Effects of αβ-Blocker Versus β1-Blocker Treatment on Heart Rate Response During Incremental Cardiopulmonary Exercise in Japanese Male Patients with Subacute Myocardial Infarction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Clinical Characteristics
2.3. Symptom-Limited CPX
2.4. Calculation of the Chronotropic Index
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Selecting Beta-Blocker Type for Subacute MI Patients
4.2. Differences in HR Response During CPX Among Subacute MI Patients Undergoing αβ-Blocker Versus β1-Blocker Treatment
4.3. Effects of αβ-Blocker Versus β1-Blocker Treatment on HR Response During CPX in Subacute MI Patients After Adjusting for Multiple Factors
4.4. Beta-Blocker Doses
4.5. Clinical Application of Study Findings
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [PubMed]
- JCS Joint Working Group. Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ. J. 2014, 78, 2022–2093. [Google Scholar] [CrossRef]
- Anderson, L.; Oldridge, N.; Thompson, D.R.; Zwisler, A.D.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2016, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K.; Beaver, W.L.; Whipp, B.J. Gas exchange theory and the lactic acidosis (anaerobic) threshold. Circulation 1990, 81, II14–II30. [Google Scholar] [PubMed]
- Adachi, H.; Koike, A.; Obayashi, T.; Umezawa, S.; Aonuma, K.; Inada, M.; Korenaga, M.; Niwa, A.; Marumo, F.; Hiroe, M. Does appropriate endurance exercise training improve cardiac function in patients with prior myocardial infarction? Eur. Heart J. 1996, 17, 1511–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brawner, C.A.; Ehrman, J.K.; Schairer, J.R.; Cao, J.J.; Keteyian, S.J. Predicting maximum heart rate among patients with coronary heart disease receiving beta-adrenergic blockade therapy. Am. Heart J. 2004, 148, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Buschmann, I.; Jaureguizar, K.V.; Calero, M.J.; Aquino, R.S. Programming exercise intensity in patients on beta-blocker treatment: The importance of choosing an appropriate method. Eur. J. Prev. Cardiol. 2014, 21, 1474–1480. [Google Scholar] [CrossRef]
- Tabet, J.Y.; Meurin, P.; Ben Driss, A.; Thabut, G.; Weber, H.; Renaud, N.; Odjinkem, N.; Solal, A.C. Determination of exercise training heart rate in patients on beta-blockers after myocardial infarction. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 538–543. [Google Scholar] [CrossRef]
- Dargie, H.J. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: The CAPRICORN randomised trial. Lancet 2001, 357, 1385–1390. [Google Scholar]
- Seo, G.W.; Kim, D.K.; Kim, K.H.; Seol, S.H.; Jin, H.Y.; Yang, T.H.; Ahn, Y.; Jeong, M.H.; Song, P.S.; Kim, D.I.; et al. Impact of Carvedilol versus beta1-selective beta blockers (bisoprolol, metoprolol, and nebivolol) in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Am. J. Cardiol. 2015, 116, 1502–1508. [Google Scholar] [CrossRef]
- Konishi, M.; Haraguchi, G.; Yoshikawa, S.; Kimura, S.; Inagaki, H.; Isobe, M. Additive Effects of β-Blockers on Renin-Angiotensin System Inhibitors for Patients After Acute Myocardial Infarction Treated with Primary Coronary Revascularization. Circ. J. 2011, 75, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Tanabe, K.; Yokoyama, Y.; Itoh, H.; Murayama, M. Influence of aerobic exercise training on brain natriuretic peptide secretion in patients in the chronic phase of myocardial infarction. Jpn. Circ. J. 1998, 62, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P. Clinical exercise testing. In American College of Sports Medicine, Resource Manual for Guidelines for Exercise Testing and Prescription; Thompson, W.R., Gordon, N.F., Pescatello, L.S., Eds.; Lea & Febiger: Philadelphia, PA, USA, 2012; pp. 205–222. [Google Scholar]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. (1985) 1986, 60, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Wilkoff, B.L.; Miller, R.E. Exercise testing for chronotropic assessment. Cardiol. Clin. 1992, 10, 705–717. [Google Scholar] [CrossRef]
- Takano, N.; Takano, H.; Fukuda, T.; Kikuchi, H.; Oguri, G.; Fukumura, K.; Iwasawa, K.; Nakajima, T. Relationship between chronotropic incompetence and beta-blockers based on changes in chronotropic response during cardiopulmonary exercise testing. Int. J. Cardiol. Heart Vasc. 2015, 6, 12–18. [Google Scholar] [PubMed]
- Brubaker, P.H.; Kitzman, D.W. Chronotropic incompetence: Causes, consequences, and management. Circulation 2011, 123, 1010–1020. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Japanese Circulation Society web. Guidelines for Secondary Prevention of Myocardial Infarction (JCS 2006). Available online: http://www.circ.or.jp/guideline/pdf/JCS2006_ishikawa_h.pdf (accessed on 21 June 2019).
