Association of School Residential PM2.5 with Childhood High Blood Pressure: Results from an Observational Study in 6 Cities in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cities Selection and Subject Recruitment
2.2. Measurements of Physical Examinations
2.3. Covariates
2.4. Ambient PM2.5 Pollutants
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.O.; Abate, K.H.; Abd-Allah, F.; Ahmed, M.; Alam, K.; Alam, T.; Alvis-Guzman, N.; Ansari, H.; Ärnlöv, J.; et al. The burden of cardiovascular diseases among US states, 1990–2016. JAMA Cardiol. 2018, 3, 375–389. [Google Scholar] [PubMed]
- Dong, Y.; Ma, J.; Song, Y.; Ma, Y.; Dong, B.; Zou, Z.; Prochaska, J.J. Secular Trends in Blood Pressure and Overweight and Obesity in Chinese Boys and Girls Aged 7 to 17 Years From 1995 to 2014. Hypertension 2018, 72, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Guo, Y.; Li, L.; Yang, K.; Liu, R.; Mao, Z.; Bie, R.; Wang, C. The burden, management rates and influencing factors of high blood pressure in a Chinese rural population: The Rural Diabetes, Obesity and Lifestyle (RuralDiab) study. J. Hum. Hypertens. 2018, 32, 236. [Google Scholar] [CrossRef] [PubMed]
- Warren, H.R.; Evangelou, E.; Cabrera, C.P.; Gao, H.; Ren, M.; Mifsud, B.; Ntalla, I.; Surendran, P.; Liu, C.; Cook, J.P.; et al. Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat. Genet. 2017, 49, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, Y. Prevention of Hypertension and Cardiovascular Diseases. Hypertension 2014, 63, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.-W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- Organization, W.H. World Health Organization Releases New Global Air Pollution Data. 2018. Available online: http://www.ccacoalition.org/en/news/world-health-organization-releases-new-global-air-pollution-data (accessed on 24 August 2018).
