Comparison of the Modulatory Effect on Intestinal Microbiota between Raw and Bran-Fried Atractylodis Rhizoma in the Rat Model of Spleen-Deficiency Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Animals and Groups
2.3. Biochemical Analysis
2.4. Fecal Sample Collection and 16S rDNA Sequencing
2.5. 16S rDNA Bioinformatics Analysis and Statistics
2.6. Correlation Analysis
2.7. Statistical Multiple Comparison
3. Results
3.1. Behavioral Comparison of Rats
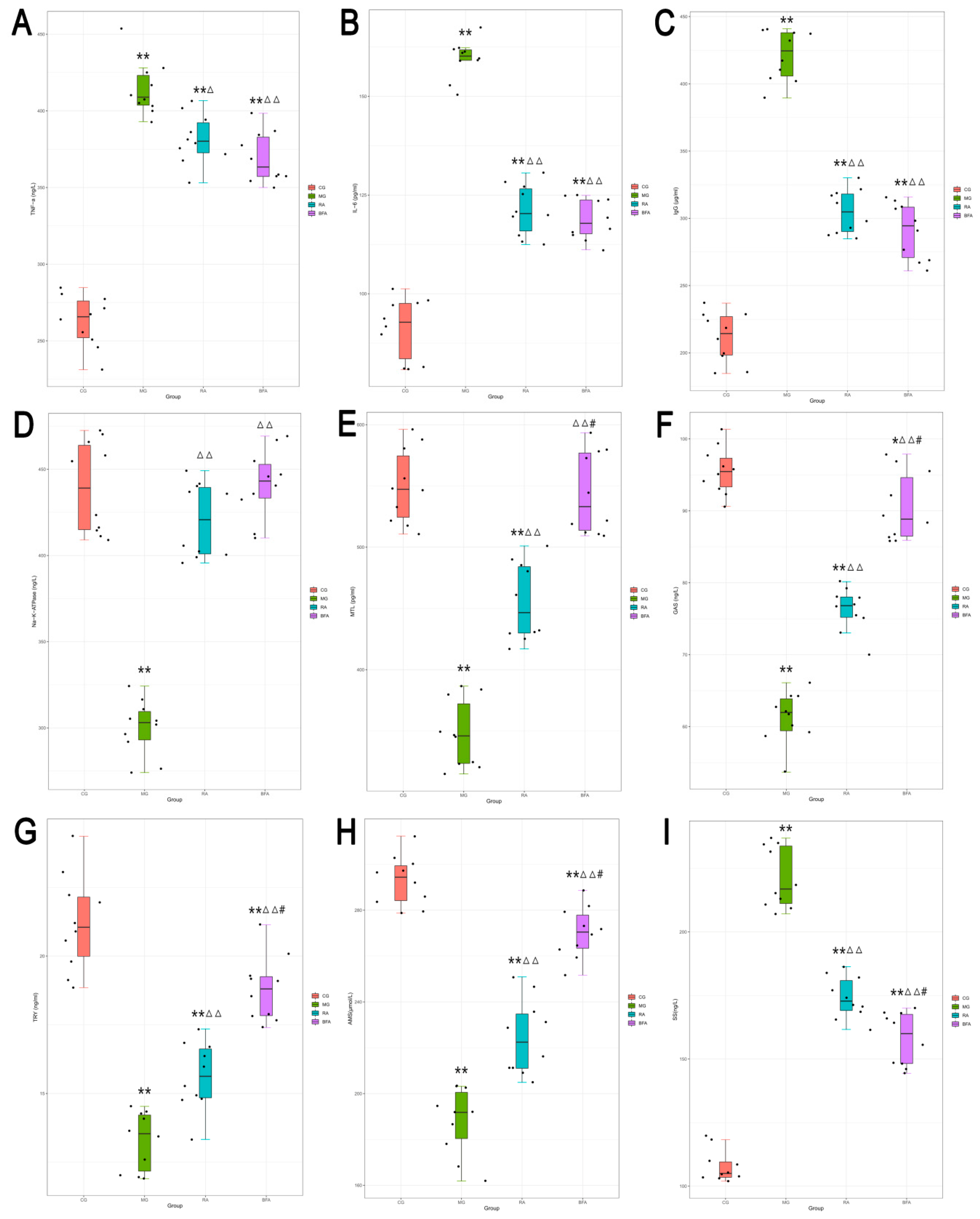
3.2. Effect of Raw and Bran-Fried AR on the Serum Levels of Biochemical Indices in SDS Rats
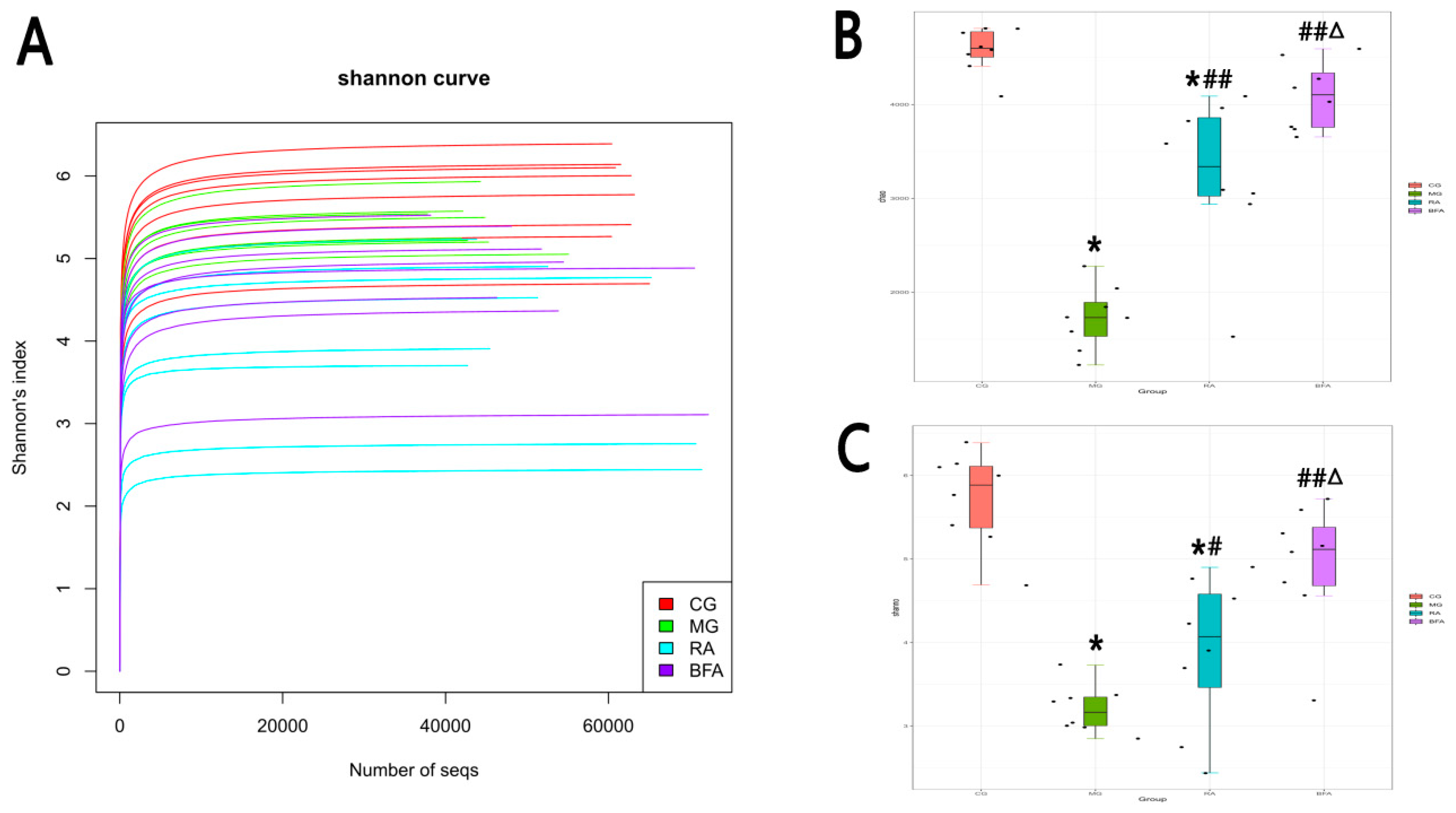
3.3. Comparison of Sequencing Depth and Alpha Diversity of Intestinal Microbiota from Fecal Samples in Rats
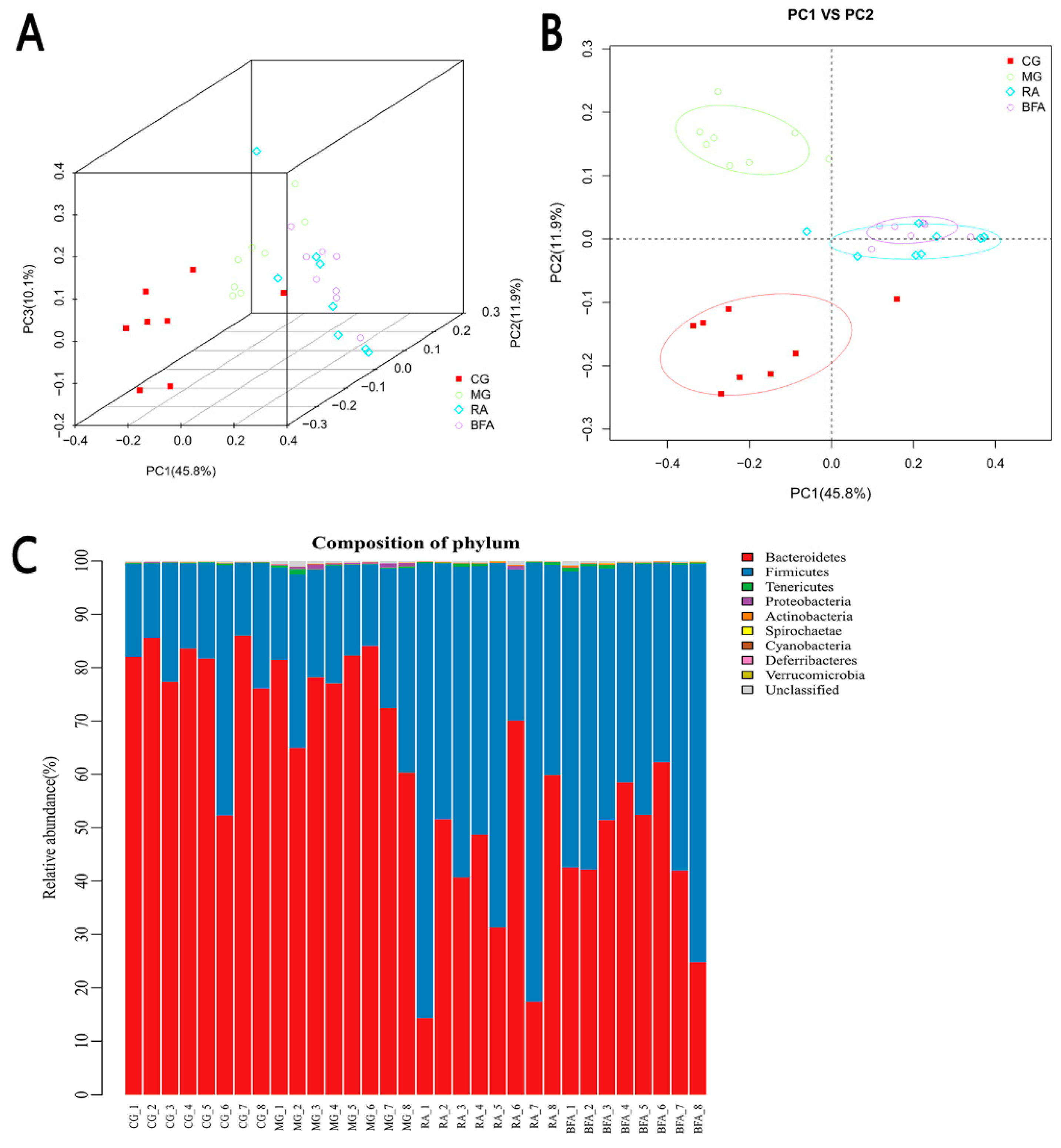
3.4. Comparison of Beta Diversity and Abundance of Intestinal Microbiota at the Phylum Level from Fecal Samples in Rats
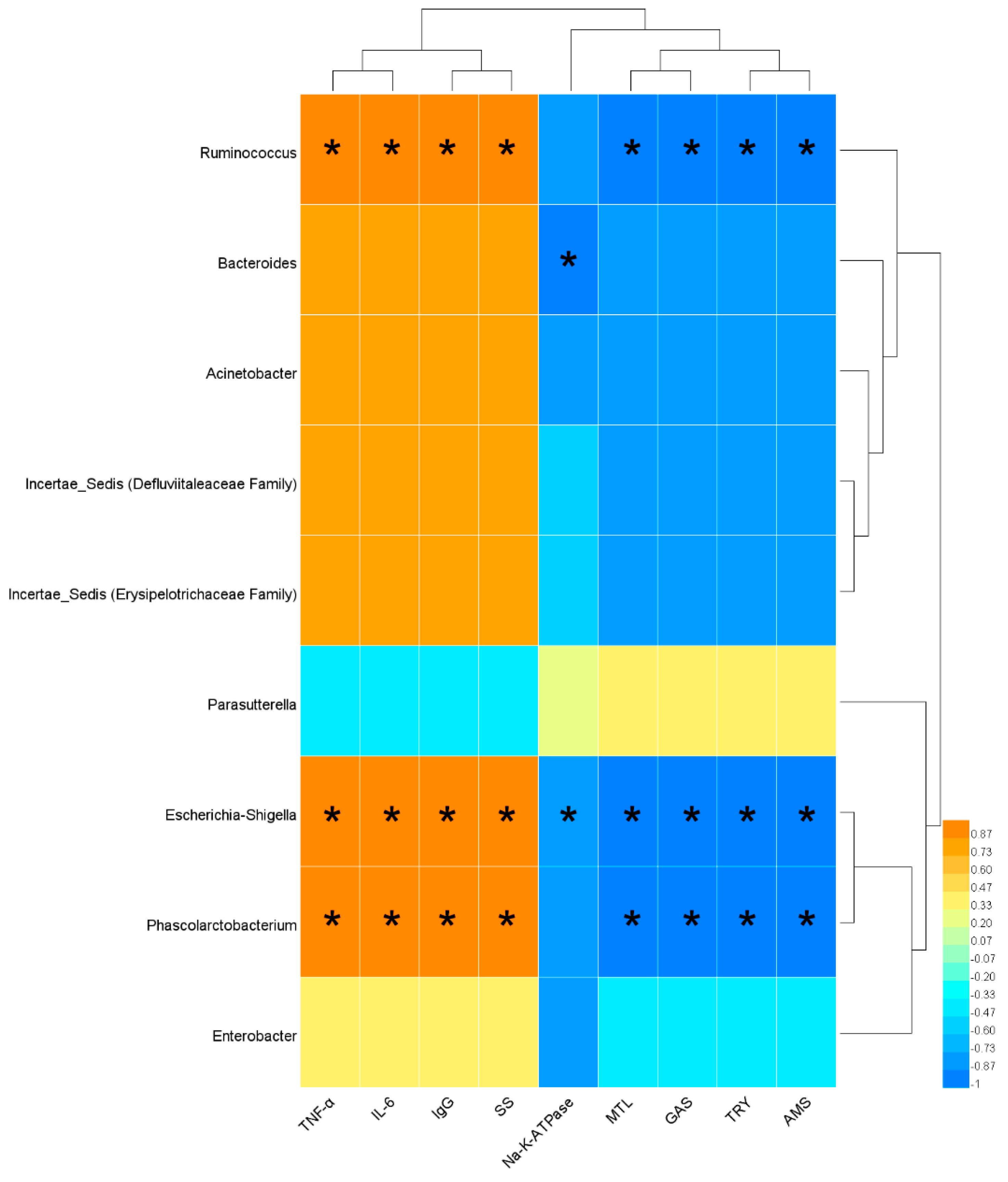
3.5. Identification of the Bacteria Closely Related to SDS and Comparison of the Modulatory Effect on these Bacteria between Raw and Bran-Fried AR
3.6. Correlation Analysis between Serum Biochemical Indices and Bacterial Abundance Indices Closely Related to SDS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sun, G.; Zheng, H. Basic Theory of Traditional Chinese Medicine; China Press of Traditional Chinese Medicine: Beijing, China, 2012. [Google Scholar]
- Yang, L.; Wang, C. Research of Spleen Governing Transformation and Transportation in the Ming Dynadsy. Lishizhen Med. Mater. Med. Res. 2016, 27, 2472–2474. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, Y. Effect of Integrated Traditional Chinese and Western Medicine Therapy on Clinical Symptoms and Curative Effect of Functional Dyspepsia with Spleen Deficiency and Food Stagnation. Chin. J. Exp. Tradit. Med. Form. 2012, 18, 277–279. [Google Scholar] [CrossRef]
