Blood Cadmium Level Is Associated with Short Progression-Free Survival in Nasopharyngeal Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Collection of General and Clinical Data
2.3. Collection of Survival Time Data
2.4. Analysis of Blood Cd Level
2.5. Statistical Analysis
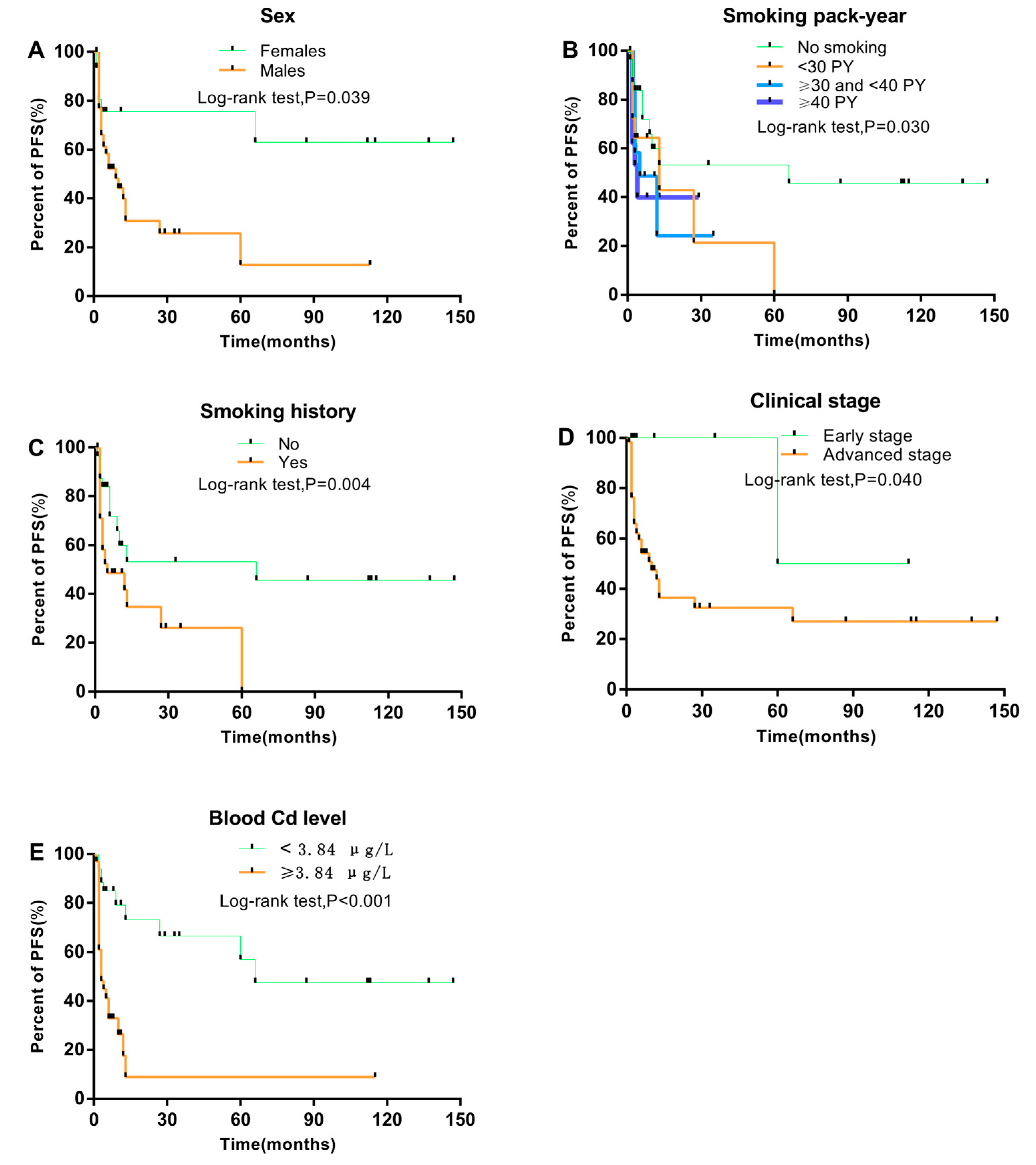
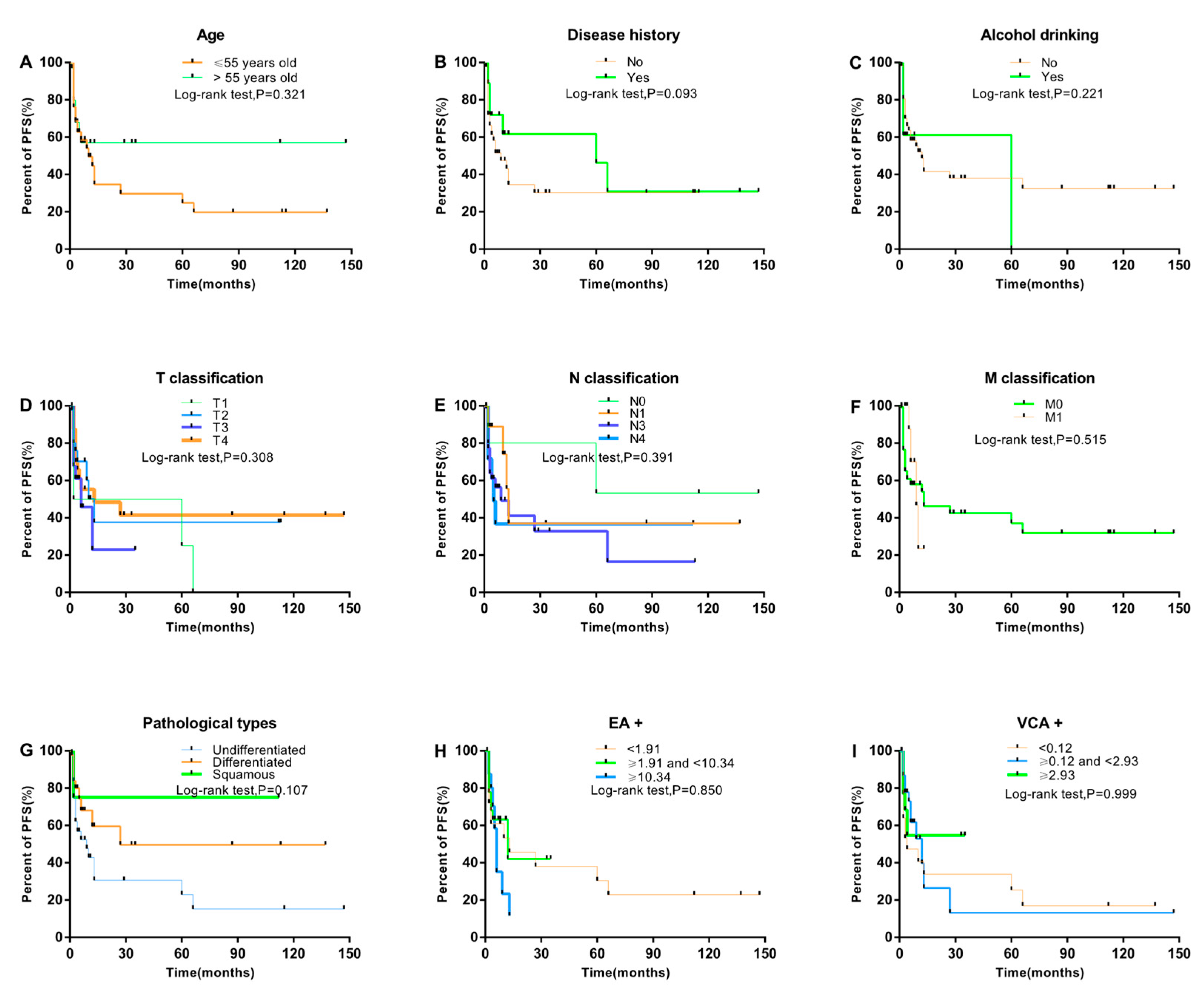
3. Results
3.1. Association between Clinical Characteristics and NPC Progression
3.2. Association between Clinical Characteristics of NPC Patients and Blood Cd Level
3.3. Prognostic Factors Associated with PFS and NPC Progression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C. Primary carcinoma of the nasopharynx. a table of cases. JAMA 1901, 37, 371–377. [Google Scholar] [CrossRef]
- Digby, K.H. Nasopharyngeal carcinoma. Br. J. Surg. 1941, 28, 517–537. [Google Scholar] [CrossRef]
- Ferlay, J.; Parkin, D.; Shin, H.; Forman, D.; Mathers, C.; Parkin, D.; Bray, F. Globocan 2000. Int. J. Cancer 2001, 94, 153–156. [Google Scholar]
- Caponigro, F.F.; Longo, F.; Perri, F. Treatment approaches to nasopharyngeal carcinoma: A review. Anticancer Drugs 2010, 21, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.W.; Huang, G.; Zeng, X.; Ma, X.; Zhou, Y.; Wang, C.; Cheng, A. Expression of the Annexin A1 gene is associated with suppression of growth, invasion and metastasis of nasopharyngeal carcinoma. Mol. Med. Rep. 2014, 10, 3059–3067. [Google Scholar] [CrossRef] [Green Version]
- Henderson, B.E.; Louie, E.; Soohoo, J.J.; Buell, P.; Gardner, M.B. Risk factors associated with nasopharyngeal carcinoma. N. Engl. J. Med. 1976, 295, 1101–1106. [Google Scholar] [CrossRef]
- Lee, A.W.; Ng, W.T.; Chan, Y.H.; Sze, H.; Chan, C.; Lam, T.H. The battle against nasopharyngeal cancer. Radiother. Oncol. 2012, 104, 272–278. [Google Scholar] [CrossRef]
- Nam, J.; Mclaughlin, J.K.; Blot, W.J. Cigarette Smoking, Alcohol, and Nasopharyngeal Carcinoma: A Case-Control Study Among U.S. Whites. J. Natl. Cancer Inst. 1992, 84, 619–622. [Google Scholar] [CrossRef]
- Purdue, M.P.; Järvholm, B.; Bergdahl, I.A.; Hayes, R.B.; Baris, D. Occupational exposures and head and neck cancers among Swedish construction workers. Scand. J. Work Environ. Health 2006, 32, 270–275. [Google Scholar] [CrossRef]
- Ulenbelt, P.; Lumens, M.E.; Géron, H.M.; Herber, R.F.; Broersen, S.; Zielhuis, R.L. Work hygienic behaviour as modifier of the lead air-lead blood relation. Int. Arch. Occup. Environ. Health 1990, 62, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Afridi, I.H.; Kazi, T.G.; Kazi, A.G.; Shah, F.; Wadhwa, S.K.; Kolachi, N.F.; Shah, A.Q.; Baig, J.A.; Kazi, N. Levels of arsenic, cadmium, lead, manganese and zinc in biological samples of paralysed steel mill workers with related to controls. Biol. Trace Elem. Res. 2011, 144, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Gil, F.; Hernández, A.F.; Márquez, C.; Femia, P.; Olmedo, P.; López-Guarnido, O.; Pla, A. Biomonitorization of cadmium, chromium, manganese, nickel and lead in whole blood, urine, axillary hair and saliva in an occupationally exposed population. Sci. Total Environ. 