- Organization, W.H. Ambient (Outdoor) Air Quality and Health. 2018. Available online: http://www.who.int/en/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 24 August 2018).
- Al-Hemoud, A.; Gasana, J.; Al-Dabbous, A.; Al-Shatti, A.; Al-Khayat, A. Disability Adjusted Life Years (DALYs) in Terms of Years of Life Lost (YLL) Due to Premature Adult Mortalities and Postneonatal Infant Mortalities Attributed to PM2.5 and PM10 Exposures in Kuwait. Int. J. Environ. Res. Public Health 2018, 15, 2609. [Google Scholar] [CrossRef]
- Collaborators GBDRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1345–1422. [Google Scholar] [CrossRef]
- Dong, G.H.; Qian, Z.M.; Xaverius, P.K.; Trevathan, E.; Maalouf, S.; Parker, J.; Yang, L.; Liu, M.M.; Wang, D.; Ren, W.H.; et al. Association between long-term air pollution and increased blood pressure and hypertension in China. Hypertension 2013, 61, 578–584. [Google Scholar] [CrossRef]
- Lin, H.; Guo, Y.; Zheng, Y.; Di, Q.; Liu, T.; Xiao, J.; Li, X.; Zeng, W.; Cummings-Vaughn, L.A.; Howard, S.W.; et al. Long-Term Effects of Ambient PM 2.5 on Hypertension and Blood Pressure and Attributable Risk Among Older Chinese Adults. Hypertension 2017, 69, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Urch, B.; Dvonch, J.T.; Bard, R.L.; Speck, M.; Keeler, G.; Morishita, M.; Marsik, F.J.; Kamal, A.S.; Kaciroti, N.; et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension 2009, 54, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Auchincloss Amy, H.; Diez Roux Ana, V.; Dvonch, J.T.; Brown Patrick, L.; Barr, R.G.; Daviglus Martha, L.; Goff David, C.; Kaufman Joel, D.; O’Neill Marie, S. Associations between Recent Exposure to Ambient Fine Particulate Matter and Blood Pressure in the Multi-Ethnic Study of Atherosclerosis (MESA). Environ. Health Perspect. 2008, 116, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Bind, M.