Remission of Type 2 Diabetes Mellitus after Bariatric Surgery: Fact or Fiction?
Abstract
1. Introduction
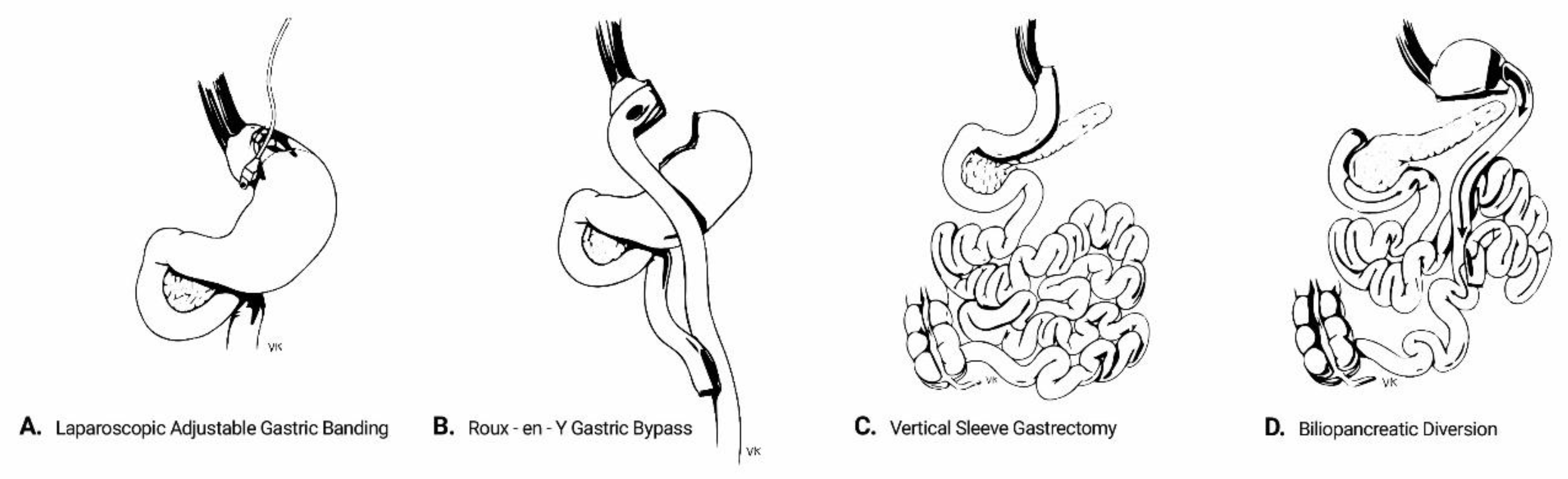
2. Overview of Interventions
- Laparoscopic adjustable gastric banding (AGB) refers to the placement of an adjustable silicon band around the upper part of the stomach. This isolates the gastric segment proximal to the band to create a small gastric pouch, thereby restricting the effective gastric volume. The size of the band and thus the degree of restriction can be adjusted by adding or removing saline solution through a subcutaneously inserted port. Despite being minimally invasive and deprived of major complications, AGB frequently fails to yield major results in magnitude and duration, owing to its one-dimensional approach and the potential for the patient to bypass the restriction by modifying diet quality towards more liquid foods. Its use is currently decreasing, being gradually displaced by the other bariatric procedures [13].
- Biliopancreatic diversion (BPD) is a mixed restrictive/malabsorptive procedure which was originally introduced by Scopinaro in 1979 [14]. It includes a partial horizontal gastrectomy and anastomosis of the gastric remnant in the distal 250 cm of the small intestine (alimentary limb), while the diverted proximal intestine carries biliopancreatic secretions. The latter is anastomosed to the alimentary limb at a varying distance from the ileocecal valve, which determines the degree of malabsorption. The procedure was later modified to include a vertical gastrectomy instead, with preservation of the pylorus and a duodeno-intestinal anastomosis, in order to prevent post-surgical dumping syndrome [15]. Although BPD has the highest success rates regarding weight loss and metabolic improvement among all bariatric procedures, its technical difficulties and high rates of perioperative and long-term complications confine its use to individuals with massive obesity or as salvage therapy after other procedures have failed.
- Roux-en-Y gastric bypass (RYGB) includes the creation of a small-volume gastric pouch which is anastomosed to the distal part of the jejunum (alimentary limb). The limb carrying biliopancreatic secretions is anastomosed typically 150 cm distally to the gastro-jejunostomy. RYGB has a balanced safety-efficacy profile and is considered to be the “gold standard” in the surgical therapy of obesity and T2DM [16].
- Vertical sleeve gastrectomy (VSG) involves the resection of the major proportion of the fundus and corpus of the stomach, leaving a tube-shaped gastric residue. It was originally the first part of a two-step approach for biliopancreatic diversion in high-risk individuals. Its effectiveness and operative simplicity have led to VSG being performed as a stand-alone procedure. It is currently the most common procedure among all bariatric modalities in the USA [13].
3. Mechanisms of Type 2 Diabetes Mellitus (T2DM) Remission Following Bariatric Surgery
4. The Challenge of Defining T2DM Remission
- Partial, when glycemic indices fall into the pre-diabetic range (HbA1c 5.7–6.4%, FPG 100–125 mg/dL) for at least 1 year;
- Complete, when normoglycemia is restored (HbA1c < 5.7%, FPG < 100 mg/dL) for at least 1 year;
- Prolonged, defined as a complete remission of at least 5 years’ duration.