- White, D.W.; Raven, P.B. Autonomic neural control of heart rate during dynamic exercise: Revisited. J. Physiol. 2014, 592, 2491–2500. [Google Scholar] [CrossRef]
- Bigger, J.T.; Fleiss, J.L.; Rolnitzky, L.M.; Steinman, R.C.; Schneider, W.J. Time course of recovery of heart period variability after myocardial infarction. J. Am. Coll. Cardiol. 1991, 18, 1643–1649. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, E.; Palau, P.; Nunez, E.; Ramon, J.M.; Lopez, L.; Melero, J.; Bellver, A.; Santas, E.; Chorro, F.J.; Nunez, J. Heart rate response and functional capacity in patients with chronic heart failure with preserved ejection fraction. ESC Heart Fail. 2018, 5, 579–585. [Google Scholar] [CrossRef]
- Robergs, R.; Landwehr, R. The surprising history of the ‘‘HRmax = 220-age’’ equation. J. Exerc. Physiol. Online 2002, 5, 1–10. [Google Scholar]
- Kasahara, Y.; Izawa, K.; Omiya, K.; Osada, N.; Watanabe, S.; Saitoh, M.; Matsunaga, A.; Masuda, T. Influence of autonomic nervous dysfunction characterizing effect of diabetes mellitus on heart rate response and exercise capacity in patients undergoing cardiac rehabilitation for acute myocardial infarction. Circ. J. 2006, 70, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Anjos-Andrade, F.D.; Sousa, A.C.; Barreto-Filho, J.A.; Alves, E.O.; Nascimento-Junior, A.C.; De Santana, N.O.; De Vasconcelos, F.L.; Garcez, F.B.; De Araujo, V.P.; De Araujo, A.C.; et al. Chronotropic incompetence and coronary artery disease. Acta Cardiol. 2010, 65, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, B.; Donato, M.; Perez, V.; Deutsch, A.C.R.; Hocht, C.; Del Mauro, J.S.; Rodriguez, M.; Gelpi, R.J. Changes in the loading conditions induced by vagal stimulation modify the myocardial infarct size through sympathetic-parasympathetic interactions. Pflugers Arch. 2015, 467, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, A.; Kodani, E.; Takano, H.; Uchida, T.; Sato, N.; Ibuki, C.; Kusama, Y.; Seino, Y.; Munakata, K.; Mizuno, K.; et al. Secondary preventive effects of a calcium antagonist for ischemic heart attack: Randomized parallel comparison with beta–blockers. Circ. J. 2011, 75, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Kawana, J.; Ohno, T.; Hanada, K.; Kaneko, M.; Mihara, K.; Shiomi, M.; Nagayama, M.; Sumiyoshi, T.; Ogata, H. Population