2011, 409, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, D.; Rossmann, A.; Wick, G. Metals in cigarette smoke. Iubmb. Life 2005, 57, 805–809. [Google Scholar]
- Eyre, H. Association. Preventing cancer, cardiovascular disease, and diabetes: A common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation 2004, 109, 3244–3255. [Google Scholar] [CrossRef] [PubMed]
- Yeon, S.K.; Wook, K.J.; Sook, K.Y.; Eun, K.J.; Yeon, C.H.; Cha, L.K.Y. Changes in lipid peroxidation and antioxidant trace elements in serum of women with cervical intraepithelial neoplasia and invasive cancer. Nutr. Cancer 2003, 47, 126–130. [Google Scholar]
- Kazi, G.T.; Memon, A.R.; Afridi, H.I.; Jamali, M.K.; Arain, M.B.; Jalbani, N.; Sarfraz, R.A. Determination of cadmium in whole blood and scalp hair samples of Pakistani male lung cancer patients by electrothermal atomic absorption spectrometer. Sci. Total Environ. 2008, 389, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Kazi, G.T.; Wadhwa, S.K.; Afridi, H.I.; Kazi, N.; Kandhro, G.A.; Baig, J.A.; Shah, A.Q.; Kolachi, N.F.; Arain, M.B. Interaction of cadmium and zinc in biological samples of smokers and chewing tobacco female mouth cancer patients. J. Hazard. Mater. 2010, 176, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.T.; Ie, B.L.; Tsai, K.Y.; Chang, T.K.; Chiang, C.T.; Su, C.C.; Hwang, Y.H. Possible association between nickel and chromium and oral cancer: A case-control study in central Taiwan. Sci. Total Environ. 2011, 409, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, K.S.; Kazi, T.G.; Kolachi, N.F.; Afridi, H.I.; Khan, S.; Chandio, A.A.; Shah, A.Q.; Kandhro, G.A.; Nasreen, S. Case-control study of male cancer patients exposed to arsenic-contaminated drinking water and tobacco smoke with relation to non-exposed cancer patients. Hum. Exp. Toxicol. 2011, 30, 2013–2022. [Google Scholar] [CrossRef]
- Tandon, M.U.; Kapil, S.; Bahadur, S.; Dwivedi, S.N.; Pathak, P. Role of micro-nutrients and trace elements in carcinoma of larynx. J. Assoc. Physic. India 2000, 48, 995–998. [Google Scholar]
- Khlifi, R.; Olmedo, P.; Gil, F.; Molka, F.T.; Hammami, B.; Ahmed, R.; Amel, H.C. Risk of laryngeal and nasopharyngeal cancer associated with arsenic and cadmium in the Tunisian population. Environ. Sci. Pollut. Res. 2014, 21, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Garnit, H.; Bouhlel, S.; Barca, D.; Chtara, C. Application of LA-ICP-MS to sedimentary phosphatic particles from Tunisian phosphorite deposits: Insights from trace elements and REE into paleo-depositional environments. Chem. Erd. 2012, 72, 127–139. [Google Scholar] [CrossRef]