5. Observational Studies and Randomized Clinical Trials (RCTs) Regarding T2DM Remission
6. Factors that Predict T2DM Remission after Bariatric Surgery
7. Sources of Heterogeneity in Reported T2DM Remission Rates among Studies
8. Effect of Bariatric Surgery on Chronic T2DM Complications, Mortality and Quality of Life
9. Short- and Long-Term Risks
10. Remission of T2DM after Bariatric Surgery: Fact or Fiction?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Field, A.E.; Coakley, E.H.; Must, A.; Spadano, J.L.; Laird, N.; Dietz, W.H.; Rimm, E.; Colditz, G.A. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch. Intern. Med. 2001, 161, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Hussain, M.E. Obesity and diabetes: An update. Diabetes Metab. Syndr. 2017, 11, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.S. The epidemic of obesity and diabetes: Trends and treatments. Tex. Heart Inst. J. 2011, 38, 142–144. [Google Scholar] [PubMed]
- Hu, F.B. Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care 2011, 34, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, R.; Pradeepa, R.; Joshi, S.R.; Mohan, V. Type 2 Diabetes: Demystifying the Global Epidemic. Diabetes 2017, 66, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Yoshida, H.; Takahashi, H.; Kawai, M. Increases in body mass index, even within non-obese levels, raise the risk for Type 2 diabetes mellitus: A follow-up study in a Japanese population. Diabet. Med. 2005, 22, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Farag, Y.M.; Gaballa, M.R. Diabesity: An overview of a rising epidemic. Nephrol. Dial. Transplant. 2011, 26, 28–35. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 8. Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S81–S89. [Google Scholar] [CrossRef]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. North Am. 2018, 102, 183–197. [Google Scholar] [CrossRef]
- Rubino, F.; Nathan, D.M.; Eckel, R.H.; Schauer, P.R.; Alberti, K.G.; Zimmet, P.Z.; Del Prato, S.; Ji, L.; Sadikot, S.M.; Herman, W.H.; et al. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes Care 2016, 39, 861–877. [Google Scholar] [CrossRef] [PubMed]
- Tadross, J.A.; le Roux, C.W. The mechanisms of weight loss after bariatric surgery. Int. J. Obes. 2009, 33, S28–S32. [Google Scholar] [CrossRef] [PubMed]
- Ponce, J.; DeMaria, E.J.; Nguyen, N.T.; Hutter, M.; Sudan, R.; Morton, J.M. American Society for Metabolic and Bariatric Surgery estimation of bariatric surgery procedures in 2015 and surgeon workforce in the United States. Surg. Obes. Relat. Dis. 2016, 12, 1637–1639. [Google Scholar] [CrossRef]
- Scopinaro, N.; Gianetta, E.; Civalleri, D.; Bonalumi, U.; Bachi, V. Bilio-pancreatic bypass for obesity: II. Initial experience in man. Br. J. Surg. 1979, 66, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, M.; Osman, S.; Robinson, T.J.; Khandelwal, S.; Bhargava, P.; Rohrmann, C.A. Evolution of bariatric surgery: A historical perspective. AJR Am. J. Roentgenol. 2013, 201, W40–W48. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, M.D.P.; Palermo, M.; Serra, E.; Ackermann, M.A. Metabolic surgery: Gastric bypass for the treatment of type 2 diabetes mellitus. Transl. Gastroenterol. Hepatol. 2017, 2, 58. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef]
- Lean, M.E.J.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019, 7, 344–355. [Google Scholar] [CrossRef]
- Miras, A.D.; le Roux, C.W. Mechanisms underlying weight loss after bariatric surgery. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 575–584. [Google Scholar] [CrossRef]
- Rabl, C.; Rao, M.N.; Schwarz, J.M.; Mulligan, K.; Campos, G.M. Thermogenic changes after gastric bypass, adjustable gastric banding or diet alone. Surgery 2014, 156, 806–812. [Google Scholar] [CrossRef]
- Faria, S.L.; Faria, O.P.; Cardeal Mde, A.; Ito, M.K.; Buffington, C. Diet-induced thermogenesis and respiratory quotient after Roux-en-Y gastric bypass surgery: A prospective study. Surg. Obes. Relat. Dis. 2014, 10, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Werling, M.; Fandriks, L.; Olbers, T.; Bueter, M.; Sjostrom, L.; Lonroth, H.; Wallenius, V.; Stenlof, K.; le Roux, C.W. Roux-en-Y Gastric Bypass Surgery Increases Respiratory Quotient and Energy Expenditure during Food Intake. PLoS ONE 2015, 10, e0129784. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Jackness, C.; Karmally, W.; Febres, G.; Conwell, I.M.; Ahmed, L.; Bessler, M.; McMahon, D.J.; Korner, J. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and beta-cell Function in type 2 diabetic patients. Diabetes 2013, 62, 3027–3032. [Google Scholar] [CrossRef] [PubMed]
- Basso, N.; Capoccia, D.; Rizzello, M.; Abbatini, F.; Mariani, P.; Maglio, C.; Coccia, F.; Borgonuovo, G.; De Luca, M.L.; Asprino, R.; et al. First-phase insulin secretion, insulin sensitivity, ghrelin, GLP-1, and PYY changes 72 h after sleeve gastrectomy in obese diabetic patients: The gastric hypothesis. Surg. Endosc. 2011, 25, 3540–3550. [Google Scholar] [CrossRef] [PubMed]
- Laferrere, B.; Teixeira, J.; McGinty, J.; Tran, H.; Egger, J.R.; Colarusso, A.; Kovack, B.; Bawa, B.; Koshy, N.; Lee, H.; et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 2479–2485. [Google Scholar] [CrossRef]
- Shah, M.; Law, J.H.; Micheletto, F.; Sathananthan, M.; Dalla Man, C.; Cobelli, C.; Rizza, R.A.; Camilleri, M.; Zinsmeister, A.R.; Vella, A. Contribution of endogenous glucagon-like peptide 1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes 2014, 63, 483–493. [Google Scholar] [CrossRef]
