The Influence on Contaminant Bioavailability and Microbial Abundance of Lake Hongze by the South-to-North Water Diversion Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Contaminant Analysis and Physicochemical Characteristics
2.2.1. In Situ Measurements
2.2.2. ExSitu Measurements
2.2.3. Statistical Analyses
3. Results
3.1. Physicochemical Characteristics
3.2. Labile Fraction Analysis
3.3. Microbial Community Composition and Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. The World Health Report. 2008. Available online: http://www.who. int/whr/2008/en/index.htm (accessed on 23 August 2008).
- Sun, W.; Xiao, T.; Sun, M.; Dong, Y.; Ning, Z.; Xiao, E.; Tang, S.; Li, J. Diversity of the Sediment Microbial Community in the Aha Watershed (Southwest China) in Response to Acid Mine Drainage Pollution Gradients. Appl. Environ. Microbiol. 2015, 81, 4874–4884. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Watzin, M.C.; Druschel, G. Relating sediment phosphorus mobility to seasonal and diel redox fluctuations at the sediment-water interface in a eutrophic freshwater lake. Limnol. Oceanogr. 2011, 56, 2251–2264. [Google Scholar] [CrossRef]
- Chen, M.; Ding, S.; Liu, L.; Xu, D.; Han, C.; Zhang, C. Iron-coupled inactivation of phosphorus in sediments by macrozoobenthos (chironomid larvae) bioturbation: Evidences from high-resolution dynamic measurements. Environ. Pollut. 2015, 204, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yao, Y.; Wang, P.F.; Hou, J.; Qian, J.; Yuan, Y.; Fan, X.L. In situ, high-resolution evaluation of labile arsenic and mercury in sediment of a large shallow lake. Sci. Total Environ. 2016, 541, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Welch, E.B.; Cooke, G.D. Effectiveness and longevity of phosphorus inactivation with alum. Lake Reserv. Manag. 1999, 15, 5–27. [Google Scholar] [CrossRef]