- Meeker, D.J.; Rossano, M.G.; Protas, B.; Diamond, M.P.; Puscheck, E.; Daly, D.; Paneth, N.; Wirth, J.J. Cadmium, Lead, and Other Metals in Relation to Semen Quality: Human Evidence for Molybdenum as a Male Reproductive Toxicant. Environ. Health Perspect. 2008, 116, 1473. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Baker, J.R.; Urbenjapol, S.; Haswell-Elkins, M.; Reilly, P.E.; Williams, D.J.; Moore, M.R. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol. Lett. 2003, 137, 65–83. [Google Scholar] [CrossRef]
- Byrne, C.; Divekar, S.D.; Storchan, G.B.; Parodi, D.A.; Martin, M.B. Metals and breast cancer. J. Mammary Gland Biol. Neoplasia 2013, 18, 63–73. [Google Scholar] [CrossRef]
- Akesson, A.; Julin, B.; Wolk, A. Long-term dietary cadmium intake and postmenopausal endometrial cancer incidence: A population-based prospective cohort study. Cancer Res. 2008, 68, 6435–6441. [Google Scholar] [CrossRef]
- Kellen, E.; Zeegers, M.P.; Hond, E.D.; Buntinx, F. Blood cadmium may be associated with bladder carcinogenesis: The Belgian case-control study on bladder cancer. Cancer Detect. Prev. 2007, 31, 77–82. [Google Scholar] [CrossRef]
- Luckett, G.B.; Su, L.J.; Rood, J.C.; Fontham, E.T. Cadmium exposure and pancreatic cancer in south Louisiana. J. Environ. Public Health 2012, 2012, 180186. [Google Scholar] [CrossRef]
- Julin, B.; Wolk, A.; Bergkvist, L.; Bottai, M.; Akesson, A. Dietary cadmium exposure and risk of postmenopausal breast cancer: A population-based prospective cohort study. Cancer Res. 2012, 72, 1459–1466. [Google Scholar] [CrossRef]
- Garcia-Esquinas, E.; Pollan, M.; Tellez-Plaza, M.; Francesconi, K.A.; Goessler, W.; Guallar, E.; Umans, J.G.; Yeh, J.; Best, L.G.; Navas-Acien, A. Cadmium exposure and cancer mortality in a prospective cohort: The strong heart study. Environ. Health Perspect. 2014, 122, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Chen, Y.; Yu, H.; Zhao, Y.; Chen, G.; Bai, L.; Liu, D.; Su, H.; Wang, H. Survival and Prognostic Analysis of Primary Nasopharyngeal Carcinoma in North China. Clin. Lab. 2015, 61, 699. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.S.; Yen, K.L.; Jian, J.J.; Tsai, S.Y.; Chu, N.M.; Leu, S.Y.; Chan, K.Y.; Tan, T.D.; Cheng, J.C.; Hsieh, C.Y. Examining prognostic factors and patterns of failure in nasopharyngeal carcinoma following concomitant radiotherapy and chemotherapy: Impact on future clinical trials. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 717–726. [Google Scholar] [CrossRef]