- Sam, A.H.; Gunner, D.J.; King, A.; Persaud, S.J.; Brooks, L.; Hostomska, K.; Ford, H.E.; Liu, B.; Ghatei, M.A.; Bloom, S.R.; et al. Selective ablation of peptide YY cells in adult mice reveals their role in beta cell survival. Gastroenterology 2012, 143, 459–468. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Wang, L.; Lin, Y.; Liu, X.; Liu, X.; Liu, L. Exendin-4 protects murine pancreatic beta-cells from free fatty acid-induced apoptosis through PI-3K signaling. Endocr. Res. 2013, 38, 40–47. [Google Scholar] [CrossRef]
- Cornu, M.; Thorens, B. GLP-1 protects beta-cells against apoptosis by enhancing the activity of an IGF-2/IGF1-receptor autocrine loop. Islets 2009, 1, 280–282. [Google Scholar] [CrossRef]
- Drucker, D.J. Glucagon-like peptide-1 and the islet beta-cell: Augmentation of cell proliferation and inhibition of apoptosis. Endocrinology 2003, 144, 5145–5148. [Google Scholar] [CrossRef] [PubMed]
- Ramracheya, R.D.; McCulloch, L.J.; Clark, A.; Wiggins, D.; Johannessen, H.; Olsen, M.K.; Cai, X.; Zhao, C.M.; Chen, D.; Rorsman, P. PYY-Dependent Restoration of Impaired Insulin and Glucagon Secretion in Type 2 Diabetes following Roux-En-Y Gastric Bypass Surgery. Cell Rep. 2016, 15, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Spegel, P.; Ekelund, M.; Garcia Vaz, E.; Pierzynowski, S.; Gomez, M.F.; Mulder, H.; Hedenbro, J.; Groop, L.; Wierup, N. Gastric bypass improves beta-cell function and increases beta-cell mass in a porcine model. Diabetes 2014, 63, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Kokkinos, A.; Peradze, N.; Tentolouris, N.; Ghaly, W.; Tsilingiris, D.; Alexandrou, A.; Mantzoros, C.S. Follistatins in glucose regulation in healthy and obese individuals. Diabetes Obes. Metab. 2019, 21, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Chondronikola, M.; Harris, L.L.; Klein, S. Bariatric surgery and type 2 diabetes: Are there weight loss-independent therapeutic effects of upper gastrointestinal bypass? J. Intern. Med. 2016, 280, 476–486. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.C.; Cull, C.A.; Frighi, V.; Holman, R.R. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: Progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 1999, 281, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Caprio, S.; Cefalu, W.T.; Ceriello, A.; Del Prato, S.; Inzucchi, S.E.; McLaughlin, S.; Phillips, G.L.; Robertson, R.P.; Rubino, F.; et al. How do we define cure of diabetes? Diabetes Care 2009, 32, 2133–2135. [Google Scholar] [CrossRef] [PubMed]
- Bruns, D.E.; Knowler, W.C. Stabilization of glucose in blood samples: Why it matters. Clin. Chem. 2009, 55, 850–852. [Google Scholar] [CrossRef]
- Weykamp, C. HbA1c: A review of analytical and clinical aspects. Ann. Lab. Med. 2013, 33, 393–400. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, H.J.; Lo Menzo, E.; Park, S.; Szomstein, S.; Rosenthal, R.J. Anemia, iron and vitamin B12 deficiencies after sleeve gastrectomy compared to Roux-en-Y gastric bypass: A meta-analysis. Surg. Obes. Relat. Dis. 2014, 10, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Pories, W.J.; Swanson, M.S.; MacDonald, K.G.; Long, S.B.; Morris, P.G.; Brown, B.M.; Barakat, H.A.; deRamon, R.A.; Israel, G.; Dolezal, J.M.; et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann. Surg. 1995, 222, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Wittgrove, A.C.; Clark, G.W. Laparoscopic gastric bypass, Roux-en-Y- 500 patients: Technique and results, with 3-60 month follow-up. Obes. Surg. 2000, 10, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.B.; O’Brien, P.E. Health outcomes of severely obese type 2 diabetic subjects 1 year after laparoscopic adjustable gastric banding. Diabetes Care 2002, 25, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Sugerman, H.J.; Wolfe, L.G.; Sica, D.A.; Clore, J.N. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann. Surg. 2003, 237, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Burguera, B.; Ikramuddin, S.; Cottam, D.; Gourash, W.; Hamad, G.; Eid, G.M.; Mattar, S.; Ramanathan, R.; Barinas-Mitchel, E.; et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann. Surg. 2003, 238, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Scopinaro, N.; Marinari, G.M.; Camerini, G.B.; Papadia, F.S.; Adami, G.F. Specific effects of biliopancreatic diversion on the major components of metabolic syndrome: A long-term follow-up study. Diabetes Care 2005, 28, 2406–2411. [Google Scholar] [CrossRef]
- Dixon, J.B.; O’Brien, P.E.; Playfair, J.; Chapman, L.; Schachter, L.M.; Skinner, S.; Proietto, J.; Bailey, M.; Anderson, M. Adjustable gastric banding and conventional therapy for type 2 diabetes: A randomized controlled trial. JAMA 2008, 299, 316–323. [Google Scholar] [CrossRef]
- Iaconelli, A.; Panunzi, S.; De Gaetano, A.; Manco, M.; Guidone, C.; Leccesi, L.; Gniuli, D.; Nanni, G.; Castagneto, M.; Ghirlanda, G.; et al. Effects of bilio-pancreatic diversion on diabetic complications: A 10-year follow-up. Diabetes Care 2011, 34, 561–567. [Google Scholar] [CrossRef]
- Kehagias, I.; Karamanakos, S.N.; Argentou, M.; Kalfarentzos, F. Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI < 50 kg/m2. Obes. Surg. 2011, 21, 1650–1656. [Google Scholar] [CrossRef]