- Bravo, A.G.; Bouchet, S.; Guedron, S.; Amouroux, D.; Dominik, J.; Zopfi, J. High methylmercury production under ferruginous conditions in sediments impacted by sewage treatment plant discharges. Water Res. 2015, 80, 245–255. [Google Scholar] [CrossRef]
- Hang, C.; Li, Q.; Gao, S.; Shang, J.K. As(III) and As(V) Adsorption by Hydrous Zirconium Oxide Nanoparticles Synthesized by a Hydrothermal Process Followed with Heat Treatment. Ind. Eng. Chem. Res. 2012, 51, 353–361. [Google Scholar] [CrossRef]
- Ding, S.M.; Han, C.; Wang, Y.P.; Yao, L.; Wang, Y.; Xu, D.; Sun, Q.; Williams, P.N.; Zhang, C.S. In situ, high-resolution imaging of labile phosphorus in sediments of a large eutrophic lake. Water Res. 2015, 74, 100–109. [Google Scholar] [CrossRef]
- Stubner, S. Quantification of Gram-negative sulphate-reducing bacteria in rice field soil by 16S rRNA gene-targeted real-time PCR. J. Microbiol. Methods 2014, 57, 219–230. [Google Scholar] [CrossRef]
- Liu, J.; Hua, Z.H.; Chen, L.X.; Kuang, J.L.; Li, S.J.; Shu, W.S.; Huang, L.N. Correlating microbial diversity patterns with geochemistry in an extreme and heterogeneous environment of mine tailings. Appl. Environ. Microbiol. 2014, 80, 3677–3686. [Google Scholar] [CrossRef]
