Proactive Telephone Smoking Cessation Counseling Tailored to Parents: Results of a Randomized Controlled Effectiveness Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Recruitment
2.2.1. Mass Media Approach
2.2.2. Health Care Approach
2.3. Sample
2.4. Data Collection
2.5. Treatment Conditions
2.5.1. Telephone Counseling (Intervention Condition)
2.5.2. Self-help Brochure (Control Condition)
2.6. Measurements
2.6.1. Primary Outcome
2.6.2. Secondary Outcomes
2.7. Statistical Analyses
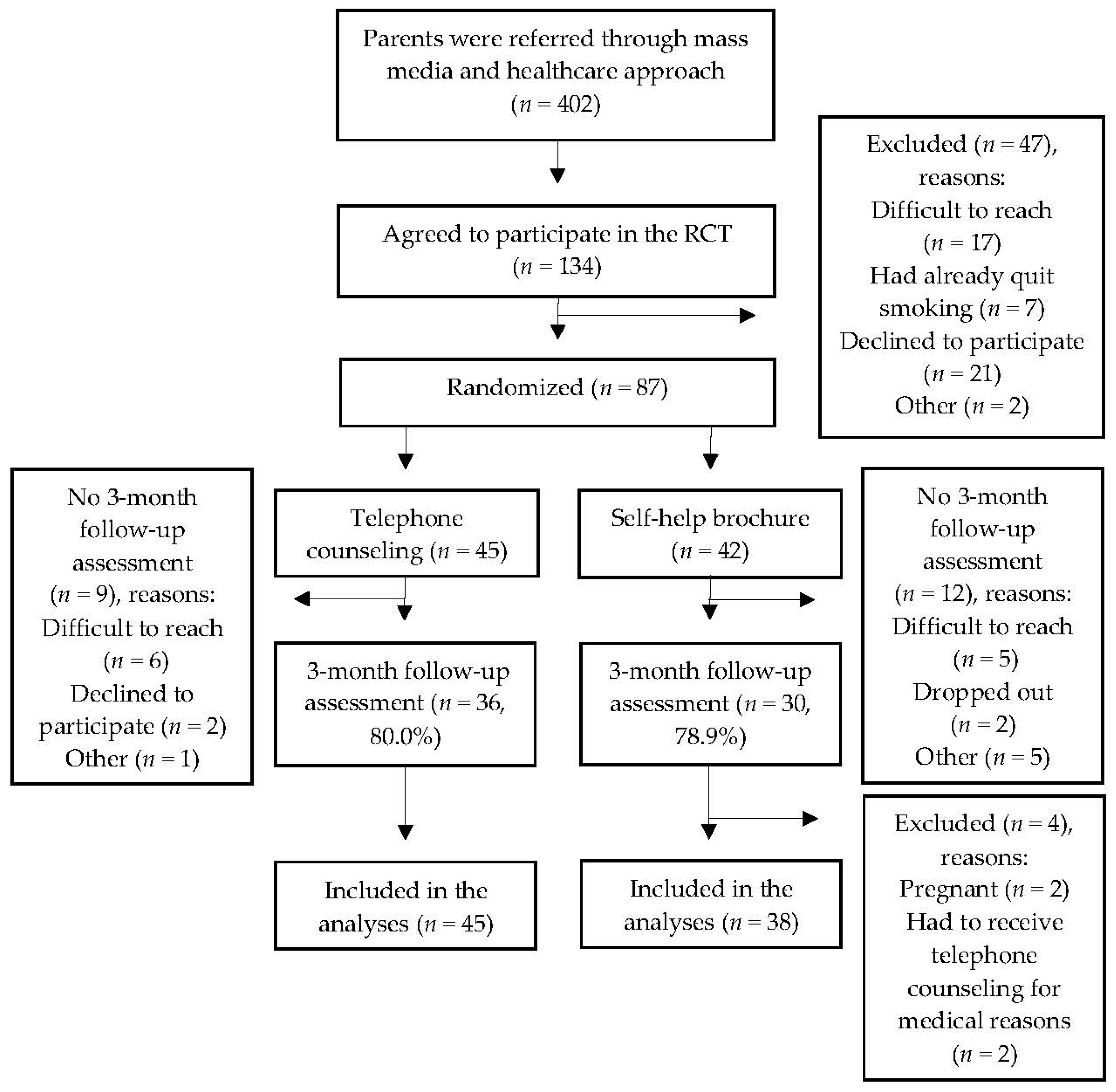
3. Results
3.1. Characteristics of the Participants
3.2. Outcomes
3.2.1. Smoking Cessation Rates
3.2.2. Use of NRT and Smoking Cessation Medication
3.2.3. Secondary Outcomes among Parents Who did not Report Abstinence
4. Discussion
4.1. Strengths and Limitations
4.2. Implications for Practice and Suggestions for Further Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Öberg, M.; Jaakkola, M.S.; Woodward, A.; Peruga, A.; Prüss-Ustün, A. Worldwide Burden of Disease from Exposure to Second-hand Smoke: A Retrospective Analysis of Data from 192 Countries. Lancet 2011, 377, 139–146. [Google Scholar] [CrossRef]
- Hofhuis, W.; De Jongste, J.C.; Merkus, M. Adverse health effects of prenatal and postnatal tobacco smoke exposure on children. Arch. Dis. Child. 2003, 88, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2006; ISBN 0-16-076152-2.
- Difranza, J.R.; Aligne, C.A.; Weitzman, M. Prenatal and Postnatal Environmental Tobacco Smoke Exposure and Children’s Health. Pediatrics 2004, 113, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.; Cheung, Y.T.; Fong, D.Y.; Emmons, K.; Leung, A.Y.; Leung, D.Y.; Lam, T.H. Family-based Smoking Cessation Intervention for Smoking Fathers and Nonsmoking Mothers with a Child: A Randomized Controlled Trial. J. Pediatr. 2017, 182, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Bricker, J.B.; Leroux, B.G.; Peterson, A.V.; Kealey, K.A.; Sarason, I.G.; Andersen, M.R.; Marek, P.M. Nine-year Prospective Relationship between Parental Smoking Cessation and Children’s Daily Smoking. Addiction 2003, 98, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.S.; Mak, Y.W.; Loke, A.Y.; Lam, T.H. Smoking Cessation Intervention in Parents of Young Children: A Randomised Controlled Trial. Addiction 2005, 100, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Severson, H.H.; Andrews, J.A.; Lichtenstein, E.; Wall, M.; Akers, L. Reducing Maternal Smoking and Relapse: Long-term Evaluation of a Pediatric Intervention. Prev. Med. (Baltim.) 