3D-Analysis of Mouth, Nose and Eye Parameters in Children with Fetal Alcohol Syndrome (FAS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting and Participants
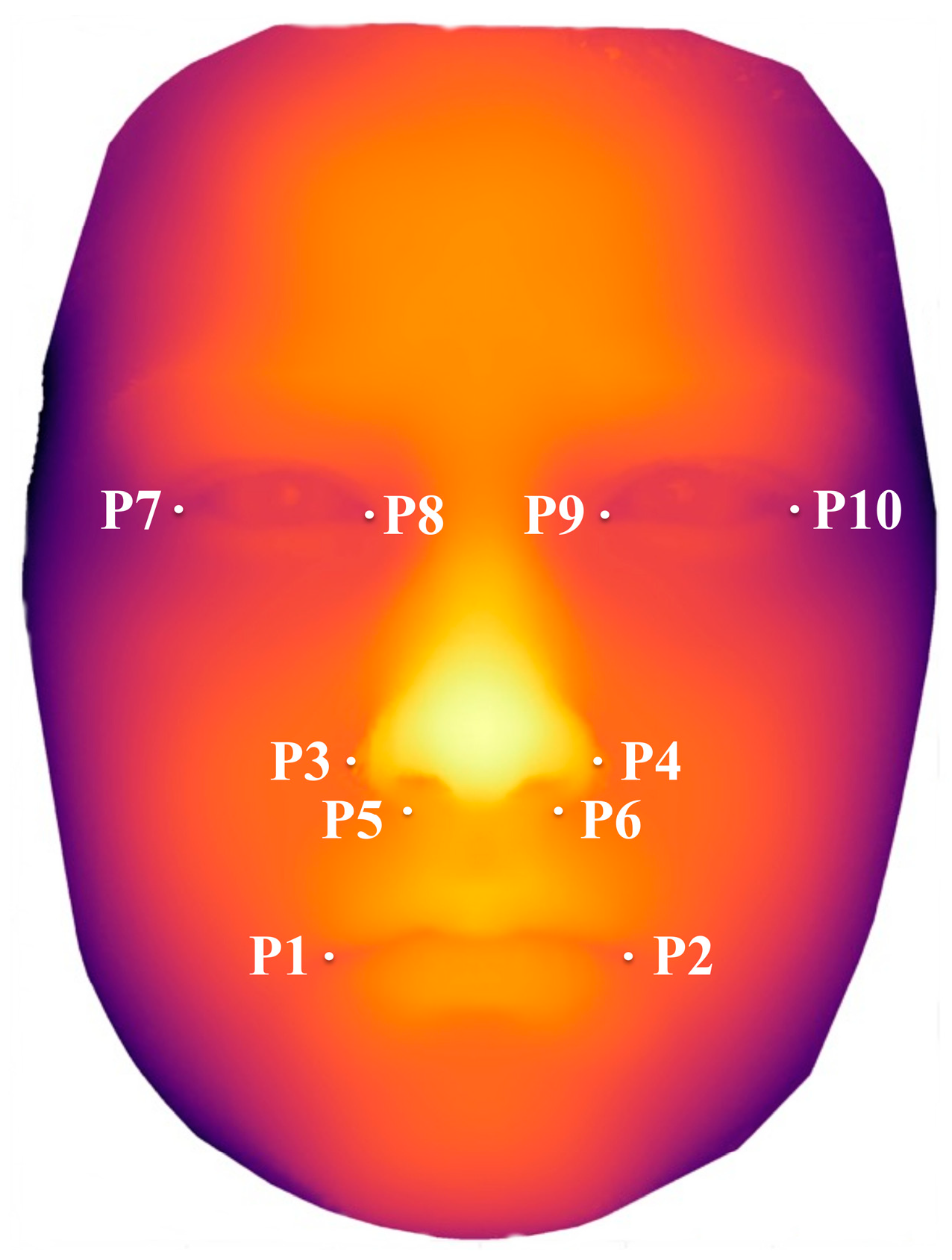
2.2. Variables and Data Sources/Management
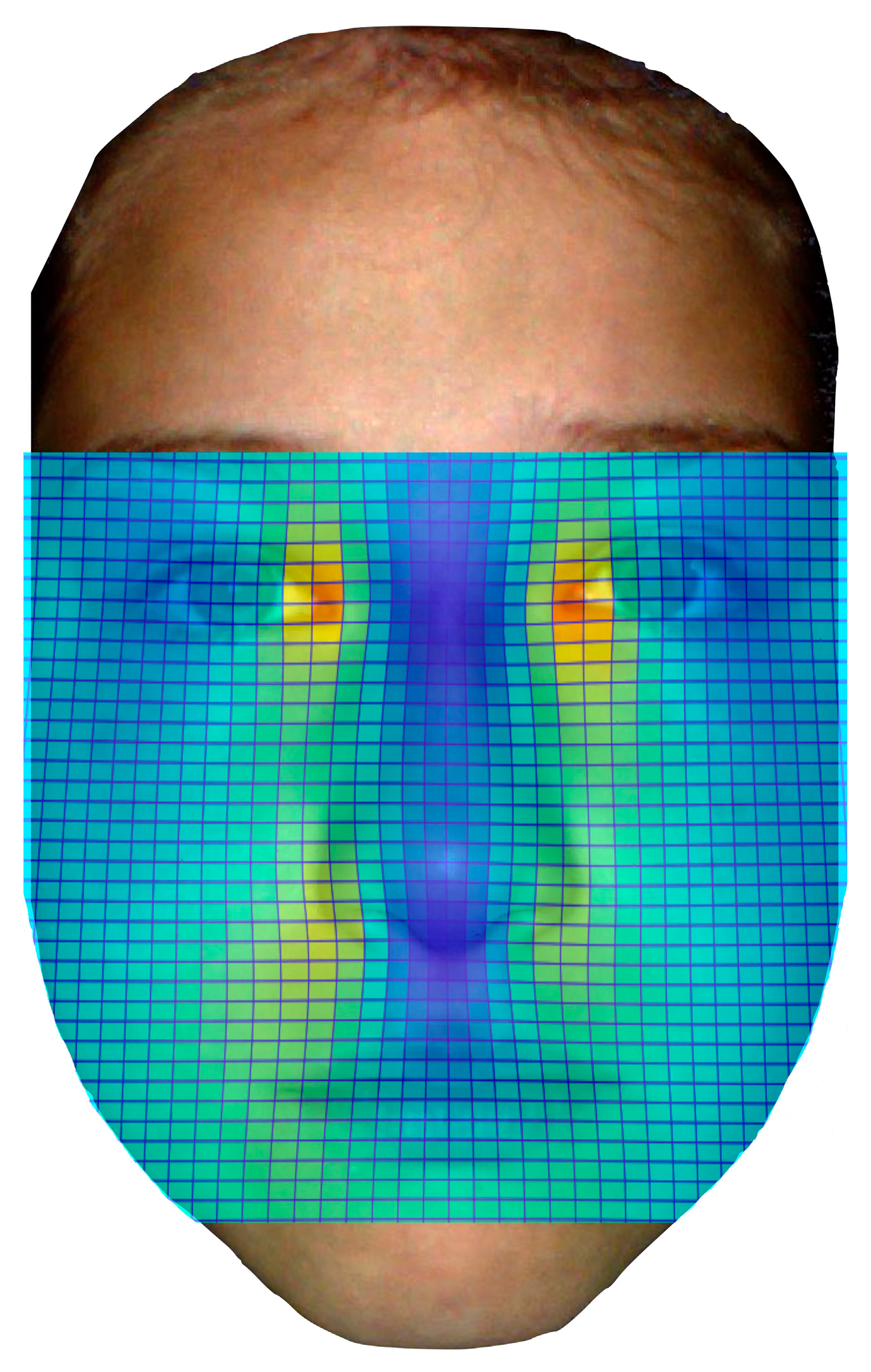
2.3. Heat Mapping
2.4. Bias
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
3.1. Study Participants
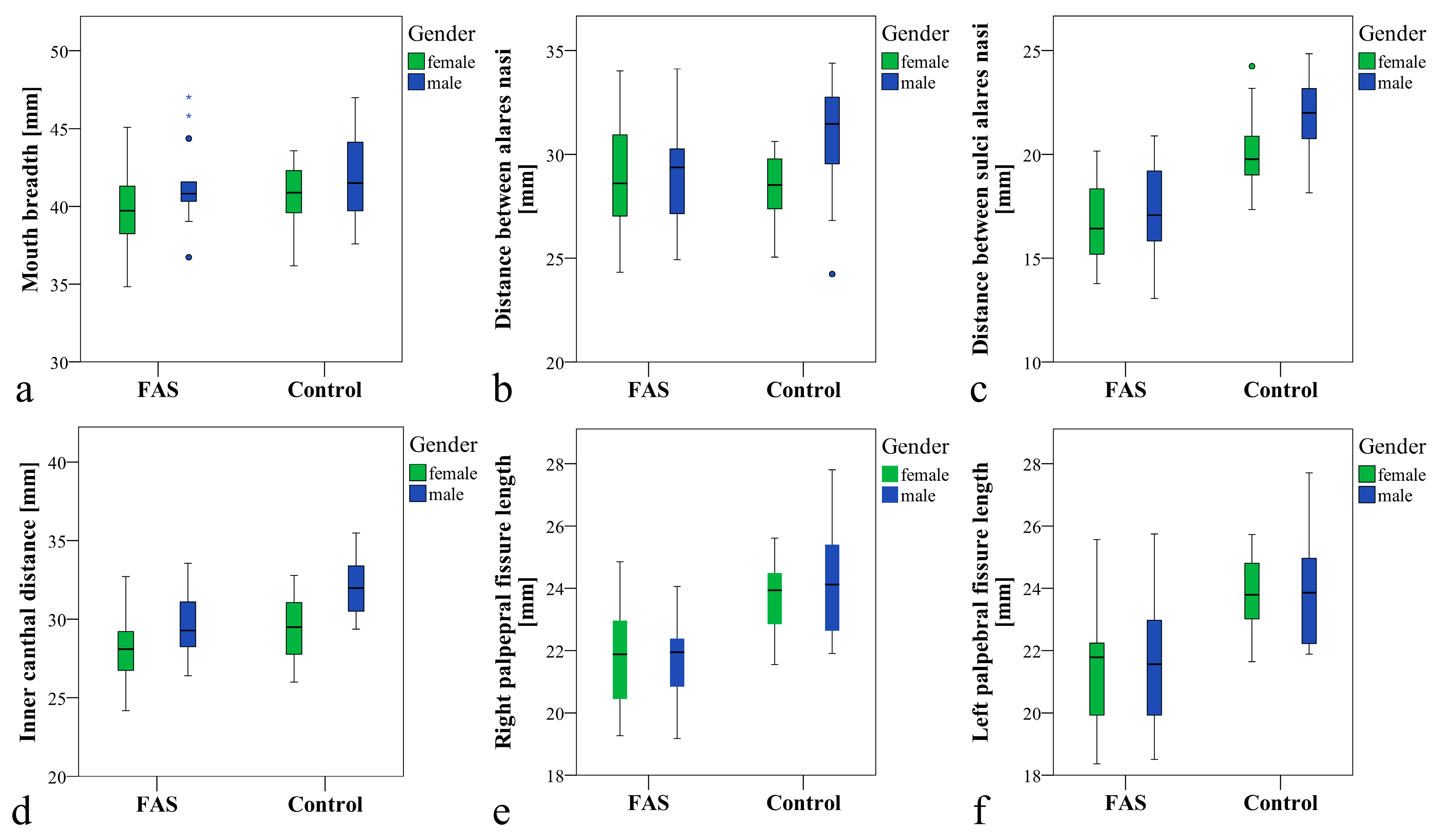
3.2. Main Results
3.3. Sensitivity and Specificity
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Landgraf, M.N.; Albers, L.; Rahmsdorf, B.; Vill, K.; Gerstl, L.; Lippert, M.; Heinen, F. Fetal alcohol spectrum disorders (FASD)—What we know and what we should know—The knowledge of German health professionals and parents. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2018, 22, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Chasnoff, I.J.; Wells, A.M.; King, L. Misdiagnosis and missed diagnoses in foster and adopted children with prenatal alcohol exposure. Pediatrics 2015, 135, 264–270. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Chambers, C.D.; Kalberg, W.O.; Zellner, J.; Feldman, H.; Buckley, D.; Kopald, D.; Hasken, J.M.; Xu, R.; Honerkamp-Smith, G.; et al. Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. JAMA 2018, 319, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Streissguth, A.P. A Long-Term Perspective of FAS. Alcohol Health Res. World 1994, 18, 74–81. [Google Scholar]
- Greenbaum, R.; Koren, G. Fetal alcohol spectrum disorder—New diagnostic initiatives. Paediatr. Child Health 2002, 7, 139–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muggli, E.; Matthews, H.; Penington, A.; Claes, P.; O’Leary, C.; Forster, D.; Donath, S.; Anderson, P.J.; Lewis, S.; Nagle, C.; et al. Association Between Prenatal Alcohol Exposure and Craniofacial Shape of Children at 12 Months of Age. JAMA Pediatrics 2017, 171, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Probst, C.; Gmel, G.; Rehm, J.; Burd, L.; Popova, S. Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth: A Systematic Review and Meta-analysis. JAMA Pediatrics 2017, 171, 948–956. [Google Scholar] [CrossRef]
- Stockwell, T.; Zhao, J.; Sherk, A.; Rehm, J.; Shield, K.; Naimi, T. Underestimation of alcohol consumption in cohort studies and implications for alcohol's contribution to the global burden of disease. Addiction 2018, 113, 2245–2249. [Google Scholar] [CrossRef]
- Ponce de Leon, R.G.; Ewerling, F.; Serruya, S.J.; Silveira, M.F.; Sanhueza, A.; Moazzam, A.; Becerra-Posada, F.; Coll, C.V.N.; Hellwig, F.; Victora, C.G.; et al. Contraceptive use in Latin America and the Caribbean with a focus on long-acting reversible contraceptives: Prevalence and inequalities in 23 countries. Lancet. Glob. Health 2019, 7, e227–e235. [Google Scholar] [CrossRef]
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. Lancet. Global Health 2017, 5, e290–e299. [Google Scholar] [CrossRef]
- Popova, S.; Lange, S.; Chudley, A.E.; Reynolds, J.N.; Rehm, J. World Health Organization International Study on the Prevalence of Fetal Alcohol Spectrum Disorder (FASD). 2018. Available online: https://canfasd.ca/wp-content/uploads/sites/35/2018/05/2018-Popova-WHO-FASD-Prevalance-Report.pdf (accessed on 14 July 2019).