pharmacokinetics of R- and S-carvedilol in Japanese patients with chronic heart failure. Biol. Pharm. Bull. 2010, 33, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Hakusui, H.; Fujimaki, M. Pharmacokinetics of carvedilol in hypertensive patients with renal failure. Drugs 1988, 36, 144–147. [Google Scholar] [CrossRef]
- Neugebauer, G.; Gabor, M.; Reiff, K. Pharmacokinetics and bio-availability of carvedilol in patients with liver cirrhosis. Drugs 1988, 36, 148–154. [Google Scholar] [CrossRef]
- Nemoto, S.; Kasahara, Y.; Izawa, K.P.; Watanabe, S.; Yoshizawa, K.; Takeichi, N.; Kamiya, K.; Suzuki, N.; Omiya, K.; Matsunaga, A.; et al. Effect of carvedilol on heart rate response to cardiopulmonary exercise up to the anaerobic threshold in patients with subacute myocardial infarction. Heart Vessel. 2019, 34, 957–964. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | αβ-Blocker Group (n = 67) | β1-Blocker Group (n = 17) | No-β-Blocker Group (n = 47) | p-Value |
|---|---|---|---|---|
| Age (years) | 64.98 ± 10.48 | 61.59 ± 8.91 | 61.62 ± 10.83 | 0.184 |
| Body mass index (kg/m2) | 23.44 ± 2.78 | 23.58 ± 3.27 | 23.12 ± 3.10 | 0.804 |
| MI | 0.621 | |||
| Inferior | 23 (37.3) | 7 (41.2) | 24 (51.1) | |
| Anterior | 34 (50.8) | 9 (52.9) | 19 (40.4) | |
| Lateral | 8 (11.9) | 1 (5.9) | 4 (8.5) | |
| Residual coronary artery stenosis | 30 (44.9) | 11 (64.7) | 19 (40.4) | 0.221 |
| Medical history | ||||
| Prior MI | 4 (5.8) | 2 (11.8) | 4 (8.5) | 0.696 |
| Hypertension | 48 (71.6) | 13 (76.5) | 31 (66.0) | 0.673 |
| Dyslipidemia | 39 (58.2) | 12 (70.6) | 30 (63.8) | 0.605 |
| Chronic kidney disease | 7 (10.5) | 2 (11.8) | 6 (12.8) | 0.929 |
| Diabetes mellitus | 19 (28.4) | 4 (23.5) | 19 (40.4) | 0.287 |
| Orthopedic disorder | 2 (3.0) | 1 (6.3) | 0 (0) | 0.309 |
| Cerebrovascular disease | 1 (1.5) | 0 (0) | 3 (6.4) | 0.241 |
| Respiratory disease | 2 (3.0) | 0 (0) | 1 (2.1) | 0.760 |
| Hyperuricemia | 7 (10.5) | 3 (17.7) | 8 (17.0) | 0.533 |
| Peripheral arterial disease | 1 (1.5) | 1 (5.9) | 1 (2.1) | 0.555 |
| Dementia | 0 (0) | 0 (0) | 1 (2.1) | 0.406 |
| Heart failure after MI | 6 (9.0) | 1 (5.9) | 5 (10.6) | 0.841 |
| Medication | ||||
| Diuretic | 9 (13.4) | 2 (11.8) | 4 (8.5) | 0.718 |