1997, 26, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, B.; McQuaid, E.L.; Tooley, E.M.; Busch, A.M.; Hammond, S.K.; Becker, B.; Dunsiger, S. Motivating Parents of Kids with Asthma to Quit Smoking: The Effect of the Teachable Moment and Increasing Intervention Intensity Using a Longitudinal Randomized Trial Design. Addiction 2016, 111, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, B.; McQuaid, E.L.; Novak, S.P.; Hammond, S.K.; Becker, B. Motivating Latino Caregivers of Children with Asthma to Quit Smoking: A Randomized Trial. J. Consult. Clin. Psychol. 2010, 78, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Curry, S.J.; Ludman, E.J.; Graham, E.; Stout, J.; Grothaus, L.; Lozano, P. Pediatric-based Smoking Cessation Intervention for Low-income Women: A Randomized Trial. Arch. Pediatr. Adolesc. Med. 2003, 157, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Groner, J.A.; Ahijevych, K.; Grossman, L.K.; Rich, L.N. The Impact of a Brief Intervention on Maternal Smoking Behavior. Pediatrics 2000, 105, 267–271. [Google Scholar] [PubMed]
- Ralston, S.; Roohi, M.A. Randomized, Controlled Trial of Smoking Cessation Counseling Provided during Child Hospitalization for Respiratory Illness. Pediatr. Pulmonol. 2008, 43, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.; Grohman, C.; Word, D.; Williams, J. A Randomized Trial of a Brief Intervention to Promote Smoking Cessation for Parents during Child Hospitalization. Pediatr. Pulmonol. 2013, 48, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Schuck, K.; Bricker, J.B.; Otten, R.; Kleinjan, M.; Brandon, T.H.; Engels, R.C. Effectiveness of Proactive Quitline Counselling for Smoking Parents Recruited through Primary Schools: Results of a Randomized Controlled Trial. Addiction 2014, 109, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Flay, B.R. Efficacy and Effectiveness Trials (and Other Phases of Research) in the Development of Health Promotion Programs. Prev. Med. (Baltim.) 1986, 15, 451–474. [Google Scholar] [CrossRef]
- Flay, B.R.; Biglan, A.; Boruch, R.F.; Castro, F.G.; Gottfredson, D.; Kellam, S.; Mo´scicki, E.K.; Schinke, S.; Valentine, J.C.; Ji, P. Standards of Evidence: Criteria for Efficacy, Effectiveness and Dissemination. Prev. Sci. 2005, 6, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.; Leung, G.M.; Wong, D.C.; Lam, T.H. Helping Chinese Fathers Quit Smoking Through Educating Their Nonsmoking Spouses: A Randomized Controlled Trial. Am. J. Health Promot. 2008, 23, 31–34. [Google Scholar] [CrossRef]
- Hannöver, W.; Thyrian, J.R.; Röske, K.; Grempler, J.; Rumpf, H.J.; John, U.; Hapke, U. Smoking Cessation and Relapse Prevention for Postpartum Women: Results from a Randomized Controlled Trial at 6, 12, 18 and 24 Months. Addict. Behav. 2009, 34, 1–8. [Google Scholar] [CrossRef]