- Coles, C.D.; Gailey, A.R.; Mulle, J.G.; Kable, J.A.; Lynch, M.E.; Jones, K.L. A Comparison Among 5 Methods for the Clinical Diagnosis of Fetal Alcohol Spectrum Disorders. Alcohol. Clin. Exp. Res. 2016, 40, 1000–1009. [Google Scholar] [CrossRef]
- Astley, S.J. Diagnostic Guide for Fetal Alcohol Spectrum Disorders: The 4- Digit Diagnostic Code., 3rd ed.; University of Washington Publication Services: Seattle, WA, USA, 2004. [Google Scholar]
- Astley, S.J.; Clarren, S.K. Diagnosing the full spectrum of fetal alcohol-exposed individuals: introducing the 4-digit diagnostic code. Alcohol Alcohol. 2000, 35, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Astley, S.J.; Clarren, S.K. Measuring the facial phenotype of individuals with prenatal alcohol exposure: Correlations with brain dysfunction. Alcohol Alcohol. 2001, 36, 147–159. [Google Scholar] [CrossRef]
- Blanck-Lubarsch, M.; Dirksen, D.; Feldmann, R.; Sauerland, C.; Kirschneck, C.; Hohoff, A. 3D Analysis of Philtrum Depth in Children with Fetal Alcohol Syndrome. Alcohol Alcohol. 2019, 54, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, M.N.; Nothacker, M.; Heinen, F. Diagnosis of fetal alcohol syndrome (FAS): German guideline version 2013. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2013, 17, 437–446. [Google Scholar] [CrossRef]
- Bischoff, G.; Borocz, Z.; Proll, C.; Kleinheinz, J.; von Bally, G.; Dirksen, D. Modular optical topometric sensor for 3D acquisition of human body surfaces and long-term monitoring of variations. Biomed. Technik. Biomed. Eng. 2007, 52, 284–289. [Google Scholar] [CrossRef]
- De Berg, M.; Cheong, O.; Van Kreveld, M.; Overmars, M. Computational Geometry: Algorithms and Applications; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Astley, S.J. Canadian palpebral fissure length growth charts reflect a good fit for two school and FASD clinic-based U.S. populations. J. Popul. Ther. Clin. Pharmacol. 2011, 18, e231–e241. [Google Scholar]
- Clarren, S.K.; Chudley, A.E.; Wong, L.; Friesen, J.; Brant, R. Normal distribution of palpebral fissure lengths in Canadian school age children. Can. J. Clin. Pharmacol. 2010, 17, e67–e78. [Google Scholar]
- Stromland, K.; Chen, Y.; Norberg, T.; Wennerstrom, K.; Michael, G. Reference values of facial features in Scandinavian children measured with a range-camera technique. Scand. J. Plast. Reconstr. Surg. Hand. Surg. 1999, 33, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Prahl-Andersen, B.; Ligthelm-Bakker, A.S.; Wattel, E.; Nanda, R. Adolescent growth changes in soft tissue profile. Am. J. Orthod. Dentofac. Orthop Off. Publ. Am. Assoc. Orthod. Const. Soc. Am. Board Orthod. 1995, 107, 476–483. [Google Scholar] [CrossRef]
- Nanda, R.S.; Meng, H.; Kapila, S.; Goorhuis, J. Growth changes in the soft tissue facial profile. Angle Orthod. 1990, 60, 177–190. [Google Scholar]
