahim, S.; Shuy, W.Z.; Ang, H.-M.; Wang, S. Preparation of bioadsorbents for effective adsorption of a reactive dye in aqueous solution. Asia Pac. J. Chem. Eng. 2010, 5, 563–569. [Google Scholar] [CrossRef]
- Alam, U.; Khan, A.; Bahnemann, D.; Muneer, M. Synthesis of Co doped ZnWO4 for simultaneous oxidation of RhB and reduction of Cr(VI) under UV-light irradiation. J. Environ. Chem. Eng. 2018, 6, 4885–4898. [Google Scholar] [CrossRef]
- Tang, L.; Feng, C.; Deng, Y.; Zeng, G.; Wang, J.; Liu, Y.; Feng, H.; Wang, J. Enhanced photocatalytic activity of ternary Ag/g-C3N4/NaTaO3 photocatalysts under wide spectrum light radiation: The high potential band protection mechanism. Appl. Catal. B Environ. 2018, 230, 102–114. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, S.; Yang, J. Preparation of Ag2O/g-C3N4/Fe3O4 composites and the application in the photocatalytic degradation of Rhodamine B under visible light. J. Alloy. Compd. 2017, 708, 1141–1149. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, H.-L.; Peng, J.; Zhai, M.; Yu, Z.Z. Cr(VI) removal from aqueous solution using chemically reduced and functionalized graphene oxide. J. Mater. Sci. 2012, 48, 1883–1889. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, H.; Hu, X.; Wang, H.; Li, J.; Xie, G.; Tan, X.; Jin, Q.; Zhou, D.; Li, C.; Qiu, G.; et al. Synergy of Photocatalysis and Adsorption for Simultaneous Removal of Hexavalent Chromium and Methylene Blue by g-C3N4/BiFeO3/Carbon Nanotubes Ternary Composites. Int. J. Environ. Res. Public Health 2019, 16, 3219. https://doi.org/10.3390/ijerph16173219
Huo H, Hu X, Wang H, Li J, Xie G, Tan X, Jin Q, Zhou D, Li C, Qiu G, et al. Synergy of Photocatalysis and Adsorption for Simultaneous Removal of Hexavalent Chromium and Methylene Blue by g-C3N4/BiFeO3/Carbon Nanotubes Ternary Composites. International Journal of Environmental Research and Public Health. 2019; 16(17):3219. https://doi.org/10.3390/ijerph16173219
Chicago/Turabian StyleHuo, Huiwen, Xinjiang Hu, Hui Wang, Jiang Li, Guangyu Xie, Xiaofei Tan, Qi Jin, Daixi Zhou, Chuang Li, Guoqiang Qiu, and et al. 2019. "Synergy of Photocatalysis and Adsorption for Simultaneous Removal of Hexavalent Chromium and Methylene Blue by g-C3N4/BiFeO3/Carbon Nanotubes Ternary Composites" International Journal of Environmental Research and Public Health 16, no. 17: 3219. https://doi.org/10.3390/ijerph16173219
APA StyleHuo, H., Hu, X., Wang, H., Li, J., Xie, G., Tan, X., Jin, Q., Zhou, D., Li, C., Qiu, G., & Liu, Y. (2019). Synergy of Photocatalysis and Adsorption for Simultaneous Removal of Hexavalent Chromium and Methylene Blue by g-C3N4/BiFeO3/Carbon Nanotubes Ternary Composites. International Journal of Environmental Research and Public Health, 16(17), 3219. https://doi.org/10.3390/ijerph16173219