- Mao, P.Y.; Xie, F.Y.; Liu, L.Z.; Sun, Y.; Li, L.; Tang, L.L.; Liao, X.B.; Xu, H.Y.; Chen, L.; Lai, S.Z. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.S.; Li, W.F.; Chen, L.; Luo, W.; Chen, Y.Y.; Liu, L.Z.; Sun, Y.; Lin, A.H.; Liu, M.Z.; Ma, J. How Does Intensity-Modulated Radiotherapy Versus Conventional Two-Dimensional Radiotherapy Influence the Treatment Results in Nasopharyngeal Carcinoma Patients? Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Khlifi, R.; Olmedo, P.; Gil, F.; Hammami, B.; Chakroun, A.; Rebai, A.; Hamza-Chaffai, A. Arsenic, cadmium, chromium and nickel in cancerous and healthy tissues from patients with head and neck cancer. Sci. Total Environ. 2013, 452, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, X.; Huo, X.; Xu, X.; Lin, K.; Zhang, J.; Huang, Y.; Wu, K. Blood cadmium burden and the risk of nasopharyngeal carcinoma: A case-control study in Chinese Chaoshan population. Environ. Sci. Pollut. Res. Int. 2015, 22, 12323–12331. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wu, K.; Li, Y.; Qi, Z.; Han, D.; Zhang, B.; Gu, C.; Chen, G.; Liu, J.; Chen, S. Blood lead and cadmium levels and relevant factors among children from an e-waste recycling town in China. Environ. Res. 2008, 108, 15–20. [Google Scholar] [CrossRef]
- Warren, W.G.; Reid, M.E.; Cummings, K.M.; Marshall, J.R. Author’s reply to: Smoking at diagnosis and survival in cancer patients. Int. J. Cancer 2013, 132, 401–410. [Google Scholar] [CrossRef]
- Xiao, W.W.; Huang, S.M.; Han, F.; Wu, S.X.; Lu, L.X.; Lin, C.G.; Deng, X.W.; Lu, T.X.; Cui, N.J.; Zhao, C. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: Long-term results of a phase 2 study. Cancer 2011, 117, 1874–1883. [Google Scholar] [CrossRef]
- Lee, W.A.; Sze, W.M.; Au, J.S.; Leung, S.F.; Leung, T.W.; Chua, D.T.; Zee, B.C.; Law, S.C.; Teo, P.M.; Tung, S.Y. Treatment results for nasopharyngeal carcinoma in the modern era: The Hong Kong experience. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Salem, F.A.; Al-Zoubi, M.S.; Whitaker-Menezes, D.; Martinez-Outschoorn, U.E.; Lamb, R.; Hulit, J.; Howell, A.; Gandara, R.; Sartini, M.; Galbiati, F. Cigarette smoke metabolically promotes cancer, via autophagy and premature aging in the host stromal microenvironment. Cell Cycle 2013, 12, 818–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browman, P.G.; Wong, G.; Hodson, I.; Sathya, J.; Russell, R.; Mcalpine, L.; Skingley, P.; Levine, M.N. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N. Engl. J. Med. 1993, 328, 159. [Google Scholar] [CrossRef] [PubMed]
- Browman, G.P.; Mohide, E.A.; Willan, A.; Hodson, I.; Wong, G.; Grimard, L.; MacKenzie, R.G.; El-Sayed, S.; Dunn, E.; Farell, S. Association between smoking during radiotherapy and prognosis in head and neck cancer: A follow-up study. Head Neck 2002, 24, 1031–1037. [Google Scholar] [CrossRef]
- Chen, A.M.; Chen, L.M.; Vaughan, A.; Sreeraman, R.; Farwell, D.G.; Luu, Q.; Lau, D.H.; Stuart, K.; Purdy, J.A.; Vijayakumar, S. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 414. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.W.; Han, F.; Lu, T.X.; Chen, C.Y.; Huang, Y.; Zhao, C. Treatment outcomes after radiotherapy alone for patients with early-stage nasopharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1070. [Google Scholar] [CrossRef]