- Weber, M.J.; Brown, M.L. Relationships among invasive common carp, native fishes and physicochemical characteristics in upper Midwest (USA) lakes. Ecol. Freshw. Fish 2011, 20, 270–278. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, C.; Wang, P.F.; Hou, J.; Wang, T.; Liu, C.; Yuan, Y. In situ, high resolution ZrO-Chelex DGT for the investigation of iron-coupled inactivation of arsenic in sediments by macrozoobenthos bioturbation and hydrodynamic interactions. Sci. Total Environ. 2016, 562, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Davison, W.; Zhang, H. In situ speciation measurements of tracecomponents in natural waters using thin-film gels. Nature 1994, 367, 546–548. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, P.F.; Wang, C.; Hou, J.; Miao, L.Z.; Yuan, Y.; Wang, T.; Liu, C. Assessment of Mobilization of Labile Phosphorus and Iron Across Sediment-water Interface in a Shallow Lake (Hongze) Based on in situ High-resolution Measurement. Environ. Pollut. 2016, 219, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Tandy, S.; Mundus, S.; Yngvesson, J. The Use of DGT for Prediction of Plant Available Copper, Zinc and Phosphorus in Agricultural Soils. Plant Soil 2011, 346, 167–180. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, L.P.; Ding, S.M.; Li, C.; Yang, J.Y.; Chen, J.; Wang, P.F. Evaluation of the diffusive gradients in thin films technique using a mixed binding gel for measuring iron, phosphorus and arsenic in the environment. Environ. Sci. Process. Impacts 2015, 17, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Chen, Y.; Ding, S.; Sun, Q.; Wang, Y.; Zhang, C. Diffusive gradients in thin films technique equippedwith a mixed binding gel for simultaneous measurements of dissolved reactive phosphorus and dissolved iron. Environ. Sci. Technol. 2013, 47, 10477–10484. [Google Scholar] [PubMed]