- Schauer, P.R.; Kashyap, S.R.; Wolski, K.; Brethauer, S.A.; Kirwan, J.P.; Pothier, C.E.; Thomas, S.; Abood, B.; Nissen, S.E.; Bhatt, D.L. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N. Engl. J. Med. 2012, 366, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Leccesi, L.; Nanni, G.; Pomp, A.; Castagneto, M.; Ghirlanda, G.; et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N. Engl. J. Med. 2012, 366, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.M.; Peltonen, M.; Ahlin, S.; Anveden, A.; Bouchard, C.; Carlsson, B.; Jacobson, P.; Lonroth, H.; Maglio, C.; Naslund, I.; et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N. Engl. J. Med. 2012, 367, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.D.; Davidson, L.E.; Litwin, S.E.; Kolotkin, R.L.; LaMonte, M.J.; Pendleton, R.C.; Strong, M.B.; Vinik, R.; Wanner, N.A.; Hopkins, P.N.; et al. Health benefits of gastric bypass surgery after 6 years. JAMA 2012, 308, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Gregg, E.W.; Chen, H.; Wagenknecht, L.E.; Clark, J.M.; Delahanty, L.M.; Bantle, J.; Pownall, H.J.; Johnson, K.C.; Safford, M.M.; Kitabchi, A.E.; et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012, 308, 2489–2496. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Bogart, A.; Sherwood, N.E.; Sidney, S.; Coleman, K.J.; Haneuse, S.; O’Connor, P.J.; Theis, M.K.; Campos, G.M.; McCulloch, D.; et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes. Surg. 2013, 23, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wu, Q.; Chen, B.; Yu, P.; Zhao, H.; Ouyang, X. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: A randomized controlled trial. Diabetes Res. Clin. Pract. 2013, 101, 50–56. [Google Scholar] [CrossRef]
- Arterburn, D.; Bogart, A.; Coleman, K.J.; Haneuse, S.; Selby, J.V.; Sherwood, N.E.; Sidney, S.; Theis, M.K.; Campos, G.M.; McCulloch, D.; et al. Comparative effectiveness of bariatric surgery vs. nonsurgical treatment of type 2 diabetes among severely obese adults. Obes. Res. Clin. Pract. 2013, 7, e258–e268. [Google Scholar] [CrossRef]
- Wentworth, J.M.; Playfair, J.; Laurie, C.; Ritchie, M.E.; Brown, W.A.; Burton, P.; Shaw, J.E.; O’Brien, P.E. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: A randomised controlled trial. Lancet Diabetes Endocrinol. 2014, 2, 545–552. [Google Scholar] [CrossRef]
- Courcoulas, A.P.; Goodpaster, B.H.; Eagleton, J.K.; Belle, S.H.; Kalarchian, M.A.; Lang, W.; Toledo, F.G.; Jakicic, J.M. Surgical vs medical treatments for type 2 diabetes mellitus: A randomized clinical trial. JAMA Surg. 2014, 149, 707–715. [Google Scholar] [CrossRef]
- Halperin, F.; Ding, S.A.; Simonson, D.C.; Panosian, J.; Goebel-Fabbri, A.; Wewalka, M.; Hamdy, O.; Abrahamson, M.; Clancy, K.; Foster, K.; et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: Feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014, 149, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Risstad, H.; Sovik, T.T.; Engstrom, M.; Aasheim, E.T.; Fagerland, M.W.; Olsen, M.F.; Kristinsson, J.A.; le Roux, C.W.; Bohmer, T.; Birkeland, K.I.; et al. Five-year outcomes after laparoscopic gastric bypass and laparoscopic duodenal switch in patients with body mass index of 50 to 60: A randomized clinical trial. JAMA Surg. 2015, 150, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Yska, J.P.; van Roon, E.N.; de Boer, A.; Leufkens, H.G.; Wilffert, B.; de Heide, L.J.; de Vries, F.; Lalmohamed, A. Remission of Type 2 Diabetes Mellitus in Patients After Different Types of Bariatric Surgery: A Population-Based Cohort Study in the United Kingdom. JAMA Surg. 2015, 150, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Arterburn, D.E.; Westbrook, E.O.; Kuzma, J.N.; Stewart, S.D.; Chan, C.P.; Bock, S.N.; Landers, J.T.; Kratz, M.; Foster-Schubert, K.E.; et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: The CROSSROADS randomised controlled trial. Diabetologia 2016, 59, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Purnell, J.Q.; Selzer, F.; Wahed, A.S.; Pender, J.; Pories, W.; Pomp, A.; Dakin, G.; Mitchell, J.; Garcia, L.; Staten, M.A.; et al. Type 2 Diabetes Remission Rates After Laparoscopic Gastric Bypass and Gastric Banding: Results of the Longitudinal Assessment of Bariatric Surgery Study. Diabetes Care 2016, 39, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Salminen, P.; Helmio, M.; Ovaska, J.; Juuti, A.; Leivonen, M.; Peromaa-Haavisto, P.; Hurme, S.; Soinio, M.; Nuutila, P.; Victorzon, M. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients With Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA 2018, 319, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.R.; Baggesen, L.M.; Richelsen, B.; Thomsen, R.W. Effect of Roux-en-Y gastric bypass surgery on diabetes remission and complications in individuals with type 2 diabetes: A Danish population-based matched cohort study. Diabetologia 2019, 62, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, L.; Lindroos, A.K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjostrom, C.D.; et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, L.; Peltonen, M.; Jacobson, P.; Ahlin, S.; Andersson-Assarsson, J.; Anveden, A.; Bouchard, C.; Carlsson, B.; Karason, K.; Lonroth, H.; et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014, 311, 2297–2304. [Google Scholar] [CrossRef]
- Adams, T.D.; Gress, R.E.; Smith, S.C.; Halverson, R.C.; Simper, S.C.; Rosamond, W.D.; Lamonte, M.J.; Stroup, A.M.; Hunt, S.C. Long-term mortality after gastric bypass surgery. N. Engl. J. Med. 2007, 357, 753–761. [Google Scholar] [CrossRef]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Brethauer, S.A.; Navaneethan, S.D.; Aminian, A.; Pothier, C.E.; Kim, E.S.; Nissen, S.E.; et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N. Engl. J. Med. 2014, 370, 2002–2013. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Nanni, G.; Castagneto, M.; Bornstein, S.; Rubino, F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015, 386, 964–973. [Google Scholar] [CrossRef]
