Dermatologic Vasculature Diseases as a Risk Factor of Subconjunctival Hemorrhage
Abstract
:1. Introduction
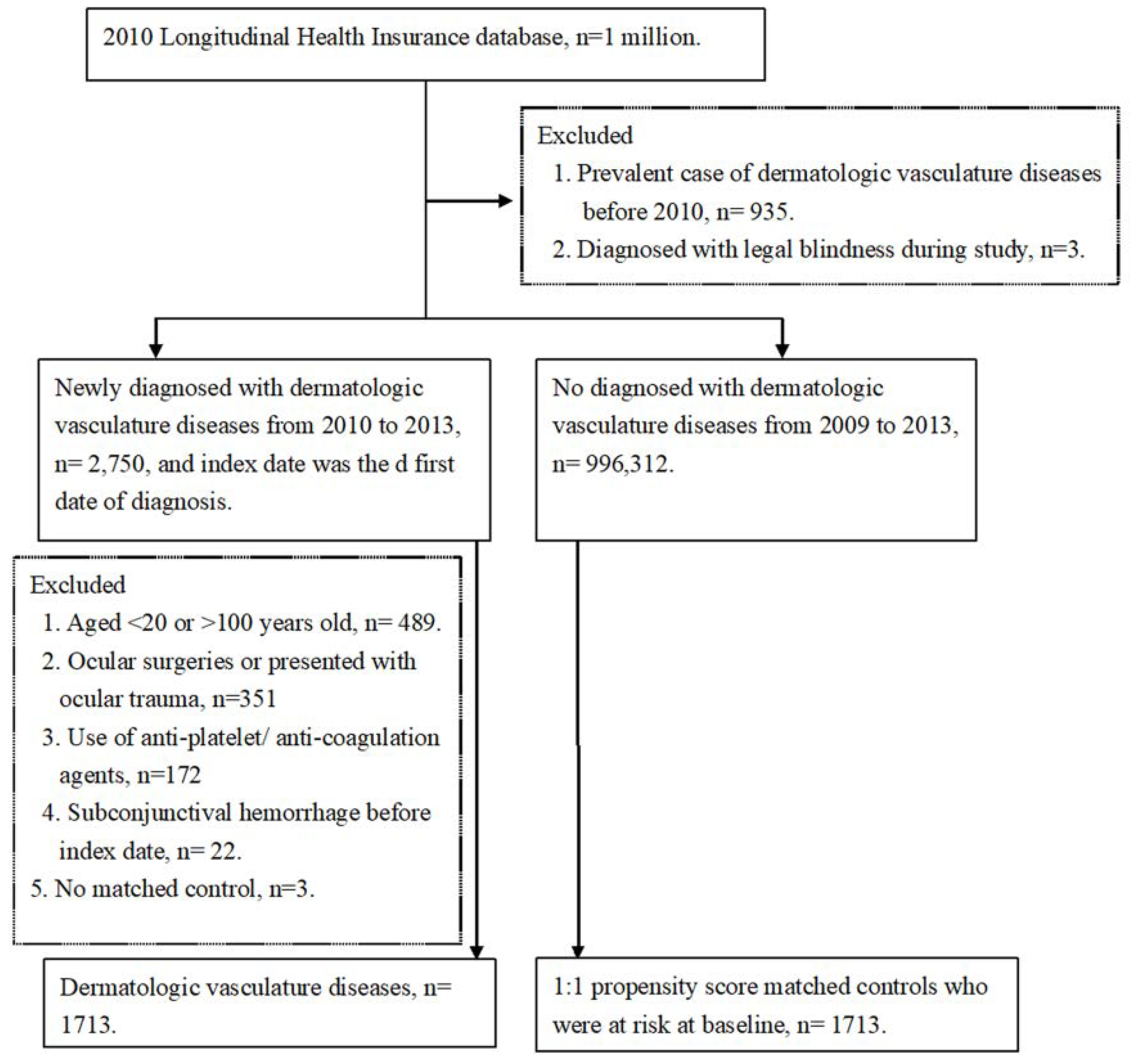
2. Materials and Methods
2.1. Data Source
2.2. Patient Selection
2.3. Main Outcome Measurement
2.4. Statistical Analysis
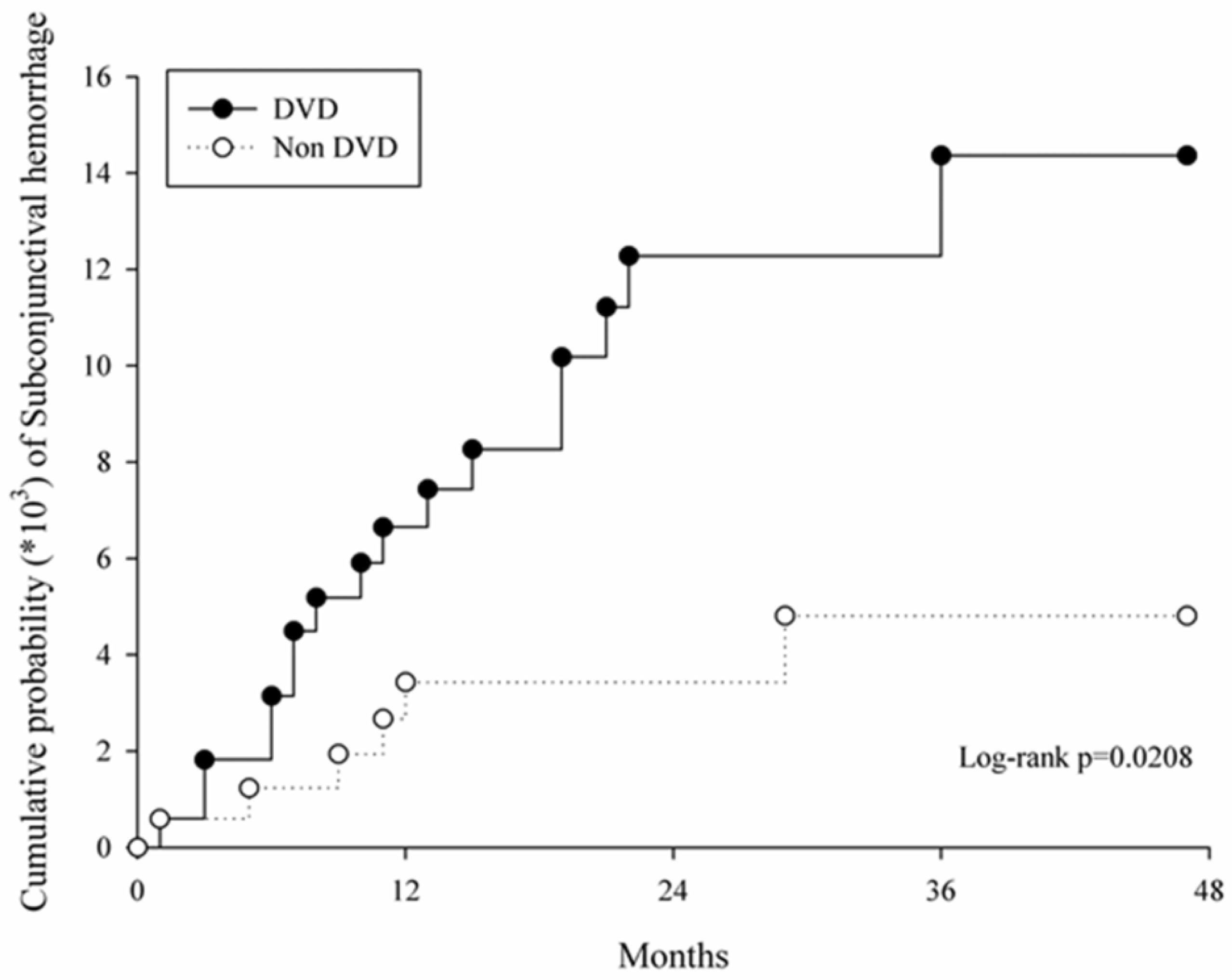
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hu, D.N.; Mou, C.H.; Chao, S.C.; Lin, C.Y.; Nien, C.W.; Kuan, P.T.; Jonas, J.B.; Sung, F.C. Incidence of non-traumatic subconjunctival hemorrhage in a nationwide study in taiwan from 2000 to 2011. PLoS ONE 2015, 10, e0132762. [Google Scholar] [CrossRef]
- Tarlan, B.; Kiratli, H. Subconjunctival hemorrhage: Risk factors and potential indicators. Clin. Ophthalmol. (Auckl. N.Z.) 2013, 7, 1163–1170. [Google Scholar]
- Mimura, T.; Yamagami, S.; Mori, M.; Funatsu, H.; Usui, T.; Noma, H.; Amano, S. Contact lens-induced subconjunctival hemorrhage. Am. J. Ophthalmol. 2010, 150, 656–665.e1. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Yokoi, N.; Ogata, M.; Shiraishi, A.; Yamaguchi, M.; Uno, T.; Inagaki, K.; Hayashi, K.; Kinoshita, S.; Ohashi, Y. Correlation between recurrent subconjunctival hemorrhages and conjunctivochalasis by clinical profile and successful surgical outcome. Eye Contact Lens 2015, 41, 367–372. [Google Scholar] [CrossRef]
- Mimura, T.; Usui, T.; Yamagami, S.; Funatsu, H.; Noma, H.; Honda, N.; Amano, S. Recent causes of subconjunctival hemorrhage. Ophthalmologica 2010, 224, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, J.; Zhang, J.; Dou, R.; Zhang, H.; Li, L.; Zhao, W.; Wei, P. Incidence and management of intraoperative complications during small-incision lenticule extraction in 3004 cases. J. Cataract Refract. Surg. 2017, 43, 796–802. [Google Scholar] [CrossRef]