- Wang, P.F.; Yao, Y.; Wang, C.; Hou, J.; Qian, J.; Miao, L.Z. Impact of macrozoobenthic bioturbation and wind fluctuation interactions on net methylmercury in freshwater lakes. Water Res. 2017, 124, 320–330. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, H.; Santner, J.; Davison, W. Performance characteristics of diffusive gradients in thin films equipped with a binding gel layer containing precipitated ferrihydrite for measuring arsenic(V), selenium(VI), vanadium(V), and antimony(V). Anal. Chem. 2010, 82, 8903–8909. [Google Scholar] [CrossRef]
- Ding, S.M.; Jia, F.; Xu, D.; Sun, Q.; Zhang, L.; Fan, C.X.; Zhang, C.S. High-Resolution, Two-Dimensional Measurement of Dissolved Reactive Phosphorus in Sediments Using the Diffusive Gradients in Thin Films Technique in Combination with a Routine Procedure. Environ. Sci. Technol. 2011, 45, 9680–9686. [Google Scholar] [CrossRef]
- Ding, S.M.; Sun, Q.; Xu, D.; Jia, F.; He, X.; Zhang, C.S. High-resolution simultaneous measurements of dissolved reactive phosphorus and dissolved sulfide: The first observation of their simultaneous release in sediments. Environ. Sci. Technol. 2012, 46, 8297–8304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lombi, E.; Smolders, E. Kinetic of Zn release in soils and prediction of Zn concentration in plants using diffusive gradients in thin films. Environ. Sci. Technol. 2004, 38, 3608–3613. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, W.B.; Lu, S.Y.; Yan, S.W.; Hu, R.J.; Chen, L.; Zhang, L.L.; Yu, J.P. Spatial Distribution Characteristics of Surface Sediments Nutrients in Lake Hongze and Their Pollution Status Evaluation. Environ. Sci. 2010, 31, 961–968. (In Chinese) [Google Scholar]