- Yeh, A.S.; Tang, Y.; Lui, C.C.; Huang, Y.J.; Huang, E.Y. Treatment outcomes and late complications of 849 patients with nasopharyngeal carcinoma treated with radiotherapy alone. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Han, F.; Zhao, C.; Lu, L.X.; Sun, Y.; Liu, X.F.; Lu, T.X. Treatment results and late complications of 556 patients with locally advanced nasopharyngeal carcinoma treated with radiotherapy alone. Br. J. Radiol. 2009, 82, 452–458. [Google Scholar] [CrossRef]
- Chen, C.Y.; Pu, Y.S.; Wu, H.C.; Wu, T.T.; Lai, M.K.; Yang, C.Y.; Sung, F.C. Cadmium burden and the risk and phenotype of prostate cancer. BMC Cancer 2009, 9, 429. [Google Scholar] [CrossRef]
- Liu, T.M.; Hsieh, C.Y.; Chang, T.H.; Lin, J.P.; Huang, C.C.; Wang, A.Y. Prognostic Factors Affecting the Outcome of Nasopharyngeal Carcinoma. Jpn. J. Clin. Oncol. 2003, 33, 501–508. [Google Scholar] [CrossRef] [Green Version]
- Zuckerman, A.J. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. J. Clin. Pathol. 1995, 48, 691. [Google Scholar] [CrossRef]
- Hartwig, A. Cadmium and Cancer; Springer: Dordrecht, The Netherlands, 2013; pp. 491–507. [Google Scholar]
- Ferguson, J.E. Heavy Elements: Chemistry, e.i.a.h.e., The Heavy Elements: Chemistry, Environmental Impact, and Health Effects; Pergamon Press: Oxford, UK, 1990; p. 69. [Google Scholar]
- Tord, K.; Lars, F.; Barbro, R. Mortality and cancer morbidity among cadmium-exposed workers. Environ. Health Perspect. 1979, 28, 199. [Google Scholar]
- Robards, K.; Worsfold, P. Cadmium: Toxicology and analysis: A review. Analyst 1991, 116, 549–568. [Google Scholar] [CrossRef] [PubMed]
- Seiler, H.G.; Sigel, H.; Sigel, A. Handbook of Toxicity of Inorganic Compounds; Taylor and Francis: Abingdon, UK, 1988; p. 1024. [Google Scholar]
- Kazantzis, G. Induction of Sarcoma in the Rat by Cadmium Sulphide Pigment. Nature 1963, 198, 1213–1214. [Google Scholar] [CrossRef]
- Friberg, L.; Piscator, M.; Nordberg, G.F.; Kjellstroem, T. Cadmium in the Environment, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1974. [Google Scholar]
- Collins, M.M.; Pang, Y.T.; Loughran, S.; Wilson, J.A. Environmental risk factors and gender in nasal polyposis. Clin. Otolaryngol. Allied Sci. 2002, 27, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Khlifi, R.; Olmedo, P.; Gil, F.; Chakroun, A.; Hammami, B.; Hamza-Chaffai, A. Heavy metals in normal mucosa and nasal polyp tissues from Tunisian patients. Environ. Sci. Pollut. Res. Int. 2015, 22, 463–471. [Google Scholar] [CrossRef]
- Pearson, C.A.; Prozialeck, W.C. E-Cadherin, beta -Catenin and cadmium carcinogenesis. Med. Hypotheses 2001, 56, 573–581. [Google Scholar] [CrossRef]