-A.; Peters, A.; Koutrakis, P.; Coull, B.; Vokonas, P.; Schwartz, J. Quantile Regression Analysis of the Distributional Effects of Air Pollution on Blood Pressure, Heart Rate Variability, Blood Lipids, and Biomarkers of Inflammation in Elderly American Men: The Normative Aging Study. Environ. Health Perspect. 2016, 124, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Requia, W.J.; Adams, M.D.; Koutrakis, P. Association of PM 2.5 with diabetes, asthma, and high blood pressure incidence in Canada: A spatiotemporal analysis of the impacts of the energy generation and fuel sales. Sci. Total Environ. 2017, 98, 1077. [Google Scholar] [CrossRef]
- Chan, S.H.; Van Hee, V.C.; Bergen, S.; Szpiro, A.A.; DeRoo, L.A.; London, S.J.; Marshall, J.D.; Kaufman, J.D.; Sandler, D.P. Long-Term Air Pollution Exposure and Blood Pressure in the Sister Study. Environ. Health Perspect. 2015, 123, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Bilenko, N.; van Rossem, L.; Brunekreef, B.; Beelen, R.; Eeftens, M.; Hoek, G.; Houthuijs, D.; de Jongste, J.C.; van Kempen, E.; Koppelman, G.H.; et al. Traffic-related air pollution and noise and children’s blood pressure: Results from the PIAMA birth cohort study. Eur. J. Prev. Cardiol. 2015, 22, 4–12. [Google Scholar] [CrossRef]
- Bilenko, N.; Brunekreef, B.; Beelen, R.; Eeftens, M.; de Hoogh, K.; Hoek, G.; Koppelman, G.H.; Wang, M.; van Rossem, L.; Gehring, U. Associations between particulate matter composition and childhood blood pressure—The PIAMA study. Environ. Int. 2015, 84, 1–6. [Google Scholar] [CrossRef]
- Sughis, M.; Nawrot, T.S.; Ihsan-ul-Haque, S.; Amjad, A.; Nemery, B. Blood pressure and particulate air pollution in schoolchildren of Lahore, Pakistan. BMC Public Health 2012, 12, 378. [Google Scholar] [CrossRef]