- Sha, W. Summary of Experience of Shenling Baizhu Powder in Treating Diarrhea due to Spleen Deficiency. World Lat. Med. Inf. 2018, 18, 180. [Google Scholar] [CrossRef]
- Chen, J. TCM master XU Jing-fan’s clinical experience in treating irritable bowel syndrome. Chin. J. Tradit. Chin. Med. Pharm. 2013, 28, 1746–1748. [Google Scholar]
- Li, Y.; Ma, J.; Li, F.; Tang, X. Modern literature research on TCM syndrome differentiation standard of functional diarrhea. Chin. J. Tradit. Chin. Med. Pharm. 2019, 34, 977–980. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; The Medicine Science and Technology Press of China: Beijing, China, 2015. [Google Scholar]
- Qu, L.; Tu, J.; Cao, G.; Zhao, J.; Pan, X.; Liu, Y. Study on dryness effect of Atractylodis Rhizoma based on theory of dry-dry and dryness-induced Yin deficiency. Chin. J. Chin. Mater. Med. 2018, 43, 2705–2712. [Google Scholar] [CrossRef]
- Chen, W.; Zeng, M.; Xu, K. Pharmacodynamics of water extracts from Atractylodes lancea before and after processing. Chin. J. Chin. Mater. Med. 2012, 37, 2276–2279. [Google Scholar] [CrossRef]
- Chang, X.; Liu, Y.; Cai, Q. Fingerprints of Raw and Processed Atractylodis Rhizoma by HPLC. Chin. J. Exp. Tradit. Med. Form. 2015, 21, 40–43. [Google Scholar] [CrossRef]
- Xue, D.; Liu, Y.; Cai, Q.; Liang, K.; Zheng, B.; Li, F.; Pang, X. Comparison of Bran-Processed and Crude Atractylodes Lancea Effects on Spleen Deficiency Syndrome in Rats. Pharmacogn. Mag. 2018, 14, 214–219. [Google Scholar] [CrossRef]