- Afridi, I.H.; Kazi, T.G.; Kazi, N.; Kandhro, G.A.; Baig, J.A.; Jamali, M.K.; Arain, M.B.; Shah, A.Q. Erratum: Interactions Between Cadmium and Zinc in the Biological Samples of Pakistani Smokers and Nonsmokers Cardiovascular Disease Patients. Biol. Trace Elem. Res. 2011, 139, 368. [Google Scholar] [CrossRef]
- El, A.O.; Gokmen, I.G. Smoking habits and cadmium intake in Turkey. Biol. Trace Elem. Res. 2002, 88, 31. [Google Scholar]
- Pappas, S.R.; Polzin, G.M.; Zhang, L.; Watson, C.H.; Paschal, D.C.; Ashley, D.L. Cadmium, lead, and thallium in mainstream tobacco smoke particulate. Food Chem. Toxicol. 2006, 44, 714–723. [Google Scholar] [CrossRef]
- Galazyn-Sidorczuk, M.; Brzoska, M.M.; Moniuszko-Jakoniuk, J. Estimation of Polish cigarettes contamination with cadmium and lead, and exposure to these metals via smoking. Environ. Monit. Assess. 2008, 137, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Waalkes, P.M.; Coogan, T.P.; Barter, R.A. Toxicological principles of metal carcinogenesis with special emphasis on cadmium. Crit. Rev. Toxicol. 1992, 22, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Nriagu, J.O. Arsenic in the Environment, Part 2, Human Health and Ecosystem Effects; Advances in Environmental Science and Technology Series; Wiley: Hoboken, NJ, USA, 1994. [Google Scholar]
- Järup, L.; Berglund, M.; Elinder, C.G.; Nordberg, G.; Vahter, M. Health effects of cadmium exposure—A review of the literature and a risk estimate. Scand. J. Work Environ. Health 1998, 24, 1–51. [Google Scholar] [PubMed]
| Clinicopathologic Characteristics | NPC Progression n = 49 | No NPC Progression n = 85 | p-Value |
|---|---|---|---|
| Age (years), mean ± SD | 54.00 ± 11.61 | 56.47 ± 12.08 | 0.250 a |
| Cd level (μg/L), median, IQR | 7.62 (3.37–9.43) | 3.09 (1.77–4.80) | <0.001 * |
| EA+ | 4.53 (0.73–10.08) | 5.99 (2.51–10.36) | 0.270 a |
| VCA+ | 0.37 (0.03–2.05) | 1.15 (0.21–3.09) | 0.092 a |
| Sex | |||
| Female | 8 (16.3) | 23 (27.1) | 0.156 b |
| Male | 41 (83.7) | 62 (72.9) | |
| Family history of cancer | |||
| Yes | 8 (16.3) | 15 (17.6) | 0.845 b |
| No | 41 (83.7) | 70 (82.4) | |
| History of disease | |||
| Yes | 12 (24.5) | 33 (38.8) | 0.091 b |
| No | 37 (75.5) | 52 (61.2) | |
| Smoking pack-years | |||
| No smoking | 14 (28.6) | 42 (49.4) | 0.118 b |
| <30 | 12 (24.5) | 17 (20.0) | |
| 30–40 | 8 (16.3) | 10 (11.8) | |
| ≥40 | 15 (30.6) | 16 (18.8) | |
| Smoking history | |||
| Yes | 35 (71.4) | 43 (50.6) | 0.018 b |
| No | 14 (28.6) | 42 (49.4) | |
| Alcohol drinking | |||
| Yes | 8 (16.3) | 10 (11.8) | 0.456 b |
| No | 41 (83.7) | 75 (88.2) | |
| T classification | |||
| T1 | 5 (10.4) | 1 (1.2) | 0.080 b |
| T2 | 14 (29.2) | 32 (37.6) | |
| T3 | 13 (27.1) | 20 (23.5) | |
| T4 | 16 (33.3) | 32 (37.6) | |
| Clinical stages | |||
| Early | 1 (2.0) | 8 (9.4) | 0.101 b |