- Liu, C.; Fuertes, E.; Tiesler, C.M.; Birk, M.; Babisch, W.; Bauer, C.P.; Koletzko, S.; von Berg, A.; Hoffmann, B.; Heinrich, J.; et al. The associations between traffic-related air pollution and noise with blood pressure in children: Results from the GINIplus and LISAplus studies. Int. J. Hyg. Environ. Health 2014, 217, 499–505. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, L.; Ma, Y.; Wang, H.; Luo, J.; Zhang, X.; Luo, C.; Wang, H.; Zhao, H.; Pan, D.; et al. A national school-based health lifestyles interventions among Chinese children and adolescents against obesity: Rationale, design and methodology of a randomized controlled trial in China. BMC Public Health 2015, 15, 210. [Google Scholar] [CrossRef]
- NHBPEP. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics 2004, 114, 24. [Google Scholar]
- Cheng Ye, J.I.; Chen, T.J.; China, W.G.O.O.I. Empirical Changes in the Prevalence of Overweight and Obesity among Chinese Students from 1985 to 2010 and Corresponding Preventive Strategies. Biomed. Environ. Sci. 2013, 26, 1–12. [Google Scholar]
- Krzyzanowski, M.; Cohen, A. Update of WHO air quality guidelines. Air Qual. Atmos. Health 2008, 1, 7–13. [Google Scholar] [CrossRef]
- Pieters, N.; Koppen, G.; Van Poppel, M.; De Prins, S.; Cox, B.; Dons, E.; Nelen, V.; Panis, L.I.; Plusquin, M.; Schoeters, G.; et al. Blood Pressure and Same-Day Exposure to Air Pollution at School: Associations with Nano-Sized to Coarse PM in Children. Environ. Health Perspect 2015, 123, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, Y.; Yang, Y.; Xu, J.; Zhang, Y.; Tang, W.; Guo, T.; Wang, Q.; Shen, H.; Zhang, Y.; et al. Long-Term Effects of Ambient Particulate Matter (With an Aerodynamic Diameter ≤ 2.5 μm) on Hypertension and Blood Pressure and Attributable Risk Among Reproductive-Age Adults in China. J. Am. Heart Assoc. 2018, 7, e008553. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Fang, X.; Wen, B.; Shan, A. Spatio-temporal variations of PM2.5 emission in China from 2005 to 2014. Chemosphere 2017, 183, 429–436. [Google Scholar] [CrossRef]