- Zhang, H.; Davison, W. Performance Characteristics of Diffusion Gradients in Thin Films for the in situ Measurement of Trace Metals in Aqueous Solution. Anal. Chem. 1995, 67, 3391–3400. [Google Scholar] [CrossRef]
- Lewandowski, J.R.; Hupfer, M. Effect of macrozoobenthos on two-dimensional small-scale heterogeneity of pore water phosphorus concentrations in lake sediments: A laboratory study. Limnol. Oceanogr. 2005, 50, 1106–1118. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, X.; Fan, C.; Shang, J.; Shen, Q.; Wang, Z.; Shen, J. Impact of different benthic animals on phosphorus dynamics across the sediment-water interface. J. Environ. Sci. 2010, 22, 1674–1682. [Google Scholar] [CrossRef]
- Loman, N.J.; Misra, R.V.; Dallman, T.J.; Constantinidou, C.; Gharbia, S.E.; Wain, J.; Pallen, M.J. Corrigendum: Performance comparison of benchtop high-throughput sequencing platforms. Nat. Biotechnol. 2012, 30, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, X.X.; Lin, X.R.; Chen, W.C.; Zhou, Q.X.; Shu, W.S.; Huang, L.N. Ecological Effects of Combined Pollution Associated with EWaste Recycling on the Composition and Diversity of Soil Microbial Communities. Environ. Sci. Technol. 2015, 49, 6438–6447. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F. Canonical correspondence analysis: A new eigenvector method for multivariate direct gradient analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef]
- Williams, P.N.; Price, A.H.; Raab, A.; Figuerola, J.; Green, A.J.; Feldmann, J.; Meharg, A.A. Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ. Sci. Technol. 2007, 41, 6854–6859. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Pehkonen, S.O.; Lin, C.J. Degradation of monomethylmercury chlorideby hydroxyl radicals in simulated natural waters. Water Res. 2003, 37, 2496–2504. [Google Scholar] [CrossRef]