- Pitts, J.F.; Jardine, A.G.; Murray, S.B.; Barker, N.H. Spontaneous subconjunctival haemorrhage–A sign of hypertension? Br. J. Ophthalmol. 1992, 76, 297–299. [Google Scholar] [CrossRef]
- Chijioke, A. Uremic bleeding with pericardial and subconjunctival hemorrhage. Saudi J. Kidney Dis. Transplant. 2011, 22, 1246–1248. [Google Scholar]
- Jain, S.; Goswami, A.; Singh, N.; Kaur, S. Bilateral eyelid ecchymosis and subconjunctival haemorrhage manifesting as presenting feature in a case of dengue haemorrhagic fever. Trop. Dr. 2015, 45, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Puca, E.; Pilaca, A.; Kalo, T.; Pipero, P.; Bino, S.; Hysenaj, Z.; Abazaj, E.; Gega, A.; Petrela, E.; Kraja, D. Ocular and cutaneous manifestation of leptospirosis acquired in albania: A retrospective analysis with implications for travel medicine. Travel Med. Infect. Dis. 2016, 14, 143–147. [Google Scholar] [CrossRef]
- Simpson, H.K.; Baird, J.; Allison, M.; Briggs, J.D.; Rowe, P.A.; Welsh, M.; Macdougall, A.I.; Grant, A.C.; Lowe, G.D.; Rumley, A.; et al. Long-term use of the low molecular weight heparin tinzaparin in haemodialysis. Haemostasis 1996, 26, 90–97. [Google Scholar]
- Tong, J.W.; Sawamura, M.H. Subconjunctival hemorrhages: Presenting sign for hereditary hemochromatosis. Optom. Vis. Sci. 2011, 88, 1133–1139. [Google Scholar] [CrossRef]
- Catt, C.J.; Hamilton, G.M.; Fish, J.; Mireskandari, K.; Ali, A. Ocular manifestations of stevens-johnson syndrome and toxic epidermal necrolysis in children. Am. J. Ophthalmol. 2016, 166, 68–75. [Google Scholar] [CrossRef]
- Schieving, J.H.; Schoenaker, M.H.D.; Weemaes, C.M.; van Deuren, M.; van der Flier, M.; Seyger, M.M.; Willemsen, M. Telangiectasias: Small lesions referring to serious disorders. Eur. J. Paediatr. Neurol. 2017, 21, 807–815. [Google Scholar] [CrossRef]