- Fang, C.; Wang, Z.; Xu, G. Spatial-temporal characteristics of PM2.5 in China: A city-level perspective analysis. J. Geogr. Sci. 2016, 26, 1519–1532. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Chen, S.Y.; Wu, C.F.; Lee, J.H.; Hoffmann, B.; Peters, A.; Brunekreef, B.; Chu, D.C.; Chan, C.C. Associations between Long-Term Air Pollutant Exposures and Blood Pressure in Elderly Residents of Taipei City: A Cross-Sectional Study. Environ. Health Perspect 2015, 123, 779–784. [Google Scholar] [CrossRef]
- Liu, C.; Chen, R.; Zhao, Y.; Ma, Z.; Bi, J.; Liu, Y.; Meng, X.; Wang, Y.; Chen, X.; Li, W.; et al. Associations between ambient fine particulate air pollution and hypertension: A nationwide cross-sectional study in China. Sci. Total Environ. 2017, 584–585, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Dvonch, J.T.; Kannan, S.; Schulz, A.J.; Keeler, G.J.; Mentz, G.; House, J.; Benjamin, A.; Max, P.; Bard, R.L.; Brook, R.D. Acute effects of ambient particulate matter on blood pressure: Differential effects across urban communities. Hypertension 2009, 53, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Coogan, P.F.; White, L.F.; Yu, J.; Burnett, R.T.; Seto, E.; Brook, R.D.; Palmer, J.R.; Rosenberg, L.; Jerrett, M. PM2.5 and Diabetes and Hypertension Incidence in the Black Women’s Health Study. Epidemiology 2015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Laden, F.; Forman, J.P.; Hart, J.E. Long-Term Exposure to Particulate Matter and Self-Reported Hypertension: A Prospective Analysis in the Nurses’ Health Study. Environ. Health Perspect. 2016, 124, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.J.; Mentz, G.B.; Sampson, N.R.; Dvonch, J.T.; Reyes, A.G.; Izumi, B. Effects of particulate matter and antioxidant dietary intake on blood pressure. Am. J. Public Health 2015, 105, 1254. [Google Scholar] [CrossRef] [PubMed]