- Brantner, J.S.; Senko, J.M. Response of soil-associated microbial communities to intrusion of coal mine-derived acid mine drainage. Environ. Sci. Technol. 2014, 48, 8556–8563. [Google Scholar] [CrossRef] [PubMed]
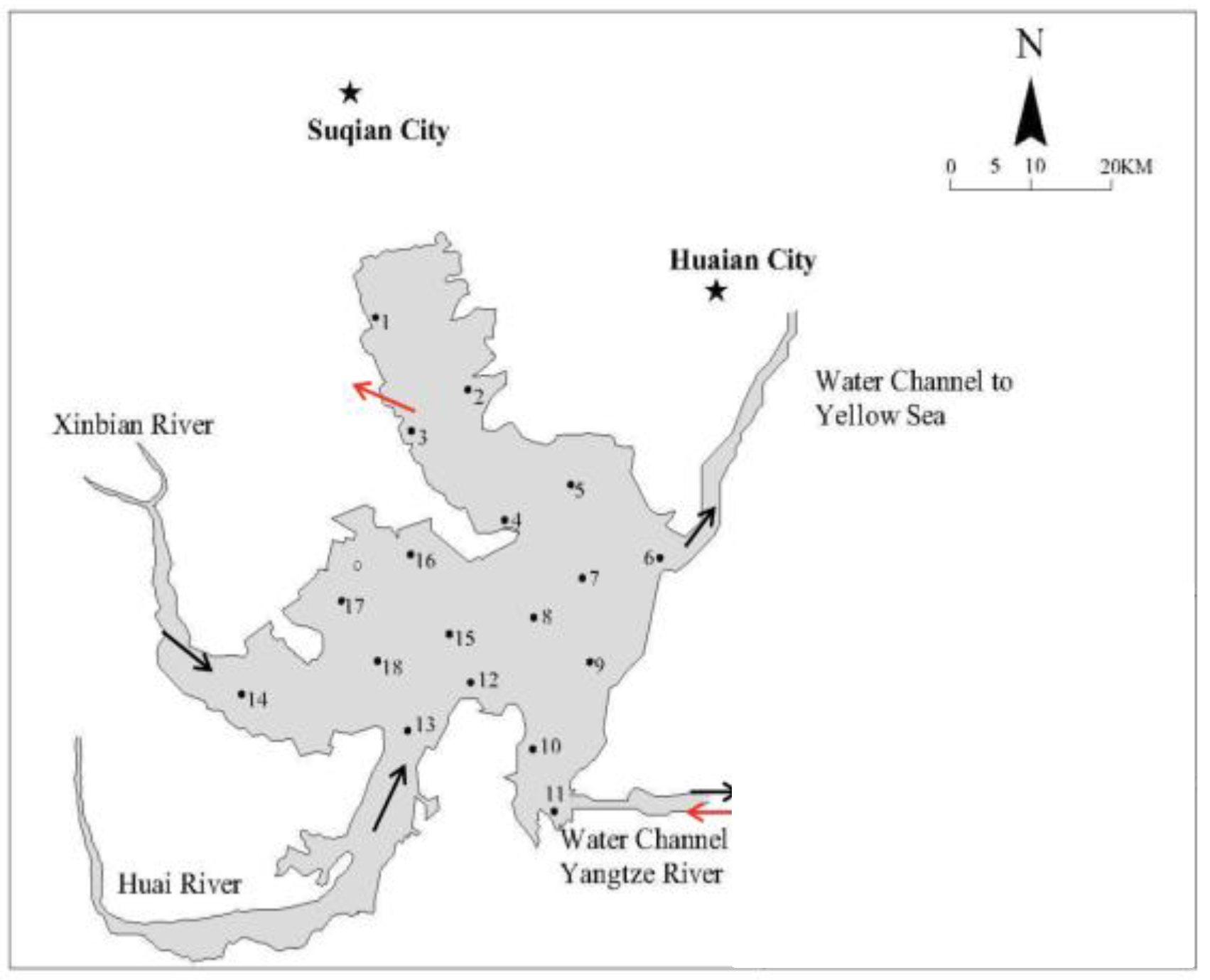

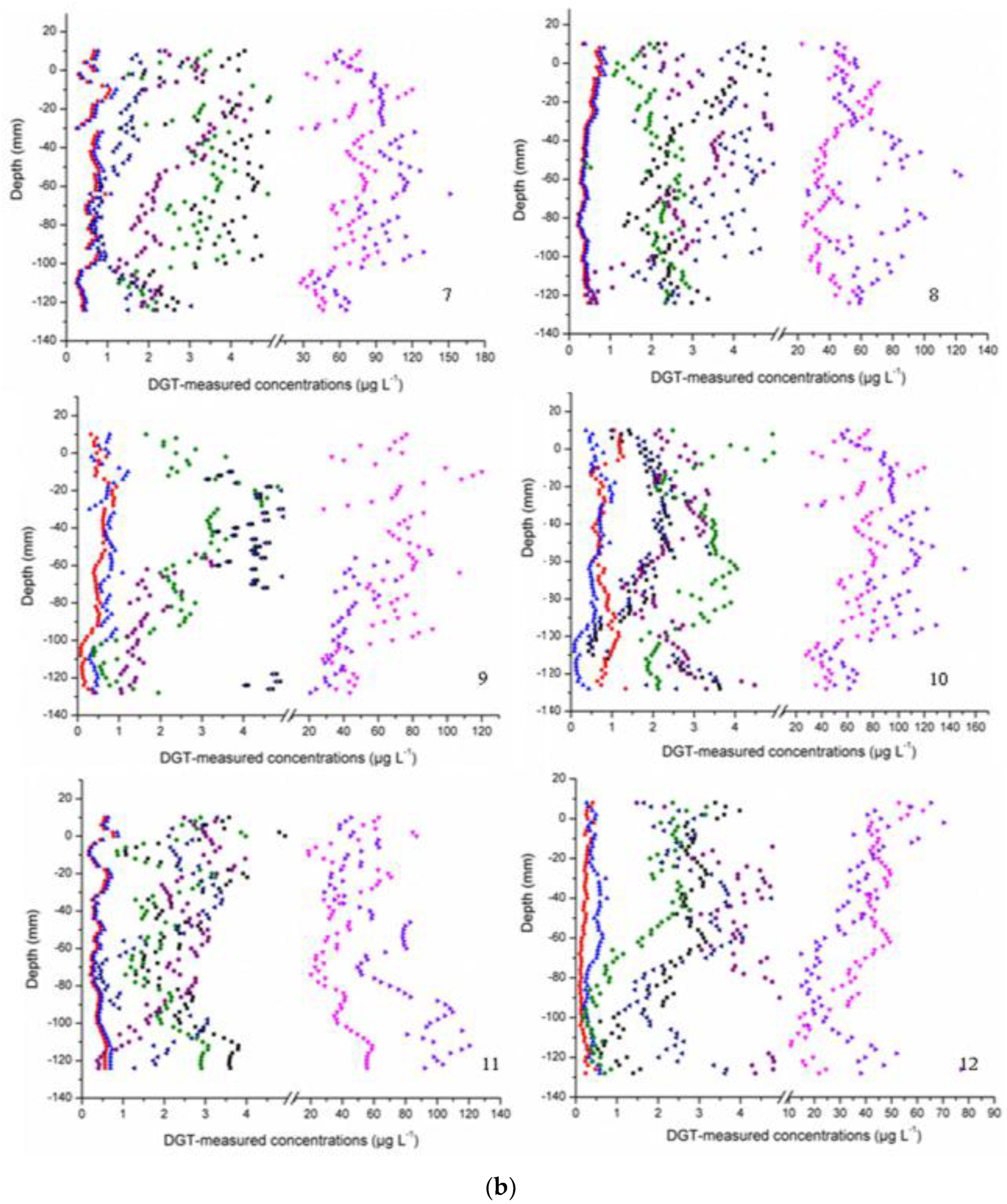
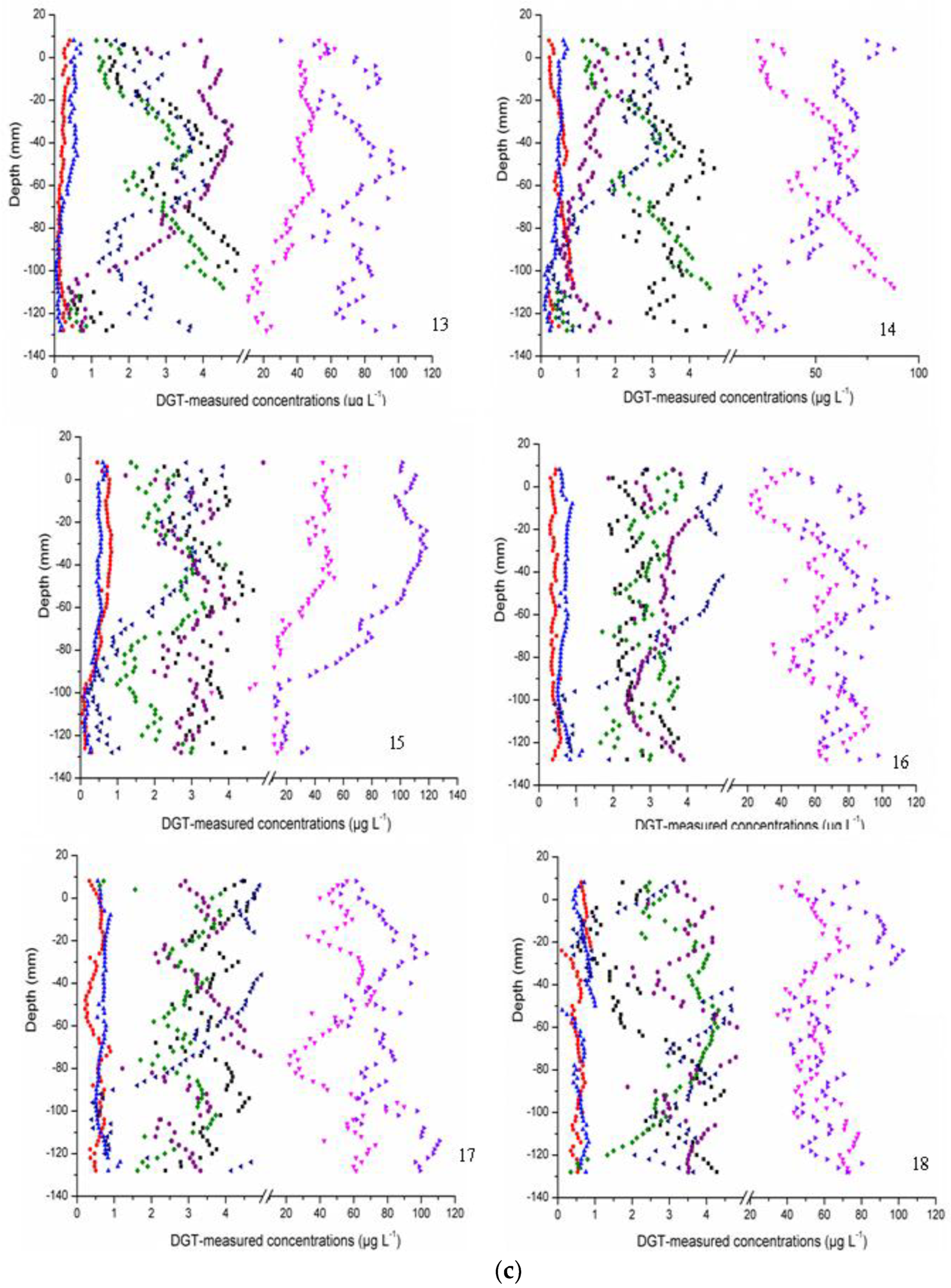
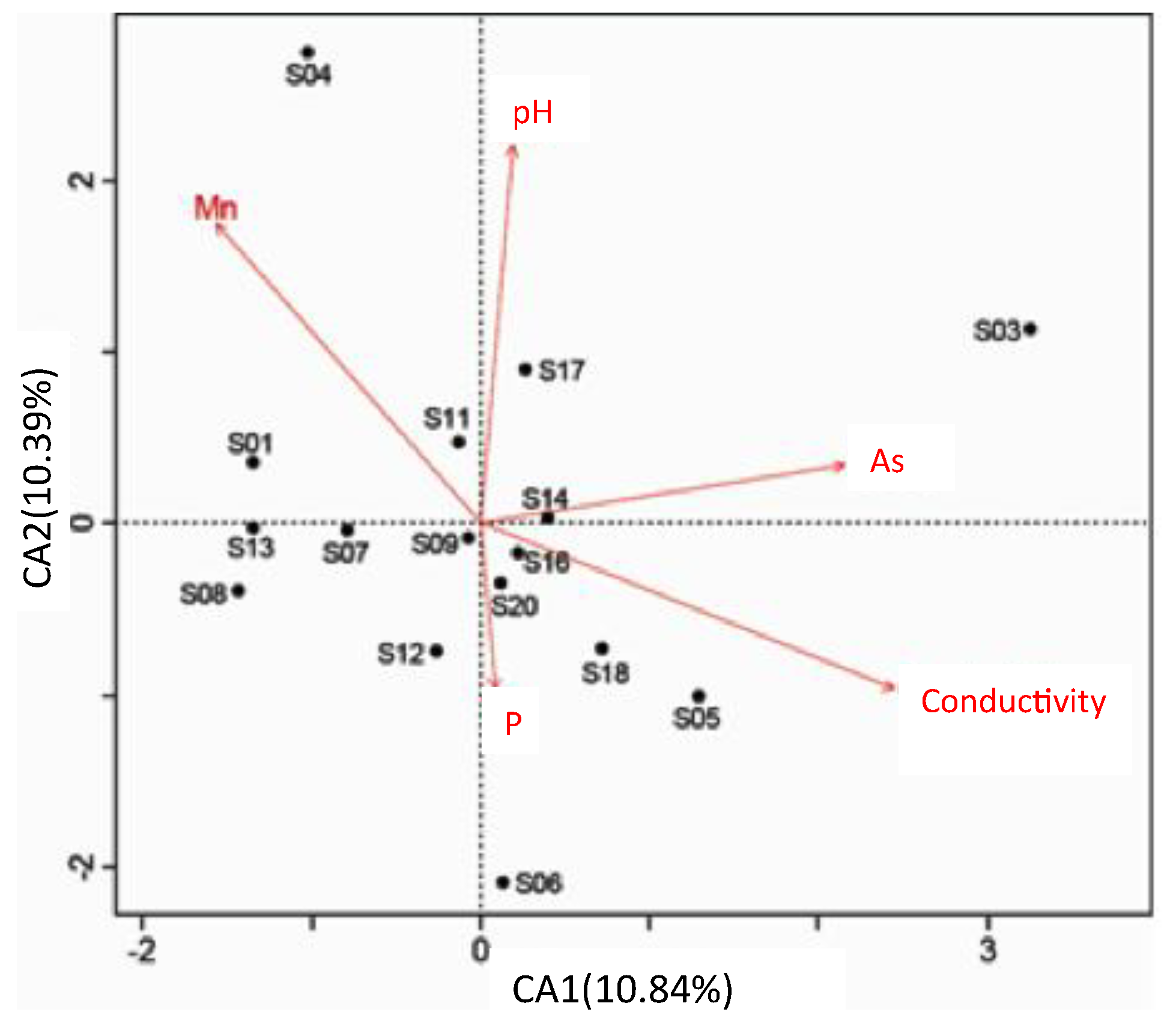

| Sites | Overlying Water | Surface Sediment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | TN (μg/L) | TP (μg/L) | DO (mg/L) | ORP (mv) | EC (μs) | pH | SS (g/L) | DOC (mg/L) | Moisture Content (%) | DO (mg/L) | pH | ORP (mv) | H2S (mg/L) | |
| 1 | 25.2 | 1.59 | 57.1 | 14.18 | 514 | 514 | 9.20 | 3.32 | 8.8 | 55.54 | 7.31 | 8.10 | 405 | 0.185 |
| 2 | 22.6 | 2.87 | 79.6 | 6.75 | 641 | 584 | 9.21 | 5.25 | 9.1 | 59.28 | 6.26 | 9.43 | 418 | 0.191 |
| 3 | 21.7 | 1.94 | 49.3 | 7.12 | 627 | 591 | 8.89 | 4.25 | 8.9 | 44.26 | 6.02 | 8.21 | 441 | 0.174 |
| 4 | 20.6 | 1.84 | 55.4 | 13.87 | 582 | 442 | 8.71 | 4.05 | 8.7 | 48.43 | 6.79 | 8.33 | 423 | 0.190 |
| 5 | 21.2 | 1.71 | 67.2 | 13.29 | 747 | 601 | 7.41 | 3.35 | 9.1 | 59.06 | 7.02 | 7.56 | 454 | 0.207 |
| 6 | 20.0 | 1.89 | 66.7 | 14.59 | 609 | 594 | 7.42 | 3.87 | 8.9 | 59.15 | 7.82 | 8.02 | 470 | 0.205 |
| 7 | 18.8 | 1.91 | 64.3 | 13.90 | 1041 | 441 | 7.51 | 3.55 | 9.4 | 56.20 | 5.98 | 7.41 | 416 | 0.209 |
| 8 | 18.1 | 2.13 | 68.9 | 14.15 | 714 | 386 | 7.69 | 3.35 | 9.3 | 58.98 | 6.71 | 8.11 | 517 | 0.192 |