- Xu, S.; Qi, X.; Liu, Y.; Liu, Y.; Lv, X.; Sun, J.; Cai, Q. UPLC-MS/MS of Atractylenolide I, Atractylenolide II, Atractylenolide III, and Atractyloside A in Rat Plasma after Oral Administration of Raw and Wheat Bran-Processed Atractylodis Rhizoma. Molecules 2018, 23, 3234. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, T.; Cai, Q.; Jiang, N.; Ma, M.; Min, D.; Yuan, Y. Comparison of the anti-ulcer activity between the crude and bran-processed Atractylodes lancea in the rat model of gastric ulcer induced by acetic acid. J. Ethnopharmacol. 2015, 160, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Y.; Cai, Q. Study on Spleen-invigorating Effect of Atractylodis Rhizoma before and after Stir-frying. Lishizhen Med. Mater. Med. Res. 2013, 24, 155–156. [Google Scholar]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Kim, B.S.; Han, K.; Kim, H. Ephedra-Treated Donor-Derived Gut Microbiota Transplantation Ameliorates High Fat Diet-Induced Obesity in Rats. Int. J. Environ. Res. Public Health 2017, 14, 555. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Li, H.; Xie, Z.; Niu, X.; Wen, C. Relationship between the syndrome of water retention due to spleen deficiency and intestinal flora disturbance based on the theory of spleen governing transportation and transformation. Chin. J. Tradit. Chin. Med. Pharm. 2014, 29, 3762–3765. [Google Scholar]
- Jin, J.; Yang, J.; Wu, C.; Li, X. Regulation of three Jianpi Buqi recipes on intestinal microflora of Piqi-deficiency rat. Chin. J. Chin. Mater. Med. 2008, 21, 2530–2534. [Google Scholar]
- Gao, Q.; Jin, X.; Ge, Y.; Li, Y.; Wu, Y.; Chen, G. Effects of two kinds of Spleen-strengthening products on intestinal flora in rat model of spleen deficiency syndrome combined with antibiotics. Chin. Tradit. Pat. Med. 2017, 39, 2155–2159. [Google Scholar] [CrossRef]