- Babisch, W.; Wolf, K.; Petz, M.; Heinrich, J.; Cyrys, J.; Peters, A. Associations between Traffic Noise, Particulate Air Pollution, Hypertension, and Isolated Systolic Hypertension in Adults: The KORA Study. Environ. Health Perspect. 2014, 122, 492–498. [Google Scholar] [CrossRef] [PubMed]
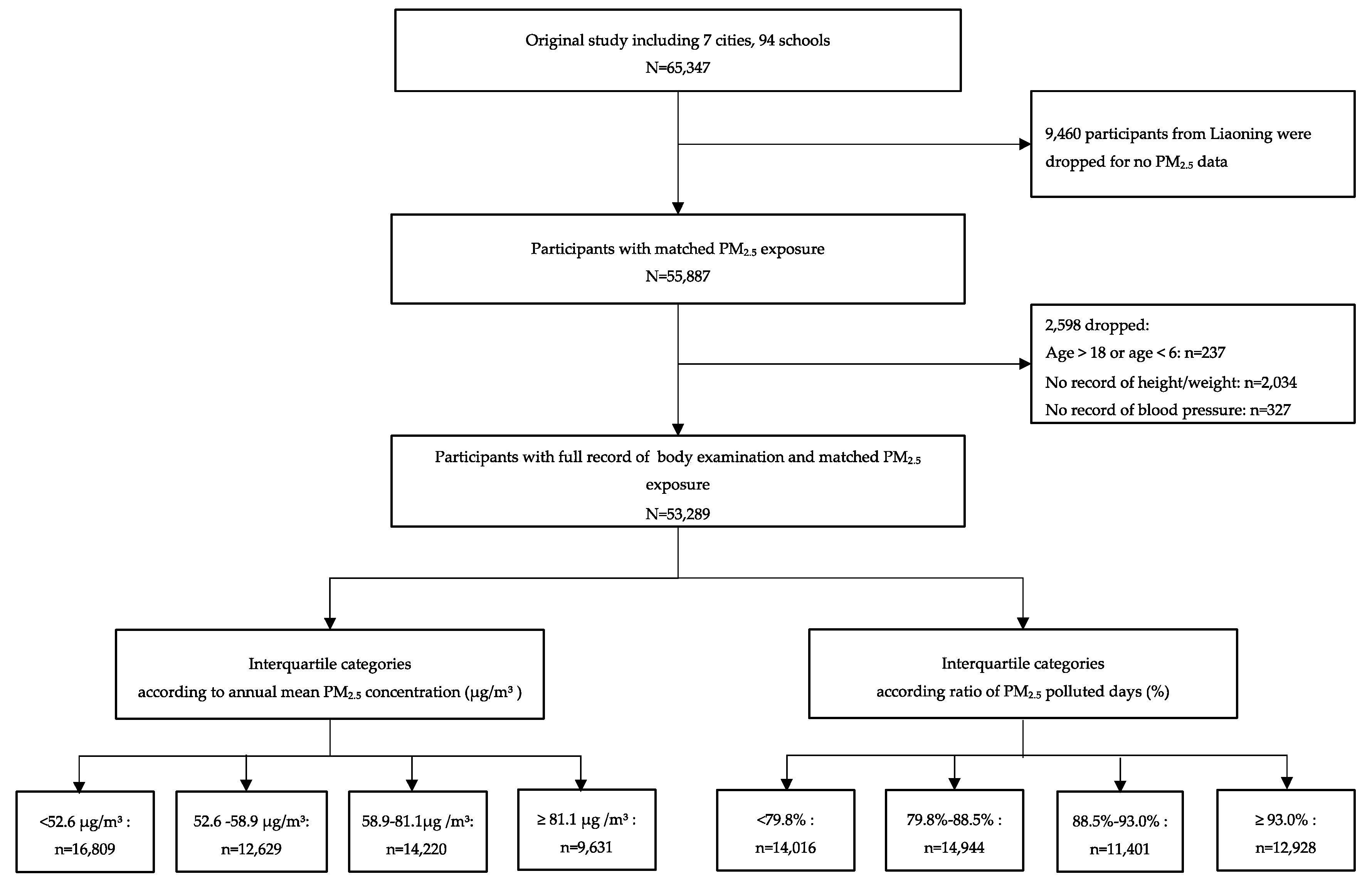
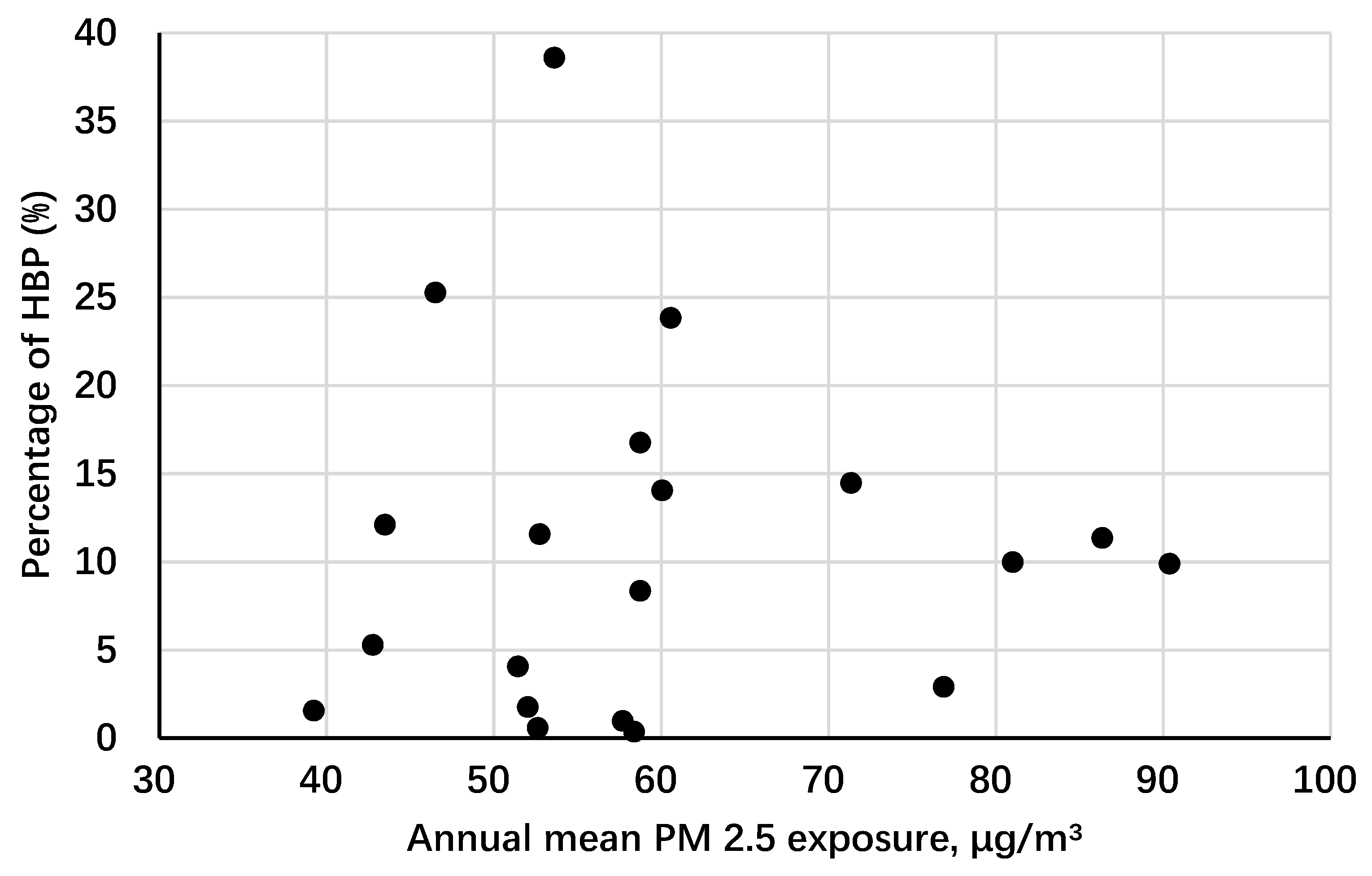
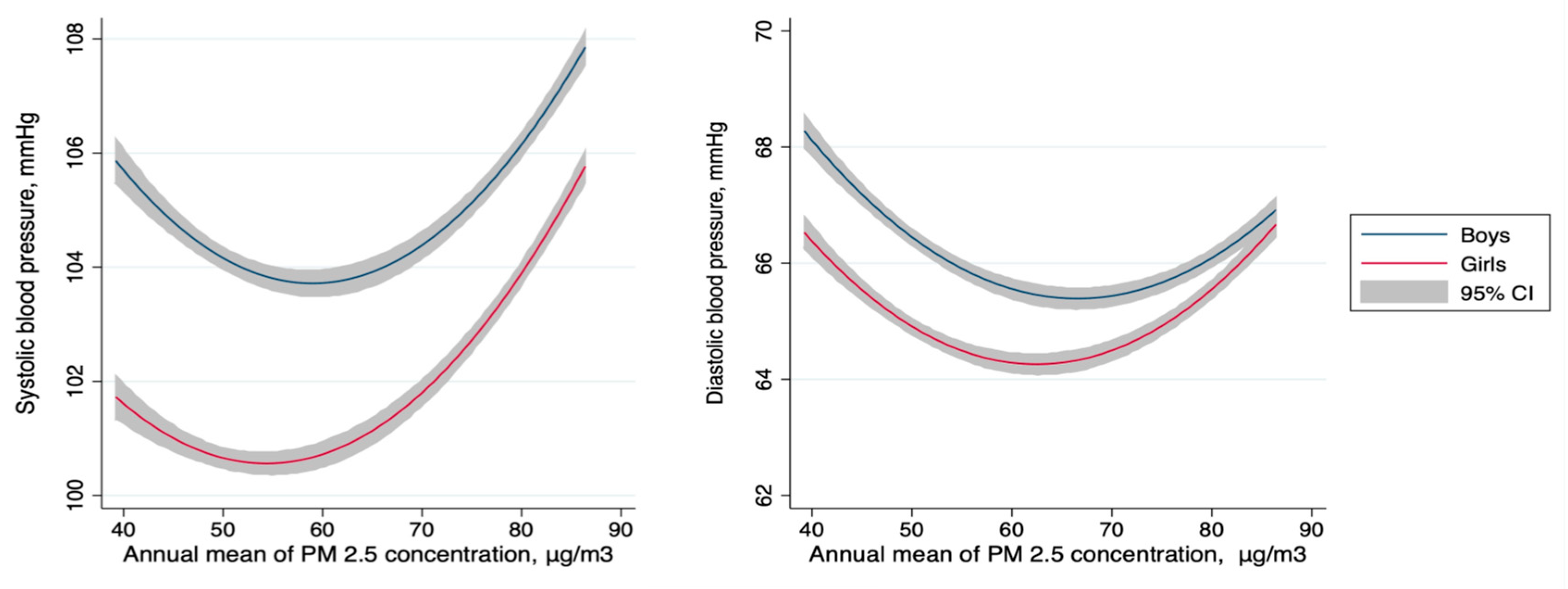
| Characteristic | Overall | Quartiles of Annual Mean PM2.5 Pollution, μg/m3 | P-Value | |||
|---|---|---|---|---|---|---|
| <52.6 | 52.6–58.9 | 58.9–81.1 | ≥81.1 | |||
| No. of observations | 53,289 | 16,809 | 12,629 | 14,220 | 9631 | |
| Age, year | 10.8 (8.0–13.0) | 11.1 (8.0–14.0) | 10.6 (8.0–13.0) | 10.6 (8.0–13.0) | 10.9 (8.0–13.0) | <0.001 |
| Average PM2.5, μg/m3 | 63.1 (51.4–81.0) | 45.3 (42.8–46.5) | 57.3 (57.7–58.8) | 73.6 (71.4–81.0) | 86.4 (86.4–86.4) | <0.001 |
| Systolic blood pressure, mmHg | 103.8 (95.0–110.0) | 102.0 (92.5–110.0) | 103.3 (94.0–111.0) | 103.2 (96.0–110.0) | 108.4 (100.0–120.0) | <0.001 |
| Diastolic blood pressure, mmHg | 65.9 (60.0–70.0) | 65.8 (60.0–71.0) | 65.3 (60.0–71.0) | 65.1 (60.0–70.0) | 68.1 (60.0–70.0) | <0.001 |
| High blood pressure, n (%) | 5222 (9.8) | 1452 (8.6) | 1103 (8.7) | 1649 (11.6) | 1018 (10.6) | <0.001 |
| BMI, kg/m2 | 18.5 (15.8–20.3) | 18.3 (15.8–20.2) | 18.3 (15.6–20.3) | 18.1 (15.7–20.0) | 19.4 (16.0–21.6) | <0.001 |
| Male, n (%) | 27,544 (51.7) | 8582 (51.1) | 6572 (52.0) | 7475 (52.6) | 4915 (51.0) | 0.025 |
| Passive smoking exposure, n (%) | 32,804 (61.6) | 11,528 (68.6) | 7088 (56.1) | 8723 (61.3) | 5465 (56.7) | <0.001 |
| Family history of hypertension, n (%) | 2847 (6.8) | 647 (6.2) | 930 (8.4) | 668 (5.7) | 602 (7.1) | <0.001 |
| Daily consumption of fruit, serving | 1.3 (0.6–2.0) | 1.3 (0.7–2.0) | 1.2 (0.6–1.7) | 1.2 (0.6–1.7) | 1.4 (0.9–2.0) | 0.002 |
| Daily consumption of vegetable, serving | 1.8 (1.0–2.0) | 2.0 (1.0–2.0) | 1.7 (1.0–2.0) | 1.7 (1.0–2.0) | 1.9 (1.0–2.0) | <0.001 |