- Mahabee-Gittens, E.M.; Gordon, J.S.; Krugh, M.E.; Henry, B.; Leonard, A.C. A Smoking Cessation Intervention Plus Proactive Quitline Referral in the Pediatric Emergency Department: A Pilot Study. Nicotine Tob. Res. 2008, 10, 1745–1751. [Google Scholar] [CrossRef]
- Yu, S.; Duan, Z.; Redmon, P.B.; Eriksen, M.P.; Koplan, J.P.; Huang, C. mHealth Intervention is Effective in Creating Smoke-free Homes for Newborns: A Randomized Controlled Trial Study in China. Sci. Rep. 2017, 7, 9276. [Google Scholar] [CrossRef]
- Caldwell, A.L.; Tingen, M.S.; Nguyen, J.T.; Andrews, J.O.; Heath, J.; Waller, J.L.; Treiber, F.A. Parental Smoking Cessation: Impacting Children’s Tobacco Smoke Exposure in the Home. Pediatrics 2018, 141, S96–S105. [Google Scholar] [CrossRef] [PubMed]
- Winickoff, J.P.; Hillist, V.J.; Palfrey, J.S.; Perrin, J.M.; Rigotti, N.A. A Smoking Cessation Intervention for Parents of Children Who Are Hospitalized for Respiratory Illness: The Stop Tobacco Outreach Program. Pediatrics 2003, 111, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Bernet, A.C.; Willens, D.E.; Bauer, M.S. Effectiveness-implementation Hybrid Designs: Implications for Quality Improvement Science. Implement. Sci. 2013, 8, S2. [Google Scholar] [CrossRef]
- Curran, G.M.; Bauer, M.; Mittman, B.; Pyne, J.M.; Stetler, C. Effectiveness-implementation Hybrid Design: Combining Elements of Clinical Effectiveness and Implementation Research to Enhance Public Health. Med. Care 2012, 50, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Scheffers-van Schayck, T.; Otten, R.; Engels, R.; Kleinjan, M. Evaluation and Implementation of a Proactive Telephone Smoking Cessation Counseling for Parents: A Study Protocol of an Effectiveness Implementation Hybrid Design. Int. J. Environ. Res. Public Health 2018, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Heckman, C.J.; Egleston, B.L.; Hofmann, M.T. Efficacy of Motivational Interviewing for Smoking Cessation. Tob. Control 2011, 19, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Hettema, J.E.; Hendricks, P.S. Motivational Interviewing for Smoking Cessation: A Meta-analytic Review. J. Consult. Clin. Psychol. 2010, 78, 868–884. [Google Scholar] [CrossRef] [PubMed]
- West, R. Assessing Smoking Cessation Performance in NHS Stop Smoking Services: The Russell Standard (Clinical); Cancer Research UK and University College London: London, UK, 2005. [Google Scholar]
- Hyland, A.; Higbee, C.; Travers, M.J.; Van Deusen, A.; Bansal-Travers, M.; King, B.; Cummings, K.M. Smoke-free Homes and Smoking Cessation and Relapse in a Longitudinal Population of Adults. Nicotine Tob. Res. 2009, 11, 614–618. [Google Scholar] [CrossRef]