- Clauss, M.; Kaiser, T.; Hummel, J. The Morphophysiological Adaptations of Browsing and Grazing Mammals. In The Ecology of Browsing and Grazing; Gordon, I.J., Prins, H.H.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 47–88. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Zhu, A.; Huang, Q. Research thinking on syndrome model defined by traditional Chinese medicine. J. Beijing Univ. Tradit. Chin. Med. 2005, 6, 18–21. [Google Scholar]
- Wang, H.; Xie, M. Immunologic Effect on the Model Rat of Spleen-qi Deficiency Induced by Complex Factors. Chin. J. Exp. Tradit. Med. Formulae 2006, 12, 41–45. [Google Scholar] [CrossRef]
- Pharmaceutical Bureau of the Ministry of Health of the People’s Republic of China. Guiding Principles of Clinical Research on the Treatment of Spleen Deficiency Syndrome with Traditional Chinese Medicine. Chin. J. Tradit. Chin. Med. Pharm. 1988, 5, 71–72. [Google Scholar]
- Pan, A.; Yi, W.; Yu, X.; Chen, K.; Li, J. Protective Effects of Total Saponins of Panax ginseng on Spleen Deficiency Model Rats. Chin. Pharm. 2013, 24, 3682–3684. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Bercier, P.; Grenier, D. TNF-α disrupts the integrity of the porcine respiratory epithelial barrier. Res. Vet. Sci. 2019, 124, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Rovin, B. A pathophysiology-based approach to the diagnosis and treatment of lupus nephritis. Kidney Int. 2016, 90, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Factor, P.; Dumasius, V.; Saldias, F.; Brown, L.A.; Sznajder, J.I. Adenovirus-mediated transfer of an Na+/K+-ATPase beta1 subunit gene improves alveolar fluid clearance and survival in hyperoxic rats. Hum. Gene Ther. 2000, 11, 2231–2242. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Song, Y. Advances in Gastrin and Related Gastrointestinal Hormones. J. Clin. Pathol. Res. 2000, 3, 229–231. [Google Scholar]
- Liu, H.; Zhu, T.; Ma, Y.; Qu, S. Effect of erythromycin on contractile response of uterine smooth muscle strips in non-pregnant rats. Pol. J. Pharmacol. 2003, 55, 57–62. [Google Scholar]
- Ma, Y.; Zhao, H.; Chen, Z. Effect of Qiangji Jianli Decoction and Astragalus Polysaccharide on Gastrointestinal hormone Levels in Rats with Spleen Deficiency Syndrome. Tradit. Chin. Drug Res. Clin. Pharm. 2011, 22, 590–593. [Google Scholar] [CrossRef]
- Hiruma-Lima, C.A.; Calvo, T.R.; Rodrigues, C.M.; Andrade, F.D.; Vilegas, W.; Brito, A.R. Antiulcerogenic activity of Alchornea castaneaefolia: Effects on somatostatin, gastrin and prostaglandin. J. Ethnopharmacol. 2006, 104, 215–224. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Kang, A.; Yao, W.; Lou, J.; Zhang, Q.; Bao, B.; Cao, Y.; Yu, S.; Guo, S.; Zhang, Y.; et al. Euphorbia kansui fry-baked with vinegar modulates gut microbiota and reduces intestinal toxicity in rats. J. Ethnopharmacol. 2018, 226, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Bashiardes, S.; Shapiro, H.; Rozin, S.; Shibolet, O.; Elinav, E. Non-alcoholic fatty liver and the gut microbiota. Mol. Metab. 2016, 5, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Kong, J.Y.; Stothard, P.; Willing, B.P. Defining the role of Parasutterella, a previously uncharacterized member of the core gut microbiota. ISME J. 2019, 13, 1520–1534. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.H.; Wang, L.H.; Tsai, T.T.; Lei, H.Y.; Liao, P.C. Secretomic Analysis of Host-Pathogen Interactions Reveals That Elongation Factor-Tu Is a Potential Adherence Factor of Helicobacter pylori during Pathogenesis. J. Proteome Res. 2017, 16, 264–273. [Google Scholar] [CrossRef]
- Gao, B.; Wang, R.; Peng, Y.; Li, X. Effects of a homogeneous polysaccharide from Sijunzi decoction on human intestinal microbes and short chain fatty acids in vitro. J. Ethnopharmacol. 2018, 224, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Liu, B.; Ye, H.; Li, Q.; Pan, L.; Zha, X.; Liu, J.; Duan, J.; Luo, J. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr. Polym. 2019, 206, 149–162. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Q. Content Determination of Polysaccharides in Atractylodis Rhizoma before and after Processing from Different Sources. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 89–91. [Google Scholar]


| Name | CG (10−3/%) | MG (10−3/%) | RA (10−3/%) | BFA (10−3/%) |
|---|---|---|---|---|
| Ruminococcus | 436.025 ± 163.965 | 4010.280 ± 1610.945 | 625.017 ± 99.849 * | 571.414 ± 139.984 * |
| Bacteroides | 81.152 ± 19.609 | 1116.391 ± 275.562 | 168.912 ± 25.552 * | 51.197 ± 11.961 *Δ |
| Parasutterella | 45.710 ± 10.285 | 1.632 ± 0.528 | 45.882 ± 5.340 * | 9.010 ± 2.544 *Δ |
| Escherichia-Shigella | 1.397 ± 0.405 | 203.602 ± 42.451 | 21.553 ± 3.667 * | 2.810 ± 1.212 *Δ |
| Phascolarctobacterium | 0 | 1924.348 ± 228.220 | 32.973 ± 7.846 * | 0.327 ± 0.126 *Δ |
| Enterobacter | 0.604 ± 0.433 | 30.883 ± 14.957 | 0.293 ± 0.182 * | 0 * |
| Acinetobacter | 0 | 35.678 ± 17.171 | 0 * | 0 * |