| 9 | 18.4 | 1.96 | 59.4 | 12.32 | 1047 | 541 | 7.70 | 1.98 | 8.9 | 53.29 | 6.88 | 7.45 | 507 | 0.194 |
| 10 | 17.2 | 1.89 | 43.5 | 9.76 | 784 | 571 | 7.90 | 3.30 | 9.1 | 33.86 | 5.31 | 8.92 | 468 | 0.187 |
| 11 | 18.0 | 2.07 | 42.9 | 12.01 | 871 | 383 | 7.91 | 2.70 | 8.6 | 47.49 | 5.78 | 8.22 | 441 | 0.175 |
| 12 | 21.3 | 1.97 | 41.2 | 13.12 | 890 | 387 | 7.92 | 2.95 | 9.0 | 27.34 | 6.51 | 7.72 | 432 | 0.166 |
| 13 | 19.1 | 2.31 | 51.4 | 12.59 | 765 | 347 | 7.83 | 1.15 | 8.8 | 49.72 | 6.09 | 7.43 | 467 | 0.165 |
| 14 | 18.9 | 2.41 | 43.5 | 11.97 | 779 | 380 | 7.93 | 3.22 | 8.9 | 41.88 | 6.41 | 7.53 | 445 | 0.190 |
| 15 | 21.7 | 2.11 | 53.6 | 12.38 | 1049 | 445 | 7.67 | 3.81 | 9.1 | 54.83 | 6.35 | 8.09 | 518 | 0.181 |
| 16 | 20.1 | 2.35 | 57.6 | 15.92 | 554 | 506 | 7.72 | 4.89 | 9.3 | 43.22 | 7.73 | 8.15 | 489 | 0.155 |
| 17 | 19.9 | 1.93 | 58.7 | 13.41 | 773 | 486 | 7.53 | 3.23 | 9.2 | 50.37 | 6.79 | 7.73 | 501 | 0.159 |
| 18 | 20.1 | 1.76 | 49.6 | 11.11 | 971 | 640 | 9.11 | 4.65 | 8.9 | 58.14 | 5.53 | 8.43 | 511 | 0.168 |
| Site | Shannon | Chao1 | ACE | Faith’s PD | Number of OTUs | Simpson | Dominance |
|---|---|---|---|---|---|---|---|
| 1 | 9.43 | 6348 | 6648 | 366 | 3916 | 0.989 | 0.011 |
| 2 | 5.51 | 596 | 593 | 90 | 577 | 0.932 | 0.068 |
| 3 | 9.05 | 5576 | 5826 | 359 | 3626 | 0.983 | 0.017 |
| 4 | 9.50 | 5168 | 5472 | 318 | 3462 | 0.993 | 0.007 |
| 5 | 9.50 | 5417 | 5577 | 335 | 3484 | 0.994 | 0.006 |
| 6 | 9.66 | 5882 | 6154 | 363 | 3730 | 0.995 | 0.005 |
| 7 | 9.51 | 6645 | 6827 | 358 | 3969 | 0.989 | 0.011 |
| 8 | 9.55 | 5741 | 5871 | 353 | 3607 | 0.993 | 0.007 |
| 9 | 9.56 | 6668 | 6676 | 367 | 3895 | 0.991 | 0.009 |
| 10 | 8.13 | 3697 | 3879 | 246 | 2420 | 0.975 | 0.025 |