- Winickoff, J.P.; Healey, E.A.; Regan, S.; Park, E.R.; Cole, C.; Friebely, J.; Rigotti, N.A. Using the Postpartum Hospital Stay to Address Mothers’ and Fathers’ Smoking: The NEWS Study. Pediatrics 2010, 125, 518–525. [Google Scholar] [CrossRef] [PubMed]
- McClure, J.B.; Greene, S.M.; Wiese, C.; Johnson, K.E.; Alexander, G.; Strecher, V. Interest in an Online Smoking Cessation Program and Effective Recruitment Strategies: Results from Project Quit. J. Med. Internet Res. 2006, 8, e14. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.M.; Cleland, J. Effectiveness Versus Efficacy: More Than a Debate Over Language. J. Orthop. Sport. Phys.Ther. 2003, 33, 163–165. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total Sample (N = 83) | Telephone Counseling (n = 45) | Self-Help Brochure (n = 38) | |
|---|---|---|---|---|
| Age (mean, SD) | 39.2 (7.20) | 39.3 (7.66) | 39.1 (6.71) | |
| Gender % (n) | ||||
| Male | 42.2 (35) | 46.7 (21) | 36.8 (14) | |
| Nationality % (n) | ||||
| Dutch | 94.0 (78) | 93.3 (42) | 94.7 (36) | |
| Educational level % (n) | ||||
| Low | 27.7 (23) | 28.9 (13) | 26.3 (10) | |
| Medium | 47.0 (39) | 46.7 (21) | 47.4 (18) | |
| High | 25.3 (21) | 24.4 (11) | 26.3 (10) | |
| Marital status % (n) | ||||
| Married/living together | 71.1 (59) | 73.3 (33) | 68.4 (26) | |
| Divorced/widowed and now single | 7.2 (6) | 4.4 (2) | 10.5 (4) | |
| Single | 16.9 (14) | 17.8 (8) | 15.8 (6) | |
| Employment status % (n) | ||||
| Unemployed | 18.1 (15) | 20.0 (9) | 15.8 (6) | |
| Homemaker | 7.2 (6) | 6.7 (3) | 7.9 (3) | |
| Paid employment | 74.7 (62) | 73.3 (33) | 76.3 (29) | |
| Medical treatment % (n) | 15.7 (13) | 15.6 (7) | 15.8 (6) | |
| Cardiovascular disease % (n) | 7.2 (6) | 11.1 (5) | 2.6 (1) | |
| Chronic respiratory illness % (n) | 12.0 (10) | 13.3 (6) | 10.5 (4) | |
| Chronic respiratory illness child % (n) | 45.8 (38) | 40.0 (18) | 52.6 (20) | |
| Cigarettes per day (mean, SD) | 15.5 (6.67) | 15.0 (5.48) | 16.0 (7.88) | |
| Years of smoking (mean, SD) | 20.4 (7.39) | 21.1 (8.0) | 19.6 (6.66) | |
| FTND score (mean, SD) | 4.3 (2.29) | 4.4 (2.19) | 4.3 (2.45) | |
| Complete home smoking ban % (n) | 83.1 (69) | 86.7 (39) | 78.9 (30) | |
| Partner smoking % (n) | ||||
| Yes | 34.9 (29) | 33.3 (15) | 36.8 (14) | |
| Smoking Cessation Outcomes | Telephone Counseling % (n) | Self-Help Brochure % (n) | OR (95% CI) * |
|---|---|---|---|
| Primary outcome | |||
| 7-day PPA at 3-month FU | 53.3 (24) | 13.2 (5) | 7.54 (2.49–22.84) |
| Secondary outcomes | |||