- Blanck-Lubarsch, M.; Flieger, S.; Feldmann, R.; Kirschneck, C.; Sauerland, C.; Hohoff, A. Malocclusion Can Give Additional Hints for Diagnosis of Fetal Alcohol Spectrum Disorder. Alcohol Alcohol. 2018, 54, 56–61. [Google Scholar] [CrossRef]
- Laestadius, N.D.; Aase, J.M.; Smith, D.W. Normal inner canthal and outer orbital dimensions. J. Pediatrics 1969, 74, 465–468. [Google Scholar] [CrossRef]
- Douglas, T.S.; Meintjes, E.M.; Vaughan, C.L.; Viljoen, D.L. Role of depth in eye distance measurements: Comparison of single and stereo-photogrammetry. Am. J. Hum. Biol. 2003, 15, 573–578. [Google Scholar] [CrossRef]
- Valentine, M.; Bihm, D.C.J.; Wolf, L.; Hoyme, H.E.; May, P.A.; Buckley, D.; Kalberg, W.; Abdul-Rahman, O.A. Computer-Aided Recognition of Facial Attributes for Fetal Alcohol Spectrum Disorders. Pediatrics 2017, 140, e20162028. [Google Scholar] [CrossRef]
- Suttie, M.; Foroud, T.; Wetherill, L.; Jacobson, J.L.; Molteno, C.D.; Meintjes, E.M.; Hoyme, H.E.; Khaole, N.; Robinson, L.K.; Riley, E.P.; et al. Facial dysmorphism across the fetal alcohol spectrum. Pediatrics 2013, 131, e779–e788. [Google Scholar] [CrossRef]
- Modabber, A.; Peters, F.; Kniha, K.; Goloborodko, E.; Ghassemi, A.; Lethaus, B.; Holzle, F.; Mohlhenrich, S.C. Ev aluation of the accuracy of a mobile and a stationary system for three-dimensional facial scanning. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2016, 44, 1719–1724. [Google Scholar] [CrossRef]

| Total | FAS-Group | C-Group | p Value | |
|---|---|---|---|---|
| Gender | 0.309 2 | |||
| Male | 32 | 14 | 18 | |
| Female | 26 | 14 | 12 | |
| Age at examination, years | 0.171 1 | |||
| Mean (SD) | 8.4 (1.6) | 8.7 (1.4) | 8.2 (1.8) | |
| Median (Range) | 8.3 (5.8–11.9) | 8.4 (6.6–11.2) | 7.6 (5.8–11.9) | |
| Mouth breadth (P1–P2), mm | 0.267 1 | |||
| Mean (SD) | 40.9 (2.8) | 40.5 (2.9) | 41.3 (2.7) | |
| Median (Range) | 40.8 (34.8–47.0) | 40.7 (34.8–47.0) | 41.2 (36.2–47.0) | |
| Mean (SD) female | 40.1 (2.5) | 39.6 (2.7) | 40.7 (2.2) | 0.252 1 |
| Median (Range) female | 40.6 (34.8–45.1) | 39.7 (34.8–45.1) | 40.9 (36.2–43.6) | |
| Mean (SD) male | 41.6 (2.8) | 41.5 (2.8) | 41.6 (2.9) | 0.767 1 |
| Median (Range) male | 41.3 (36.7–47.0) | 40.8 (36.7–47.0) | 41.5 (37.6–47.0) | |
| Breadth between alares nasi (P3–P4), mm | 0.260 1 | |||
| Mean (SD) | 29.5 (2.6) | 29.1 (2.6) | 29.9 (2.6) | |
| Median (Range) | 29.5 (24.2–34.4) | 29.0 (24.3–34.1) | 29.6 (24.2–34.4) | |
| Mean (SD) female | 28.8 (2.3) | 29.0 (2.8) | 28.5 (1.7) | 0.595 1 |
| Median (Range) female | 28.6 (24.3–34.0) | 28.6 (24.3–34.0) | 28.5 (25.1–30.6) | |
| Mean (SD) male | 30.2 (2.7) | 29.2 (2.5) | 30.9 (2.7) | 0.106 1 |
| Median (Range) male | 30.2 (24.2–34.4) | 29.4 (24.9–34.1) | 31.5 (24.2–34.4) | |
| Breadth between sulci alares (P5–P6), mm | <0.001 1 | |||
| Mean (SD) | 19.2 (3.0) | 16.9 (2.2) | 21.2 (2.0) | |
| Median (Range) | 19.7 (13.1–24.9) | 16.5 (13.1–20.9) | 20.9 (17.4–24.9) | |
| Mean (SD) female | 18.3 (2.7) | 16.7 (2.0) | 20.2 (1.9) | <0.001 1 |