- Courcoulas, A.P.; Belle, S.H.; Neiberg, R.H.; Pierson, S.K.; Eagleton, J.K.; Kalarchian, M.A.; DeLany, J.P.; Lang, W.; Jakicic, J.M. Three-Year Outcomes of Bariatric Surgery vs Lifestyle Intervention for Type 2 Diabetes Mellitus Treatment: A Randomized Clinical Trial. JAMA Surg. 2015, 150, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Panunzi, S.; Carlsson, L.; De Gaetano, A.; Peltonen, M.; Rice, T.; Sjostrom, L.; Mingrone, G.; Dixon, J.B. Determinants of Diabetes Remission and Glycemic Control After Bariatric Surgery. Diabetes Care 2016, 39, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Debedat, J.; Sokolovska, N.; Coupaye, M.; Panunzi, S.; Chakaroun, R.; Genser, L.; de Turenne, G.; Bouillot, J.L.; Poitou, C.; Oppert, J.M.; et al. Long-term Relapse of Type 2 Diabetes After Roux-en-Y Gastric Bypass: Prediction and Clinical Relevance. Diabetes Care 2018, 41, 2086–2095. [Google Scholar] [CrossRef]
- Blackstone, R.; Bunt, J.C.; Cortes, M.C.; Sugerman, H.J. Type 2 diabetes after gastric bypass: Remission in five models using HbA1c, fasting blood glucose, and medication status. Surg. Obes. Relat. Dis. 2012, 8, 548–555. [Google Scholar] [CrossRef]
- Chikunguwo, S.M.; Wolfe, L.G.; Dodson, P.; Meador, J.G.; Baugh, N.; Clore, J.N.; Kellum, J.M.; Maher, J.W. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2010, 6, 254–259. [Google Scholar] [CrossRef]
- Cotillard, A.; Poitou, C.; Duchateau-Nguyen, G.; Aron-Wisnewsky, J.; Bouillot, J.L.; Schindler, T.; Clement, K. Type 2 Diabetes Remission After Gastric Bypass: What Is the Best Prediction Tool for Clinicians? Obes. Surg. 2015, 25, 1128–1132. [Google Scholar] [CrossRef]
- Aminian, A.; Brethauer, S.A.; Kashyap, S.R.; Kirwan, J.P.; Schauer, P.R. DiaRem score: External validation. Lancet Diabetes Endocrinol. 2014, 2, 12–13. [Google Scholar] [CrossRef]
- Honarmand, K.; Chetty, K.; Vanniyasingam, T.; Anvari, M.; Chetty, V.T. Type 2 diabetes remission rates 1-year post-Roux-en-Y gastric bypass and validation of the DiaRem score: The Ontario Bariatric Network experience. Clin. Obes. 2017, 7, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Neto, J.; Nassif, L.S.; Branco-Filho, A.J.; Bolfarini, L.A.; Loro, L.S.; de Souza, M.P.; Bianco, T. External Validation of the Diarem Score as Remission Predictor of Diabetes Mellitus Type 2 in Obese Patients Undergoing Roux-En-Y Gastric Bypass. Arq. Bras. Cir. Dig. 2015, 28, 19–22. [Google Scholar] [CrossRef]
- Pucci, A.; Tymoszuk, U.; Cheung, W.H.; Makaronidis, J.M.; Scholes, S.; Tharakan, G.; Elkalaawy, M.; Guimaraes, M.; Nora, M.; Hashemi, M.; et al. Type 2 diabetes remission 2 years post Roux-en-Y gastric bypass and sleeve gastrectomy: The role of the weight loss and comparison of DiaRem and DiaBetter scores. Diabet. Med. 2018, 35, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Craig Wood, G.; Horwitz, D.; Still, C.D.; Mirshahi, T.; Benotti, P.; Parikh, M.; Hirsch, A.G. Performance of the DiaRem Score for Predicting Diabetes Remission in Two Health Systems Following Bariatric Surgery Procedures in Hispanic and non-Hispanic White Patients. Obes. Surg. 2018, 28, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Sokolovska, N.; Liu, Y.; Comaneshter, D.S.; Vinker, S.; Pecht, T.; Poitou, C.; Oppert, J.M.; Bouillot, J.L.; Genser, L.; et al. The advanced-DiaRem score improves prediction of diabetes remission 1 year post-Roux-en-Y gastric bypass. Diabetologia 2017, 60, 1892–1902. [Google Scholar] [CrossRef]
- Dicker, D.; Golan, R.; Aron-Wisnewsky, J.; Zucker, J.D.; Sokolowska, N.; Comaneshter, D.S.; Yahalom, R.; Vinker, S.; Clement, K.; Rudich, A. Prediction of Long-Term Diabetes Remission After RYGB, Sleeve Gastrectomy, and Adjustable Gastric Banding Using DiaRem and Advanced-DiaRem Scores. Obes. Surg. 2019, 29, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Segal-Lieberman, G.; Segal, P.; Dicker, D. Revisiting the Role of BMI in the Guidelines for Bariatric Surgery. Diabetes Care 2016, 39, S268–S273. [Google Scholar] [CrossRef] [PubMed]
- Panunzi, S.; De Gaetano, A.; Carnicelli, A.; Mingrone, G. Predictors of remission of diabetes mellitus in severely obese individuals undergoing bariatric surgery: Do BMI or procedure choice matter? A meta-analysis. Ann. Surg. 2015, 261, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Astiarraga, B.; Gastaldelli, A.; Muscelli, E.; Baldi, S.; Camastra, S.; Mari, A.; Papadia, F.; Camerini, G.; Adami, G.; Scopinaro, N.; et al. Biliopancreatic diversion in nonobese patients with type 2 diabetes: Impact and mechanisms. J. Clin. Endocrinol. Metab. 2013, 98, 2765–2773. [Google Scholar] [CrossRef]
- Adami, G.F.; Camerini, G.; Papadia, F.; Catalano, M.F.; Carlini, F.; Cordera, R.; Scopinaro, N. Type 2 Diabetes Remission and Control in Overweight and in Mildly Obese Diabetic Patients at Long-Term Follow-Up After Biliopancreatic Diversion. Obes. Surg. 2019, 29, 239–245. [Google Scholar] [CrossRef]
- Vetter, M.L.; Ritter, S.; Wadden, T.A.; Sarwer, D.B. Comparison of Bariatric Surgical Procedures for Diabetes Remission: Efficacy and Mechanisms. Diabetes Spectr. 2012, 25, 200–210. [Google Scholar] [CrossRef]
- Buchwald, H.; Estok, R.; Fahrbach, K.; Banel, D.; Jensen, M.D.; Pories, W.J.; Bantle, J.P.; Sledge, I. Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am. J. Med. 2009, 122, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Liaskos, C.; Koliaki, C.; Alexiadou, K.; Argyrakopoulou, G.; Tentolouris, N.; Diamantis, T.; Alexandrou, A.; Katsilambros, N.; Kokkinos, A. Roux-en-Y Gastric Bypass Is More Effective than Sleeve Gastrectomy in Improving Postprandial Glycaemia and Lipaemia in Non-diabetic Morbidly Obese Patients: A Short-term Follow-up Analysis. Obes. Surg. 2018, 28, 3997–4005. [Google Scholar] [CrossRef] [PubMed]