| Renin-angiotensin system inhibitor | 63 (94.0) | 12 (70.6) | 41 (87.2) | 0.024 |
| Calcium antagonist | 12 (17.9) | 1 (5.9) | 3 (6.4) | 0.125 |
| Aldosterone antagonist | 5 (7.5) | 3 (17.6) | 2 (4.3) | 0.204 |
| Anticlotting drug | 2 (3.0) | 1 (5.9) | 1 (2.1) | 0.742 |
| Antiplatelet drug | 67 (100) | 17 (100) | 47 (100) | 1.00 |
| αβ-blocker, Carvedilol | 67 (100) | - | - | - |
| β1-blocker, Bisoprolol/Atenolol | - | 11 (64.7)/6 (35.3) | - | - |
| Beta-blocker dose | ||||
| Carvedilol (mg/day) | 5.48 ± 2.99 | - | - | - |
| Bisoprolol/Atenolol (mg/day) | - | 2.50 ± 1.40/33.33 ± 12.91 | - | - |
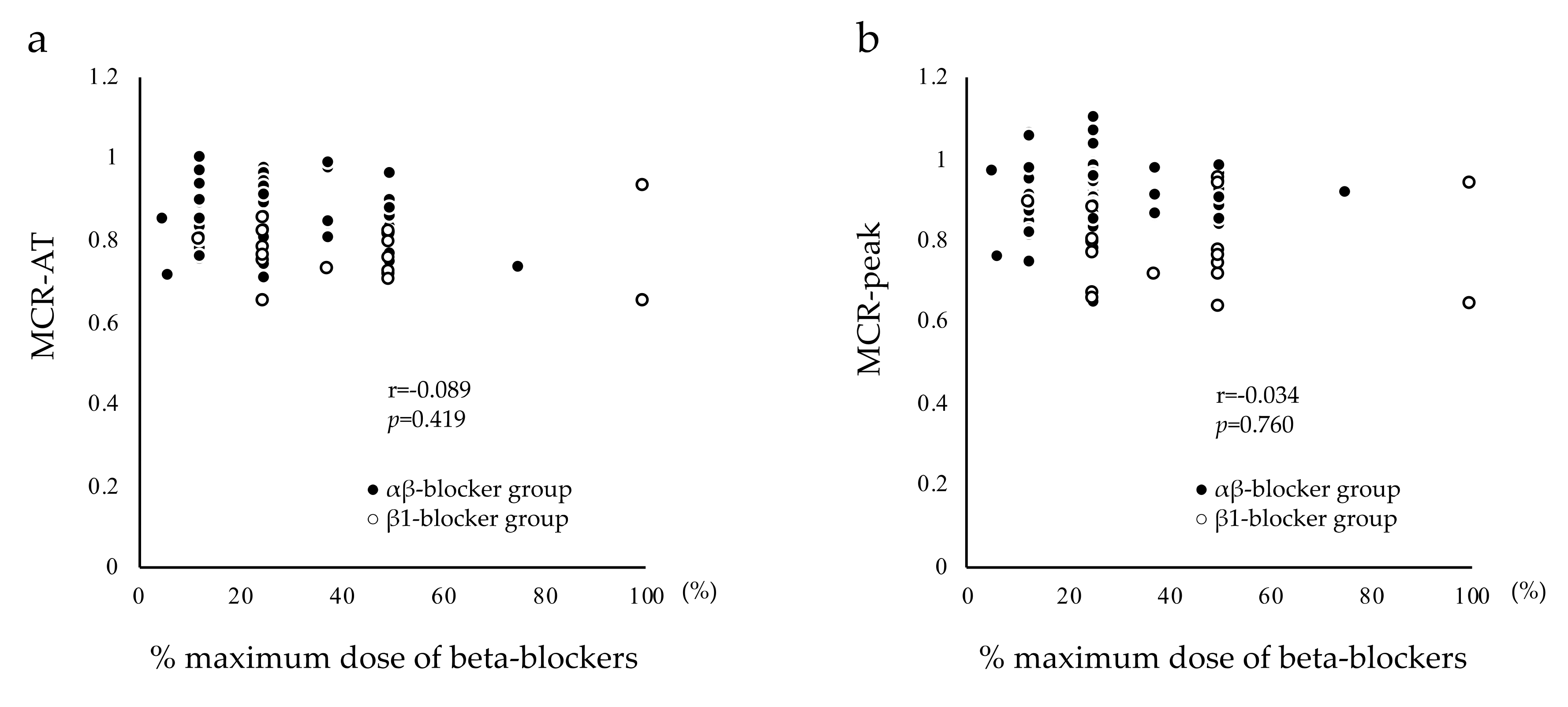
| % maximum dose of beta-blocker (%) | 27.41 ± 14.94 | 44.12 ± 24.65 | - | <0.001 |
| Max CK-MB (ng/ml) | 306.70 ± 228.05 | 232.01 ± 214.07 | 275.43 ± 225.89 | 0.477 |
| Log max CK-MB | 5.40 ± 0.90 | 5.03 ± 1.08 | 5.18 ± 0.10 | 0.262 |
| LVEF (%) | 52.91 ± 8.81 | 57.94 ± 13.60 | 54.34 ± 12.28 | 0.230 |
| Time between MI and CPX (days) | 32.09 ± 11.50 | 27.76 ± 8.72 | 31.19 ± 12.35 | 0.387 |
| Hospitalization before 2006 | 7 (10.4%) | 10 (58.8%) | 40 (85.1%) | <0.001 |
| Cardiopulmonary Exercise Testing Data | β-Blocker Group (n = 67) | β1-Blocker Group (n = 17) | No-β-Blocker Group (n = 47) | F-Value | p-Value |
|---|---|---|---|---|---|
| Average time of exercise (min) | 7.03 ± 1.57 | 7.27 ± 1.63 | 7.53 ± 1.61 | 1.372 | 0.402 |
| AT (ml/kg/min) | 15.92 ± 3.24 | 14.72 ± 2.24 | 15.91 ± 2.73 | 1.226 | 0.297 |
| Peak VO2 (ml/kg/min) | 23.27 ± 5.32 | 21.47 ± 3.72 | 23.66 ± 4.42 | 1.302 | 0.276 |
| RERAT | 0.89 ± 0.05 | 0.89 ± 0.05 | 0.89 ± 0.07 | 0.033 | 0.968 |
| RERpeak | 1.20 ± 0.07 | 1.20 ± 0.07 | 1.18 ± 0.06 | 2.086 | 0.128 |
| HRrest (bpm) | 71.49 ± 9.86 †† | 66.65 ± 11.14 †† | 79.02 ± 11.39 | 11.147 | <0.001 |
| HRAT (bpm) | 105.76 ± 11.19 ‡‡ | 95.47 ± 10.20 †† | 111.19 ± 12.24 § | 11.940 | <0.001 |
| HRpeak (bpm) | 138.76 ± 14.59 ‡‡ | 123.77 ± 18.80 †† | 148.38 ± 16.21 §§ | 15.865 | <0.001 |
| SBPrest (mmHg) | 127.43 ± 16.14 † | 129.77 ± 18.91 | 119.64 ± 17.23 | 3.734 | 0.027 |
| SBPAT (mmHg) | 158.49 ± 21.23 | 151.77 ± 27.66 | 149.30 ± 29.78 | 1.904 | 0.153 |
| SBPpeak (mmHg) | 185.93 ± 28.23 | 180.00 ± 29.19 | 183.40 ± 32.46 | 0.295 | 0.745 |
| DBPrest (mmHg) | 77.03 ± 12.16 | 84.29 ± 10.73 †† | 74.02 ± 10.39 | 5.099 | 0.007 |