| 24 h PPA at 3-month FU | 60.0 (27) | 18.4 (7) | 6.64 (2.41–18.31) |
| 14-day PPA at 4-week after the designated quit date | 55.6 (25) | 13.2 (5) | 8.23 (2.72–25.02) |
| Smoking Cessation Outcomes | Telephone Counseling % (n) | Self-Help Brochure % (n) | OR (95% CI) | Adjusted OR (95% CI) 1 | |||
|---|---|---|---|---|---|---|---|
| Health Care (n = 29) | Mass Media (n = 16) | Health Care (n = 24) | Mass Media (n = 14) | ||||
| Primary outcome | |||||||
| 7-day PPA at 3-month FU | 37.9 (11) | 81.3 (13) | 12.5 (3) | 14.3 (2) | 2.79 (1.09–7.14) | 2.90 (0.88–9.56) | |
| Secondary outcomes | |||||||
| 24 h PPA at 3-month FU | 48.3 (14) | 81.3 (13) | 16.7 (4) | 21.4 (3) | 2.22 (0.89 –5.55) | 1.63 (0.52–5.04) | |
| 14-day PPA at 4-week after the designated quit date | 44.8 (13) | 75.0 (12) | 8.3 (2) | 21.4 (3) | 2.53 (0.997–6.44) | 1.91 (0.60–6.03) | |
| Secondary Outcomes | Telephone Counseling | Self-Help Brochure | p | |
|---|---|---|---|---|
| Increase in motivation to quit during the study (M, SE) | −1.17 (0.76) | −1.00 (0.42) | >0.05 | |
| Number of quit attempts during the study (M, SE) | 2.2 (0.37) | 1.7 (0.21) | >0.05 | |
| Duration of longest quit attempt during the study * | >0.05 | |||
| Less than one week % (n) | 25.0 (4) | 48.3 (14) | ||
| One week or more % (n) | 75.0 (12) | 51.7 (15) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheffers-van Schayck, T.; Otten, R.; Engels, R.C.M.E.; Kleinjan, M. Proactive Telephone Smoking Cessation Counseling Tailored to Parents: Results of a Randomized Controlled Effectiveness Trial. Int. J. Environ. Res. Public Health 2019, 16, 2730. https://doi.org/10.3390/ijerph16152730
Scheffers-van Schayck T, Otten R, Engels RCME, Kleinjan M. Proactive Telephone Smoking Cessation Counseling Tailored to Parents: Results of a Randomized Controlled Effectiveness Trial. International Journal of Environmental Research and Public Health. 2019; 16(15):2730. https://doi.org/10.3390/ijerph16152730
Chicago/Turabian StyleScheffers-van Schayck, Tessa, Roy Otten, Rutger C.M.E. Engels, and Marloes Kleinjan. 2019. "Proactive Telephone Smoking Cessation Counseling Tailored to Parents: Results of a Randomized Controlled Effectiveness Trial" International Journal of Environmental Research and Public Health 16, no. 15: 2730. https://doi.org/10.3390/ijerph16152730
APA StyleScheffers-van Schayck, T., Otten, R., Engels, R. C. M. E., & Kleinjan, M. (2019). Proactive Telephone Smoking Cessation Counseling Tailored to Parents: Results of a Randomized Controlled Effectiveness Trial. International Journal of Environmental Research and Public Health, 16(15), 2730. https://doi.org/10.3390/ijerph16152730