| Advanced | 48 (98.0) | 77 (90.6) | |
| N classification | |||
| N0 | 2 (4.2) | 3 (3.5) | 0.573 b |
| N1 | 5 (10.4) | 14 (16.5) | |
| N2 | 34 (70.8) | 61 (71.8) | |
| N3 | 7 (14.6) | 7 (8.2) | |
| M classification | |||
| M0 | 44 (91.7) | 77 (90.6) | 0.835 b |
| M1 | 4 (8.3) | 8 (9.4) | |
| Pathological types | |||
| Undifferentiated | 36 (73.5) | 50 (58.8) | 0.234 b |
| Differentiated | 12 (24.5) | 32 (37.6) | |
| Squamous | 1 (2.0) | 3 (3.5) |
| Clinicopathologic Characteristics | No. of Patients | Blood Cd Level | p-Value | |
|---|---|---|---|---|
| <3.84 μg/L (n = 67) | ≥3.84 μg/L (n = 67) | |||
| Age (years), mean ± SD | 134 | 55.40 ± 11.66 | 55.73 ± 12.28 | 0.631 a |
| EA+ | 106 | 5.13 (1.73–9.97) | 7.10 (2.49–10.71) | 0.185 a |
| VCA+ | 107 | 0.81 (0.12–2.40) | 0.89 (0.21–3.35) | 0.432 a |
| Sex | ||||
| Female | 103 | 49 (73.1) | 54 (80.6) | 0.306b |
| Male | 31 | 18 (26.9) | 13 (19.4) | |
| Family history of cancer | ||||
| Yes | 23 | 8 (11.9) | 15 (22.4) | 0.109 b |
| No | 111 | 59 (88.1) | 52 (77.6) | |
| Disease history | ||||
| Yes | 45 | 29 (43.3) | 16 (23.9) | 0.017 b |
| No | 89 | 38 (56.7) | 51 (76.1) | |
| Smoking pack-years | ||||
| No smoking | 56 | 35 (52.2) | 21 (31.3) | 0.003 b |
| <30 | 29 | 17 (25.4) | 12 (17.9) | |
| 30–40 | 18 | 8 (11.9) | 10 (14.9) | |
| ≥40 | 31 | 7 (10.4) | 24 (35.8) | |
| Smoking history | ||||
| Yes | 78 | 32 (47.8) | 46 (68.7) | 0.014 b |
| No | 56 | 35 (52.2) | 21 (31.3) | |
| Alcohol drinking | ||||
| Yes | 18 | 7 (10.4) | 11 (16.4) | 0.311 b |
| No | 116 | 60 (89.6) | 56 (83.6) | |
| Clinical stage | ||||
| Early | 9 | 8 (11.9) | 1 (1.5) | 0.016 b |
| Advanced | 125 | 59 (88.1) | 66 (98.5) | |
| T classification | ||||
| T1 | 6 | 2 (3.0) | 4 (6.1) | 0.356 b |
| T2 | 46 | 25 (37.3) | 21 (31.8) | |
| T3 | 33 | 13 (19.4) | 20 (30.3) | |
| T4 | 48 | 27 (40.3) | 21 (31.8) | |
| N classification | ||||
| N0 | 5 | 3 (4.5) | 2 (3.0) | 0.345 b |
| N1 | 19 | 13 (19.4) | 6 (9.1) | |
| N2 | 95 | 45 (67.2) | 50 (75.8) | |
| N3 | 14 | 6 (9.0) | 8 (12.1) | |
| M classification | ||||
| M0 | 121 | 62 (92.5) | 59 (89.4) | 0.527 b |
| M1 | 12 | 5 (7.5) | 7 (10.6) | |
| Pathological types | ||||
| Undifferentiated | 86 | 40 (59.7) | 46 (68.7) | 0.410 b |
| Differentiated | 44 | 24 (35.8) | 20 (29.9) | |
| Squamous | 4 | 3 (4.5) | 1 (1.5) | |
| Variables | Blood Cd Level | OR (95%CI) | p-Value | |
|---|---|---|---|---|
| <3.84 μg/L n = 67 | ≥3.84 μg/L n = 67 | |||
| Family History of Cancer | ||||
| No | 59 (88.1) | 52 (77.6) | Reference | |
| Yes | 8 (11.9) | 15 (22.4) | 1.52 (0.53–4.40) | 0.437 |
| Smoking pack-years | ||||
| No smoking | 35 (52.2) | 21 (28.8) | Reference | |
| <30 | 17 (25.4) | 12 (16.4) | 1.14 (0.44–2.96) | 0.790 |
| 30–40 | 8 (11.9) | 16 (21.9) | 2.01 (0.64–6.32) | 0.228 |
| ≥40 | 7 (10.4) | 24 (32.9) | 4.83 (1.68–13.91) | 0.004 |
| History of disease | ||||