| DBPAT (mmHg) | 76.00 ± 17.21 | 80.24 ± 10.13 | 76.81 ± 11.83 | 0.575 | 0.564 |
| DBPpeak (mmHg) | 84.51 ± 18.20 | 85.65 ± 15.96 | 82.77 ± 12.83 | 0.2576 | 0.773 |
| ΔAT HR (bpm) | 34.27 ± 8.79 ‡ | 28.82 ± 8.10 | 32.17 ± 7.76 | 3.098 | 0.049 |
| Δpeak HR (bpm) | 67.27 ± 13.43 ‡ | 57.12 ± 13.97 † | 69.36 ± 16.44 | 4.464 | 0.013 |
| ΔAT-peak HR (bpm) | 32.76 ± 9.99 | 28.29 ± 11.46 † | 37.41 ± 12.11 | 4.965 | 0.008 |
| MCR-AT | 0.85 ± 0.07 ‡‡ | 0.77 ± 0.07 †† | 0.87 ± 0.07 | 13.122 | <0.001 |
| MCR-peak | 0.90 ± 0.08 ‡‡ | 0.78 ± 0.11 †† | 0.94 ± 0.09 § | 19.205 | <0.001 |
| Independent Variables | Dependent Variable: MCR-AT | |||
|---|---|---|---|---|
| B ± SE | β | 95% CI of B | p-Value | |
| Inferior infarct * | −0.002 ± 0.012 | −0.025 | −0.025 to 0.021 | 0.865 |
| Anterior infarct * | −0.001 ± 0.011 | −0.001 | −0.023 to 0.022 | 0.946 |
| Residual coronary artery stenosis | −0.009 ± 0.007 | −0.117 | −0.022 to 0.004 | 0.170 |
| Heart failure after MI | −0.007 ± 0.011 | −0.053 | −0.029 to 0.015 | 0.518 |
| Diabetes mellitus | 0.001 ± 0.007 | 0.016 | −0.012 to 0.015 | 0.836 |
| Renin-angiotensin system inhibitor | −0.004 ± 0.010 | −0.031 | −0.025 to 0.017 | 0.719 |
| αβ-blocker treatment ** | −0.008 ± 0.010 | −0.108 | −0.028 to 0.011 | 0.394 |
| β1-blocker treatment ** | −0.050 ± 0.011 | −0.432 | −0.071 to −0.028 | <0.001 |
| Hospitalization before 2006 | 0.000 ± 0.001 | 0.003 | −0.018 to 0.018 | 0.979 |
| Constant | 0.807 ± 0.016 | 0.000 | 0.776 to 0.839 | <0.001 |
| Coefficient of determination R2 = 0.187, F = 3.092, p = 0.002 | ||||
| Independent Variables | Dependent Variable: MCR-Peak | |||
|---|---|---|---|---|
| B ± SE | β | 95% CI of B | p-Value | |
| Inferior infarct * | −0.002 ± 0.015 | −0.017 | −0.031 to 0.027 | 0.907 |
| Anterior infarct * | −0.001 ± 0.001 | −0.007 | −0.029 to 0.028 | 0.962 |
| Residual coronary artery stenosis | −0.012 ± 0.008 | −0.114 | −0.028 to 0.005 | 0.166 |
| Heart failure after MI | 0.002 ± 0.015 | 0.012 | −0.026 to 0.031 | 0.883 |
| Diabetes mellitus | 0.004 ± 0.009 | 0.038 | −0.013 to 0.021 | 0.642 |
| Log max CK-MB | 0.018 ± 0.024 | 0.067 | −0.029 to 0.064 | 0.449 |
| LVEF | −0.027 ± 0.025 | −0.100 | −0.077 to 0.021 | 0.266 |
| Renin-angiotensin system inhibitor | 0.003 ± 0.013 | 0.021 | −0.023 to 0.030 | 0.800 |