- Pournaras, D.J.; Aasheim, E.T.; Sovik, T.T.; Andrews, R.; Mahon, D.; Welbourn, R.; Olbers, T.; le Roux, C.W. Effect of the definition of type II diabetes remission in the evaluation of bariatric surgery for metabolic disorders. Br. J. Surg. 2012, 99, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Mas-Lorenzo, A.; Benaiges, D.; Flores-Le-Roux, J.A.; Pedro-Botet, J.; Ramon, J.M.; Parri, A.; Villatoro, M.; Chillaron, J.; Pera, M.; Grande, L.; et al. Impact of different criteria on type 2 diabetes remission rate after bariatric surgery. Obes. Surg. 2014, 24, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- van de Laar, A.W.; Acherman, Y.I. Weight loss percentile charts of large representative series: A benchmark defining sufficient weight loss challenging current criteria for success of bariatric surgery. Obes. Surg. 2014, 24, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Puzziferri, N.; Nakonezny, P.A.; Livingston, E.H.; Carmody, T.J.; Provost, D.A.; Rush, A.J. Variations of weight loss following gastric bypass and gastric band. Ann. Surg. 2008, 248, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Admiraal, W.M.; Celik, F.; Gerdes, V.E.; Dallal, R.M.; Hoekstra, J.B.; Holleman, F. Ethnic differences in weight loss and diabetes remission after bariatric surgery: A meta-analysis. Diabetes Care 2012, 35, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.M. Ethnic Considerations for Metabolic Surgery. Diabetes Care 2016, 39, 949–953. [Google Scholar] [CrossRef]
- Coleman, K.J.; Haneuse, S.; Johnson, E.; Bogart, A.; Fisher, D.; O’Connor, P.J.; Sherwood, N.E.; Sidney, S.; Theis, M.K.; Anau, J.; et al. Long-term Microvascular Disease Outcomes in Patients With Type 2 Diabetes After Bariatric Surgery: Evidence for the Legacy Effect of Surgery. Diabetes Care 2016, 39, 1400–1407. [Google Scholar] [CrossRef]
- Billeter, A.T.; Eichel, S.; Scheurlen, K.M.; Probst, P.; Kopf, S.; Muller-Stich, B.P. Meta-analysis of metabolic surgery versus medical treatment for macrovascular complications and mortality in patients with type 2 diabetes. Surg. Obes. Relat. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, B.; Liakopoulos, V.; Franzen, S.; Naslund, I.; Svensson, A.M.; Ottosson, J.; Gudbjornsdottir, S. Cardiovascular disease and mortality in patients with type 2 diabetes after bariatric surgery in Sweden: A nationwide, matched, observational cohort study. Lancet Diabetes Endocrinol. 2015, 3, 847–854. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Franzen, S.; Svensson, A.M.; Zethelius, B.; Ottosson, J.; Naslund, I.; Gudbjornsdottir, S.; Eliasson, B. Changes in risk factors and their contribution to reduction of mortality risk following gastric bypass surgery among obese individuals with type 2 diabetes: A nationwide, matched, observational cohort study. BMJ Open Diabetes Res. Care 2017, 5, e000386. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Taft, C.; Ryden, A.; Sjostrom, L.; Sullivan, M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: The SOS intervention study. Int. J. Obes. 2007, 31, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Ikramuddin, S.; Gourash, W.; Ramanathan, R.; Luketich, J. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann. Surg. 2000, 232, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Aminian, A.; Brethauer, S.A.; Kirwan, J.P.; Kashyap, S.R.; Burguera, B.; Schauer, P.R. How safe is metabolic/diabetes surgery? Diabetes Obes. Metab. 2015, 17, 198–201. [Google Scholar] [CrossRef]
- Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N. Engl. J. Med. 2009, 361, 445–454. [Google Scholar] [CrossRef]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric surgery: A systematic review and meta-analysis. JAMA 2004, 292, 1724–1737. [Google Scholar] [CrossRef]
- Kim, J.H.; Wolfe, B. Bariatric/metabolic surgery: Short- and long-term safety. Curr. Atheroscler. Rep. 2012, 14, 597–605. [Google Scholar] [CrossRef]
- Lupoli, R.; Lembo, E.; Saldalamacchia, G.; Avola, C.K.; Angrisani, L.; Capaldo, B. Bariatric surgery and long-term nutritional issues. World J. Diabetes 2017, 8, 464–474. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Franzen, S.; Svensson, A.M.; Miftaraj, M.; Ottosson, J.; Naslund, I.; Gudbjornsdottir, S.; Eliasson, B. Pros and cons of gastric bypass surgery in individuals with obesity and type 2 diabetes: Nationwide, matched, observational cohort study. BMJ Open 2019, 9, e023882. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.; Kang, S.; Lee, Y.; Rosenblat, J.D.; Brietzke, E.; Zuckerman, H.; McIntyre, R.S. The long-term effect of bariatric surgery on depression and anxiety. J. Affect. Disord. 2019, 246, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Backman, O.; Stockeld, D.; Rasmussen, F.; Naslund, E.; Marsk, R. Alcohol and substance abuse, depression and suicide attempts after Roux-en-Y gastric bypass surgery. Br. J. Surg. 2016, 103, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Dawes, A.J.; Maggard-Gibbons, M.; Maher, A.R.; Booth, M.J.; Miake-Lye, I.; Beroes, J.M.; Shekelle, P.G. Mental Health Conditions Among Patients Seeking and Undergoing Bariatric Surgery: A Meta-analysis. JAMA 2016, 315, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Lagerros, Y.T.; Brandt, L.; Hedberg, J.; Sundbom, M.; Boden, R. Suicide, Self-harm, and Depression After Gastric Bypass Surgery: A Nationwide Cohort Study. Ann. Surg. 2017, 265, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.B.C.; Zhang, M.W.B.; Ho, R.C.M. Prevalence of All-Cause Mortality and Suicide among Bariatric Surgery Cohorts: A Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 15, 1519. [Google Scholar] [CrossRef] [PubMed]
- Neovius, M.; Bruze, G.; Jacobson, P.; Sjoholm, K.; Johansson, K.; Granath, F.; Sundstrom, J.; Naslund, I.; Marcus, C.; Ottosson, J.; et al. Risk of suicide and non-fatal self-harm after bariatric surgery: Results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018, 6, 197–207. [Google Scholar] [CrossRef]