| 11 | 8.90 | 5786 | 5988 | 339 | 3521 | 0.979 | 0.021 |
| 12 | 9.52 | 5853 | 6036 | 350 | 3709 | 0.992 | 0.008 |
| 13 | 9.95 | 6189 | 6460 | 370 | 3959 | 0.996 | 0.004 |
| 14 | 10.1 | 6882 | 7165 | 383 | 4281 | 0.996 | 0.004 |
| 15 | 9.93 | 5975 | 6217 | 353 | 3782 | 0.996 | 0.004 |
| 16 | 10.1 | 6751 | 6937 | 384 | 4201 | 0.996 | 0.004 |
| 17 | 9.93 | 4741 | 4916 | 320 | 3391 | 0.997 | 0.003 |
| 18 | 9.42 | 5626 | 5835 | 328 | 3477 | 0.993 | 0.007 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Wang, P.; Wang, C. The Influence on Contaminant Bioavailability and Microbial Abundance of Lake Hongze by the South-to-North Water Diversion Project. Int. J. Environ. Res. Public Health 2019, 16, 3068. https://doi.org/10.3390/ijerph16173068
Yao Y, Wang P, Wang C. The Influence on Contaminant Bioavailability and Microbial Abundance of Lake Hongze by the South-to-North Water Diversion Project. International Journal of Environmental Research and Public Health. 2019; 16(17):3068. https://doi.org/10.3390/ijerph16173068
Chicago/Turabian StyleYao, Yu, Peifang Wang, and Chao Wang. 2019. "The Influence on Contaminant Bioavailability and Microbial Abundance of Lake Hongze by the South-to-North Water Diversion Project" International Journal of Environmental Research and Public Health 16, no. 17: 3068. https://doi.org/10.3390/ijerph16173068
APA StyleYao, Y., Wang, P., & Wang, C. (2019). The Influence on Contaminant Bioavailability and Microbial Abundance of Lake Hongze by the South-to-North Water Diversion Project. International Journal of Environmental Research and Public Health, 16(17), 3068. https://doi.org/10.3390/ijerph16173068