| Median (Range) female | 18.5 (13.8–24.3) | 16.4 (13.8–20.2) | 19.8 (17.4–24.3) | |
| Mean (SD) male | 20.0 (3.1) | 17.2 (2.4) | 21.9 (1.8) | <0.0011 |
| Median (Range) male | 20.7 (13.1–24.9) | 17.1 (13.1–20.9) | 22.0 (18.2–24.9) | |
| Inner canthal distance(P8–P9), mm | 0.001 1 | |||
| Mean (SD) | 30.0 (2.5) | 28.8 (2.3) | 31.0 (2.3) | |
| Median (Range) | 29.7 (24.2–35.5) | 28.7 (24.2–33.6) | 30.8 (26.0–35.5) | |
| Mean (SD) female | 28.7 (2.3) | 28.0 (2.8) | 29.4 (2.3) | 0.145 1 |
| Median (Range) female | 28.6 (24.2–32.8) | 28.1 (24.2–32.7) | 29.5 (26–32.8) | |
| Mean (SD) male | 31.0 (2.2) | 29.7 (2.0) | 32.0 (1.8) | 0.004 1 |
| Median (Range) male | 30.8 (26.4–35.5) | 29.3 (26.4–33.6) | 32.0 (29.4–35.5) | |
| Palpebral fissure length right(P7–P8), mm | <0.001 1 | |||
| Mean (SD) | 22.9 (1.9) | 21.7 (1.5) | 24.0 (1.6) | |
| Median (Range) | 22.8 (19.2–27.8) | 21.9 (19.2–24.9) | 24.1 (21.6–27.8) | |
| Mean (SD) female | 22.6 (1.8) | 21.6 (1.6) | 23.7 (1.2) | 0.003 1 |
| Median (Range) female | 22.9 (19.3–25.6) | 21.9 (19.3–24.9) | 23.9 (21.6–25.6) | |
| Mean (SD) male | 23.1 (2.0) | 21.8 (1.4) | 24.1 (1.8) | 0.001 1 |
| Median (Range) male | 22.7 (19.2–27.8) | 21.9 (19.2–24.1) | 24.1 (21.9–27.8) | |
| Palpebral fissure length left (P9–P10), mm | <0.001 1 | |||
| Mean (SD) | 22.8 (2.1) | 21.6 (2.0) | 23.9 (1.6) | |
| Median (Range) | 22.4 (18.4–27.7) | 21.8 (18.4–25.7) | 23.9 (21.6–27.7) | |
| Mean (SD) female | 22.6 (1.9) | 21.5 (1.7) | 23.8 (1.3) | 0.001 1 |
| Median (Range) | 22.3 (18.4–25.7) | 21.8 (18.4–25.6) | 23.8 (21.6–25.7) | |
| Mean (SD) female | 23.0 (2.3) | 21.6 (2,2) | 24.0 (1.9) | 0.004 1 |
| Median (Range) male | 22.9 (18.5–27.7) | 21.6 (18.5–25.7) | 23.9 (21.9–27.7) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanck-Lubarsch, M.; Dirksen, D.; Feldmann, R.; Sauerland, C.; Hohoff, A. 3D-Analysis of Mouth, Nose and Eye Parameters in Children with Fetal Alcohol Syndrome (FAS). Int. J. Environ. Res. Public Health 2019, 16, 2535. https://doi.org/10.3390/ijerph16142535
Blanck-Lubarsch M, Dirksen D, Feldmann R, Sauerland C, Hohoff A. 3D-Analysis of Mouth, Nose and Eye Parameters in Children with Fetal Alcohol Syndrome (FAS). International Journal of Environmental Research and Public Health. 2019; 16(14):2535. https://doi.org/10.3390/ijerph16142535
Chicago/Turabian StyleBlanck-Lubarsch, Moritz, Dieter Dirksen, Reinhold Feldmann, Cristina Sauerland, and Ariane Hohoff. 2019. "3D-Analysis of Mouth, Nose and Eye Parameters in Children with Fetal Alcohol Syndrome (FAS)" International Journal of Environmental Research and Public Health 16, no. 14: 2535. https://doi.org/10.3390/ijerph16142535
APA StyleBlanck-Lubarsch, M., Dirksen, D., Feldmann, R., Sauerland, C., & Hohoff, A. (2019). 3D-Analysis of Mouth, Nose and Eye Parameters in Children with Fetal Alcohol Syndrome (FAS). International Journal of Environmental Research and Public Health, 16(14), 2535. https://doi.org/10.3390/ijerph16142535