- Turner, P.L.; Saager, L.; Dalton, J.; Abd-Elsayed, A.; Roberman, D.; Melara, P.; Kurz, A.; Turan, A. A nomogram for predicting surgical complications in bariatric surgery patients. Obes. Surg. 2011, 21, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Sugerman, H.J.; DeMaria, E.J.; Kellum, J.M.; Sugerman, E.L.; Meador, J.G.; Wolfe, L.G. Effects of bariatric surgery in older patients. Ann. Surg. 2004, 240, 243–247. [Google Scholar] [CrossRef]
- Ramirez, A.; Roy, M.; Hidalgo, J.E.; Szomstein, S.; Rosenthal, R.J. Outcomes of bariatric surgery in patients >70 years old. Surg. Obes. Relat. Dis. 2012, 8, 458–462. [Google Scholar] [CrossRef]
- Marihart, C.L.; Brunt, A.R.; Geraci, A.A. Older adults fighting obesity with bariatric surgery: Benefits, side effects, and outcomes. SAGE Open Med. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Karter, A.J.; Nundy, S.; Parker, M.M.; Moffet, H.H.; Huang, E.S. Incidence of remission in adults with type 2 diabetes: The diabetes & aging study. Diabetes Care 2014, 37, 3188–3195. [Google Scholar] [CrossRef] [PubMed]
- Keidar, A. Bariatric surgery for type 2 diabetes reversal: The risks. Diabetes Care 2011, 34, S361-S266. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Sun, Z.; Zhang, N.; Xu, G.; Song, P.; Xu, L.; Tang, W. Cost-Effectiveness of Bariatric Surgery for Type 2 Diabetes Mellitus: A Randomized Controlled Trial in China. Medicine 2016, 95, e3522. [Google Scholar] [CrossRef] [PubMed]
- Gulliford, M.C.; Charlton, J.; Prevost, T.; Booth, H.; Fildes, A.; Ashworth, M.; Littlejohns, P.; Reddy, M.; Khan, O.; Rudisill, C. Costs and Outcomes of Increasing Access to Bariatric Surgery: Cohort Study and Cost-Effectiveness Analysis Using Electronic Health Records. Value Health 2017, 20, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Hoerger, T.J.; Zhang, P.; Segel, J.E.; Kahn, H.S.; Barker, L.E.; Couper, S. Cost-effectiveness of bariatric surgery for severely obese adults with diabetes. Diabetes Care 2010, 33, 1933–1939. [Google Scholar] [CrossRef]
- Keating, C.; Neovius, M.; Sjoholm, K.; Peltonen, M.; Narbro, K.; Eriksson, J.K.; Sjostrom, L.; Carlsson, L.M. Health-care costs over 15 years after bariatric surgery for patients with different baseline glucose status: Results from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol. 2015, 3, 855–865. [Google Scholar] [CrossRef]
- Villamizar, N.; Pryor, A.D. Safety, effectiveness, and cost effectiveness of metabolic surgery in the treatment of type 2 diabetes mellitus. J. Obes. 2011, 2011, 790683. [Google Scholar] [CrossRef]
- Miras, A.D.; Perez-Pevida, B.; Aldhwayan, M.; Kamocka, A.; McGlone, E.R.; Al-Najim, W.; Chahal, H.; Batterham, R.L.; McGowan, B.; Khan, O.; et al. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 549–559. [Google Scholar] [CrossRef]
| Study | Study Population Characteristics | Study Design | Intervention | T2DM Remission Endpoint |
|---|---|---|---|---|
| Pories et al. (1995) [42] | morbidly obese, T2DM, prediabetes | Retrospective cohort | RYGB | “Normal” levels of FPG, HbA1c |
| Wittgrove et al. (2000) [43] | Morbidly obese | Prospective cohort | RYGB | Medication withdrawal and “normal” HbA1c |
| Dixon et al. (2002) [44] | BMI > 35 kg/m2, T2DM | Prospective cohort | AGB | “Normal” levels of FPG, HbA1c, fasting insulin, c-peptide |
| Sugerman et al. (2003) [45] | Morbidly obese | Retrospective cohort | GBP | FPG ≤ 120 mg/dL off medication |
| Schauer et al. (2003) [46] | morbidly obese, T2DM | Prospective cohort | RYGB | “Normal” levels of FPG, HbA1c, medication withdrawal |
| Scopinaro et al. (2005) [47] | obese, T2DM | Retrospective cohort | BPD | FPG < 110 mg/dL, ≥ 125 mg/dL for relapse |
| Dixon et al. (2008) [48] | BMI 30–40 kg/m2 T2DM duration < 2 years | RCT | AGB | FPG < 126 mg/dL, HbA1c < 6.2% off medication |
| Studies after ADA consensus panel definition (2009) | ||||
| Iaconelli et al. (2011) [49] | BMI > 35 kg/m2 newly diagnosed T2DM | Open case-control | BPD | ADA definition # |
| Kehagias et al. (2011) [50] | BMI < 50 kg/m2 | RCT | VSG, RYGB | Glucose values below diabetic range during 2h-OGTT, off medication |
| Schauer et al. (2012) (STAMPEDE) [51] | BMI ≥ 30 kg/m2 uncontrolled T2DM | RCT | VSG, RYGB | HbA1c < 6% |
| Mingrone et al. (2012) [52] | BMI ≥ 5 kg/m2 T2DM duration ≥ 5 years, HbA1c ≥7% | RCT | BPD, RYGB | FPG < 100 mg/dL and HbA1c < 6.5% off medication for ≥ 1 year |
| Carlsson et al. (2012) (SOS cohort) [53] | BMI ≥ 30 kg/m2 | Prospective cohort | RYGB, AGB or VBG | FPG < 110 mg/dL off medication |
| Adams et al. (2012) [54] | BMI ≥ 35 kg/m2 | Prospective cohort | RYGB | “Normal” levels of FPG, HbA1c off medication |
| Gregg et al. (2012) (Look AHEAD) [55] | BMI ≥ 25 kg/m2 | RCT | ILI | FPG < 126 mg/dL and HbA1c < 6.5% off medication |
| Arteburn et al. (2013) [56] | T2DM | Retrospective cohort | RYGB | ADA definition # |
| Liang et al. (2013) [57] | BMI ≥ 25 kg/m2 T2DM duration 5–10 years | RCT | RYGB, exenatide | Normal FPG, HbA1c off medication |
| Arteburn et al. (2013) [58] | BMI ≥ 35 kg/m2 T2DM | Retrospective cohort | RYGB, AGB, VSG, other | FPG < 126 mg/dL and/or HbA1c<6.5% off medication for ≥90 days |
| Wentworth et al. (2014) [59] | BMI 25–30 kg/m2 | RCT | AGB | Glucose values below diabetic range during 2h-OGTT, 2 days off medication |
| Courcoulas et al. (2014) [60] | BMI 30–40 kg/m2 | RCT | AGB, RYGB | ADA definition # |
| Halperin et al. (2014) [61] | BMI 30–42 kg/m2 T2DM duration ≥ 1 year | RCT | RYGB | FPG < 126mg/dL and HbA1c < 6.5% |
| Risstad et al. (2015) [62] | BMI 50–60 kg/m2 | RCT | RYGB, BPD | ADA definition # |
| Yska et al. (2015) [63] | BMI ≥ 35 kg/m2 | Retrospective cohort | RYGB, VSG, AGB, other | HbA1c < 6% off medication |