- Giblin, A.V.; Clover, A.J.; Athanassopoulos, A.; Budny, P.G. Pyogenic granuloma-the quest for optimum treatment: Audit of treatment of 408 cases. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2007, 60, 1030–1035. [Google Scholar] [CrossRef]
- Hochman, M. Infantile hemangiomas: Current management. Facial Plast. Surg. Clin. 2014, 22, 509–521. [Google Scholar] [CrossRef]
- Ruocco, E.; Ruocco, V.; Tornesello, M.L.; Gambardella, A.; Wolf, R.; Buonaguro, F.M. Kaposi’s sarcoma: Etiology and pathogenesis, inducing factors, causal associations, and treatments: Facts and controversies. Clin. Dermatol. 2013, 31, 413–422. [Google Scholar] [CrossRef]
- Quezada, A.A.; Shields, C.L.; Wagner, R.S.; Demirci, H.; Caputo, A.R.; Shields, J.A. Lymphangioma of the conjunctiva and nasal cavity in a child presenting with diffuse subconjunctival hemorrhage and nosebleeds. J. Pediatric Ophthalmol. Strabismus 2007, 44, 180–182. [Google Scholar] [CrossRef]
- Kiratli, H.; Uzun, S.; Tarlan, B.; Tanas, O. Recurrent subconjunctival hemorrhage due to cavernous hemangioma of the conjunctiva. Can. J. Ophthalmol. J. Can. Ophtalmol. 2012, 47, 318–320. [Google Scholar] [CrossRef]
- De Angelis, R.; Bugatti, L.; Del Medico, P.; Filosa, G. Hereditary benign telangiectasia: Videocapillaroscopic findings. Dermatology 2003, 206, 260–262. [Google Scholar] [CrossRef]
- Hurabielle, C.; Avouac, J.; Lepri, G.; de Risi, T.; Kahan, A.; Allanore, Y. Skin telangiectasia and the identification of a subset of systemic sclerosis patients with severe vascular disease. Arthritis Care Res. 2016, 68, 1021–1027. [Google Scholar] [CrossRef]
- Gupta, R.; Gautam, R.K.; Bhardwaj, M.; Chauhan, A. A clinical approach to diagnose patients with localized telangiectasia. Int. J. Dermatol. 2015, 54, e294–e301. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Choe, S.W.; Kim, Y.H.; Acharya, A.P.; Keselowsky, B.G.; Sorg, B.S.; Lee, Y.J.; Oh, S.P. Vegf neutralization can prevent and normalize arteriovenous malformations in an animal model for hereditary hemorrhagic telangiectasia 2. Angiogenesis 2014, 17, 823–830. [Google Scholar] [CrossRef]