| αβ-blocker treatment ** | −0.019 ± 0.012 | −0.185 | −0.043 to 0.006 | 0.134 |
| β1-blocker treatment ** | −0.071 ± 0.014 | −0.473 | −0.099 to −0.044 | <0.001 |
| Hospitalization before 2006 | −0.004 ± 0.012 | −0.043 | −0.019 to 0.027 | 0.706 |
| Constant | 0.844 ± 0.020 | 0.000 | 0.840 to 0.884 | <0.001 |
| Coefficient of determination R2 = 0.263, F = 3.865, p < 0.001 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemoto, S.; Kasahara, Y.; Izawa, K.P.; Watanabe, S.; Yoshizawa, K.; Takeichi, N.; Kamiya, K.; Suzuki, N.; Omiya, K.; Matsunaga, A.; et al. Effects of αβ-Blocker Versus β1-Blocker Treatment on Heart Rate Response During Incremental Cardiopulmonary Exercise in Japanese Male Patients with Subacute Myocardial Infarction. Int. J. Environ. Res. Public Health 2019, 16, 2838. https://doi.org/10.3390/ijerph16162838
Nemoto S, Kasahara Y, Izawa KP, Watanabe S, Yoshizawa K, Takeichi N, Kamiya K, Suzuki N, Omiya K, Matsunaga A, et al. Effects of αβ-Blocker Versus β1-Blocker Treatment on Heart Rate Response During Incremental Cardiopulmonary Exercise in Japanese Male Patients with Subacute Myocardial Infarction. International Journal of Environmental Research and Public Health. 2019; 16(16):2838. https://doi.org/10.3390/ijerph16162838
Chicago/Turabian StyleNemoto, Shinji, Yusuke Kasahara, Kazuhiro P. Izawa, Satoshi Watanabe, Kazuya Yoshizawa, Naoya Takeichi, Kentaro Kamiya, Norio Suzuki, Kazuto Omiya, Atsuhiko Matsunaga, and et al. 2019. "Effects of αβ-Blocker Versus β1-Blocker Treatment on Heart Rate Response During Incremental Cardiopulmonary Exercise in Japanese Male Patients with Subacute Myocardial Infarction" International Journal of Environmental Research and Public Health 16, no. 16: 2838. https://doi.org/10.3390/ijerph16162838
APA StyleNemoto, S., Kasahara, Y., Izawa, K. P., Watanabe, S., Yoshizawa, K., Takeichi, N., Kamiya, K., Suzuki, N., Omiya, K., Matsunaga, A., & Akashi, Y. J. (2019). Effects of αβ-Blocker Versus β1-Blocker Treatment on Heart Rate Response During Incremental Cardiopulmonary Exercise in Japanese Male Patients with Subacute Myocardial Infarction. International Journal of Environmental Research and Public Health, 16(16), 2838. https://doi.org/10.3390/ijerph16162838