| Cummings et al. (2016) (CROSSROADS) [64] | BMI 30–45 kg/m2 | RCT | RYGB | HbA1c < 6% off medication |
| Purnell et al. (2016) (LABS-2) [65] | BMI ≥ 30 kg/m2 | Prospective cohort | RYGB, AGB | HbA1c < 6.5% or FPG ≤ 6.9 mmol/L off medication |
| Salminen, et al. (2018) (SLEEVEPASS) [66] | Morbidly obese | RCT | VSG, RYGB | ADA definition # |
| Lean et al. (2018) (DiRECT) [17] | BMI ≥ 30 kg/m2 | RCT | ILI | HbA1c < 6.5%, at least 2 months off medication |
| Madesin et al. (2019) [67] | BMI ≥ 35 kg/m2 | Population-based cohort | RYGB | HbA1c < 6.5% off medication or HbA1c < 6% on metformin monotherapy |
| Study | Factors Predicting Remission | Factors Exerting a Neutral Effect |
|---|---|---|
| Pories et al. (1995) [42] |
| |
| Dixon et al. (2002) [44] |
| |
| Schauer et al. (2003) [46] |
| |
| Dixon et al. (2008) [48] |
|
|
| Schauer et al. (2012) (STAMPEDE) [51] |
|
|
| Mingrone et al. (2012) [52] |
| |
| Carlsson et al. (2012) (SOS cohort) [53] |
|
|
| Gregg et al. (2012) (Look AHEAD) [55] |
|
|
| Arteburn et al. (2013) [56] |
|
|
| Arteburn et al. (2013) [58] |
|
|
| Cummings et al. (2016) (CROSSROADS) [64] |
| |
| Purnell et al. (2016) (LABS-2) [65] |
|
|
| Madesin et al. (2019) [67] |
|
|
| Study | Follow up | Diabetes Complications | Quality of Life * |
|---|---|---|---|
| Dixon et al. (2002) [44] | 1 year | (Beck’s depression inventory, SF-36) Significant improvements in depression Significant improvement on physical health subscales | |
| Schauer et al. (2003) [46] | 20 months (median) | 50% (self-reported) improvement in diabetic neuropathy symptoms | |
| Schauer et al. (2000) [105] | 16.9 months (mean) | (Moorehead-Ardelt QOL Questionnaire) Quality of life 58% greatly improved, 37% improved, 5% no change | |
| Iaconelli et al. (2011) [49] | 10 years | All cases with microalbuminuria at baseline regressed by year 10. 2 new cases. Prevalence increased in the control group; 39.2% vs. 9% new nephropathy cases 4 major CV events vs. none | |
| Schauer et al. (2012) (STAMPEDE) [51] | 5 years | No effect on ophthalmologic outcomes vs. conservative treatment Significantly lower albumin-to-creatinine ratio from baseline in the SG group vs. conservative No change in albuminuria status in any group | (SF-36) Significant improvements in both surgical groups in the physical functioning, general health, and energy–fatigue subscales. Emotional well-being worsened significantly among patients in the gastric bypass group |
| Mingrone et al. (2012) [73] | 5 years | 5 major diabetic complications in the medically treated group (including 1 fatal myocardial infarction) vs. one in the RYGB arms | (SF-36) Better scores in physical and emotional aspects of QOL in both surgical arms compared to the medically treated arm |
| Carlsson et al. (2012) (SOS cohort) [53] | 18 years | Reduced rates of chronic diabetes complications in the surgical vs. control groups (HRs 0.44 and 0.65 for incident microvascular and macrovascular complications, respectively) | |
| Karlsson et al. (2007) (SOS cohort) [104] | 10 years | (SOS quality of life survey) at 0.5, 1, 2, 3, 4, 6, 8 and 10 years. Substantial early gain trends in QOL that parallel weight loss. Net gains at 10 years in all QOL domains. Greater improvements in social interaction in surgical than conventional arm at 10 years. Better overall mood scores in surgical group up to 2 years post op. Significantly better depression outcomes in surgical group vs. conventional at 10 years. Non-significant improvement in anxiety scores by year 10. | |
| Adams et al. (2012) [54] | 6 years | (SF-36) Marked improvement in physical QOL components compared to controls. No significant changes in mental QOL components | |
| Halperin et al. (2014) [61] | 1 year | (SF-36, PAID, EQ-5D, IWQOL) No significant differences between RYGB and intensive lifestyle management in components of SF36, PAID, EQ-5D. Greater improvement in IWQOL in RYGB correlated with BMI change | |
| Risstad et al. (2015) [62] | 5 years | (SF-36) Similar improvements for RYGB and BPD in components of the SF36 and Obesity–related Problems Scale | |
| Cummings et al. (2016) (CROSSROADS) [64] | 1 year | (EQ-5D) Similarly significant QOL improvements for RYGB and ILMI | |
| Salminen et al. (2018) (SLEEVEPASS) [66] | 5 years | (Moorehead-Ardelt QOL questionnaire) Similar improvements regarding QOL in VSG and RYGB | |
| Madesin et al. (2019) [67] | 5 years | 47% lower risk of microvascular complications in RYBG vs. controls Statistically non-significant 24% reduction in macrovascular complications in RYGB vs. controls |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsilingiris, D.; Koliaki, C.; Kokkinos, A. Remission of Type 2 Diabetes Mellitus after Bariatric Surgery: Fact or Fiction? Int. J. Environ. Res. Public Health 2019, 16, 3171. https://doi.org/10.3390/ijerph16173171
Tsilingiris D, Koliaki C, Kokkinos A. Remission of Type 2 Diabetes Mellitus after Bariatric Surgery: Fact or Fiction? International Journal of Environmental Research and Public Health. 2019; 16(17):3171. https://doi.org/10.3390/ijerph16173171
Chicago/Turabian StyleTsilingiris, Dimitrios, Chrysi Koliaki, and Alexander Kokkinos. 2019. "Remission of Type 2 Diabetes Mellitus after Bariatric Surgery: Fact or Fiction?" International Journal of Environmental Research and Public Health 16, no. 17: 3171. https://doi.org/10.3390/ijerph16173171
APA StyleTsilingiris, D., Koliaki, C., & Kokkinos, A. (2019). Remission of Type 2 Diabetes Mellitus after Bariatric Surgery: Fact or Fiction? International Journal of Environmental Research and Public Health, 16(17), 3171. https://doi.org/10.3390/ijerph16173171