- Khan, J.A.; Maki, R.G.; Ravi, V. Pathologic angiogenesis of malignant vascular sarcomas: Implications for treatment. J. Clin. Oncol. 2017, 36, 194–201. [Google Scholar] [CrossRef]
- Kaimbo Wa Kaimbo, D. Epidemiology of traumatic and spontaneous subconjunctival haemorrhages in congo. Bull. Soc. Belg. Ophtalmol. 2009, 311, 31–36. [Google Scholar]
- Demisse, A.G.; Greffie, E.S.; Abebe, S.M.; Bulti, A.B.; Alemu, S.; Abebe, B.; Mesfin, N. High burden of hypertension across the age groups among residents of gondar city in ethiopia: A population based cross sectional study. BMC Public Health 2017, 17, 647. [Google Scholar] [CrossRef]
- Hashemi, H.; Rastad, H.; Emamian, M.H.; Fotouhi, A. Conjunctivochalasis and related factors in an adult population of iran. Eye Contact Lens 2017, 44, s206–s209. [Google Scholar] [CrossRef]
| DVD n = 1713 | Control n = 1713 | ASD | |
|---|---|---|---|
| Age at baseline, Mean ± SD | 46.96 ± 14.45 | 46.27 ± 14.34 | 0.046 |
| <40 | 571(33.33%) | 606(35.38%) | |
| 40–59 | 821(47.93%) | 805(46.99%) | |
| ≥60 | 321(18.74%) | 302(17.63%) | |
| Sex | 0.043 | ||
| Female | 952(55.58%) | 978(57.09%) | |
| Male | 761(44.42%) | 735(42.91%) | |
| Urbanization | 0.062 | ||
| Urban | 1133(66.14%) | 1179(68.83%) | |
| Sub-urban | 448(26.15%) | 422(24.64%) | |
| Rural | 132(7.71%) | 112(6.54%) | |
| Low income | 18(1.05%) | 17(0.99%) | 0.006 |
| CCIs | 0.024 | ||
| 0 | 789(46.06%) | 801(46.76%) | |
| 1–2 | 660(38.53%) | 651(38.00%) | |
| ≥3 | 264(15.41%) | 261(15.24%) | |
| Co-morbidities | |||
| Corneal disease | 81(4.73%) | 74(4.32%) | 0.020 |
| Cataract | 93(5.43%) | 82(4.79%) | 0.029 |
| Glaucoma | 31(1.81%) | 24(1.40%) | 0.033 |
| AMD | 8(0.47%) | 2(0.12%) | 0.065 |