| No | 38 (56.7) | 51 (76.1) | Reference | |
| Yes | 29 (43.3) | 16 (23.9) | 0.39 (0.17–0.89) | 0.025 |
| Clinical stage | ||||
| Early | 8 (11.9) | 1 (1.5) | Reference | |
| Advanced | 59 (88.1) | 66 (98.5) | 5.50 (0.62–48.54) | 0.125 |
| Investigated Factors | SPY | Age | Sex | FHC | DH | AD | CS | PT | SH | BCL | VCA+ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EA+ | −0.025 | 0.204 * | −0.108 | −0.045 | −0.129 | −0.268 ** | 0.032 | 0.207 * | −0.080 | 0.167 | 0.476 ** |
| VCA+ | -0.082 | 0.149 | 0.179 | −0.039 | −0.014 | −0.066 | −0.095 | −0.105 | −0.100 | 0.065 | |
| BCL | 0.314 ** | −0.006 | −0.056 | 0.146 | −0.202 * | −0.088 | 0.192 * | −0.155 | 0.224 ** | ||
| SH | 0.899 ** | 0.064 | −0.612 ** | 0.145 | −0.006 | 0.289 ** | 0.135 | 0.017 | |||
| PT | 0.031 | 0.017 | −0.074 | 0.020 | −0.051 | −0.100 | −0.003 | ||||
| CS | 0.143 | −0.064 | −0.065 | 0.122 | −0.125 | 0.018 | |||||
| AD | 0.269 ** | 0.048 | −0.216 * | 0.111 | 0.183* | ||||||
| DH | −0.023 | 0.056 | −0.053 | 0.137 | |||||||
| FHC | 0.241 ** | 0.107 | −0.109 | ||||||||
| Sex | −0.539 ** | −0.039 | |||||||||
| Age | 0.204 * |
| Variables | PFS | ||
|---|---|---|---|
| HR | 95% CI | p-Value | |
| Sex (male vs. female) | 1.25 | 0.45–3.42 | 0.671 |
| Residence | |||
| Chaozhou | Reference | ||
| Shantou | 0.70 | 0.28–1.74 | 0.443 |
| Jieyang | 0.99 | 0.39–2.48 | 0.976 |
| Other area | 0 | 0 | 0.976 |
| Family history of cancer (yes vs. no) | 1.11 | 0.49–2.53 | 0.800 |
| History of disease (yes vs. no) | 0.65 | 0.31–1.34 | 0.240 |
| Alcohol drinking (yes vs. no) | 1.11 | 0.46–2.71 | 0.817 |
| Smoking pack-years | |||
| No smoking | Reference | ||
| <30 | 2.32 | 0.87–6.22 | 0.093 |
| 30–40 | 1.47 | 0.52–4.18 | 0.468 |
| ≥40 | 1.76 | 0.70–4.42 | 0.230 |
| Clinical stage (advanced vs early) | 3.03 | 0.37–24.65 | 0.301 |
| Blood Cd level (≥3.84 vs. <3.84 μg/L) | 4.11 | 1.92–8.81 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, T.; Huang, W.; Zheng, S.; Bao, M.; Huang, Y.; Li, A.; He, M.; Wu, K. Blood Cadmium Level Is Associated with Short Progression-Free Survival in Nasopharyngeal Carcinoma. Int. J. Environ. Res. Public Health 2019, 16, 2952. https://doi.org/10.3390/ijerph16162952
Du T, Huang W, Zheng S, Bao M, Huang Y, Li A, He M, Wu K. Blood Cadmium Level Is Associated with Short Progression-Free Survival in Nasopharyngeal Carcinoma. International Journal of Environmental Research and Public Health. 2019; 16(16):2952. https://doi.org/10.3390/ijerph16162952
Chicago/Turabian StyleDu, Taifeng, Wenlong Huang, Shukai Zheng, Mian Bao, Yuanni Huang, Anna Li, Meirong He, and Kusheng Wu. 2019. "Blood Cadmium Level Is Associated with Short Progression-Free Survival in Nasopharyngeal Carcinoma" International Journal of Environmental Research and Public Health 16, no. 16: 2952. https://doi.org/10.3390/ijerph16162952