| Incertae_Sedis (Defluviitaleaceae Family) | 13.682 ± 5.953 | 58.341 ± 24.318 | 98.844 ± 45.533 * | 34.307 ± 15.597 *Δ |
| Incertae_Sedis (Erysipelotrichaceae Family) | 1.005 ± 0.691 | 55.507 ± 23.189 | 72.524 ± 34.369 * | 7.281 ± 5.162 *Δ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Jiang, Y.; Zhang, B.; Pang, J.; Xu, X.; Sun, J.; Lv, X.; Cai, Q. Comparison of the Modulatory Effect on Intestinal Microbiota between Raw and Bran-Fried Atractylodis Rhizoma in the Rat Model of Spleen-Deficiency Syndrome. Int. J. Environ. Res. Public Health 2019, 16, 3183. https://doi.org/10.3390/ijerph16173183
Ma S, Jiang Y, Zhang B, Pang J, Xu X, Sun J, Lv X, Cai Q. Comparison of the Modulatory Effect on Intestinal Microbiota between Raw and Bran-Fried Atractylodis Rhizoma in the Rat Model of Spleen-Deficiency Syndrome. International Journal of Environmental Research and Public Health. 2019; 16(17):3183. https://doi.org/10.3390/ijerph16173183
Chicago/Turabian StyleMa, Shanpeng, Yujun Jiang, Beixue Zhang, Jian Pang, Xiaoying Xu, Jianzhi Sun, Xin Lv, and Qian Cai. 2019. "Comparison of the Modulatory Effect on Intestinal Microbiota between Raw and Bran-Fried Atractylodis Rhizoma in the Rat Model of Spleen-Deficiency Syndrome" International Journal of Environmental Research and Public Health 16, no. 17: 3183. https://doi.org/10.3390/ijerph16173183
APA StyleMa, S., Jiang, Y., Zhang, B., Pang, J., Xu, X., Sun, J., Lv, X., & Cai, Q. (2019). Comparison of the Modulatory Effect on Intestinal Microbiota between Raw and Bran-Fried Atractylodis Rhizoma in the Rat Model of Spleen-Deficiency Syndrome. International Journal of Environmental Research and Public Health, 16(17), 3183. https://doi.org/10.3390/ijerph16173183