| Blepharitis | 29(1.69%) | 32(1.87%) | 0.013 |
| Chronic conjunctivitis | 314(18.33%) | 270(15.76%) | 0.068 |
| Noninfectious dermatitis of eyelid | 3(0.18%) | 2(0.12%) | 0.015 |
| Hypertension | 304(17.75%) | 295(17.22%) | 0.014 |
| DM | 145(8.46%) | 142(8.29%) | 0.006 |
| Renal disease | 49(2.86%) | 53(3.09%) | 0.014 |
| Incidence | DVD (n = 1713) | Control (n = 1713) |
|---|---|---|
| Follow up person months | 41,339 | 41,659 |
| Event of Subconjunctival hemorrhage | 131 | 98 |
| Incidence rate * (95% CI) | 3.17 (2.67–3.76) | 2.35 (1.93–2.87) |
| Crude HR (95% CI) | 2.85 (1.13–7.24) | Reference |
| Adjusted HR (95% CI) | 2.69 (1.05–6.84) | Reference |
| Subgroups | aHR | 95% CI | p Value |
|---|---|---|---|
| Sub-group-sex | |||
| Female | 2.97 | 0.94–9.36 | 0.0628 |
| Male | 3.89 | 0.60–25.11 | 0.1540 |
| Sub-group-age | |||
| <40 | Cannot estimated | - | - |
| 40–59 | 2.31 | 0.72–7.43 | 0.1595 |
| ≥60 | 2.17 | 0.4–11.91 | 0.3730 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-Y.; Chen, H.-C.; Huang, J.-Y.; Sun, C.-C.; Yeh, C.-B.; Lin, H.-Y.; Yang, S.-F. Dermatologic Vasculature Diseases as a Risk Factor of Subconjunctival Hemorrhage. Int. J. Environ. Res. Public Health 2019, 16, 2865. https://doi.org/10.3390/ijerph16162865
Lee C-Y, Chen H-C, Huang J-Y, Sun C-C, Yeh C-B, Lin H-Y, Yang S-F. Dermatologic Vasculature Diseases as a Risk Factor of Subconjunctival Hemorrhage. International Journal of Environmental Research and Public Health. 2019; 16(16):2865. https://doi.org/10.3390/ijerph16162865
Chicago/Turabian StyleLee, Chia-Yi, Hung-Chi Chen, Jing-Yang Huang, Chi-Chin Sun, Chao-Bin Yeh, Hung-Yu Lin, and Shun-Fa Yang. 2019. "Dermatologic Vasculature Diseases as a Risk Factor of Subconjunctival Hemorrhage" International Journal of Environmental Research and Public Health 16, no. 16: 2865. https://doi.org/10.3390/ijerph16162865