| Daily physical activity time, minutes | 70.1 (25.7–87.9) | 67.2 (25.7–85.7) | 66.6 (25.7–83.6) | 68.4 (24.3–85.7) | 84.0 (28.6–98.6) | <0.001 |
| City (No. of environmental monitoring stations near the selected schools) | ||||||
| Yinchuan (2) | 8119 (15.2) | 8119 (15.2) | - | - | - | |
| Shanghai (5) | 9059 (17.0) | 2861 (5.4) | 6198 (11.6) | - | - | |
| Guangzhou (3) | 8601 (16.1) | 5829(10.9) | 2772 (5.2) | - | - | |
| Chongqing (3) | 10,353 (19.4) | - | 3659 (6.9) | 6694 (12.5) | - | |
| Changsha (2) | 7526 (14.1) | - | - | 7526 (14.1) | - | |
| Tianjin (2) | 9631 (18.1) | - | - | - | 9631 (18.1) | |
| Level of PM2.5 Pollution | Effect Estimate (95% CI) | ||
|---|---|---|---|
| HBP (Odds Ratio) | SBP (mmHg) | DBP (mmHg) | |
| Quartile categories for annual mean PM2.5, μg/m3 | |||
| <52.6 | 1 (Reference) | 0 (Reference) | 0 (Reference) |
| 52.6–58.9 | 0.86 (0.33, 2.22) | 1.63 (−1.37, 4.62) | −0.11 (−2.72, 2.50) |
| 58.9–81.1 | 2.24 (0.87, 5.80) | 1.27 (−1.72, 4.26) | −0.53 (−3.13, 2.07) |
| ≥81.1 | 2.13 (0.71, 6.34) | 5.59 (2.13, 9.04) | 2.60 (−0.41, 5.61) |
| P for trend | 0.056 | 0.007 | 0.246 |
| Quartile categories for ratio of polluted days, % a | |||
| <79.8% | 1 (Reference) | 0 (Reference) | 0 (Reference) |
| 79.9–88.5% | 0.65 (0.25, 1.65) | −2.00 (−4.97, 0.96) | −2.58 (−5.03, −0.12) |
| 88.6–93.0% | 0.45 (0.15, 1.33) | −1.27 (-4.70, 2.15) | −3.30 (−6.14, −0.47) |
| ≥93.0% | 1.24 (0.44, 3.51) | 2.54 (−0.76, 5.84) | 0.67 (−2.01, 3.39) |
| P for trend | 0.868 | 0.145 | 0.849 |
| Variable | No. of Observations with HBP, N (%) | Population Attributable Risk % and 95% CI | |
|---|---|---|---|
| Annual Mean Pollution Level a | Ratio of Polluted Days b | ||
| Overall | 5222 (9.8) | 1.16 (0.80, 1.52) | 2.85 (2.42, 3.29) |
| Sex | |||
| Boys | 2913 (10.6) | 1.17 (0.81, 1.53) | 2.85 (2.41, 3.29) |
| Girls | 2309 (9.0) | 1.15 (0.79, 1.51) | 2.85 (2.43, 3.28) |
| Age group | |||
| ≤9 years old | 2065 (11.5) | 1.16 (0.78, 1.54) | 2.68 (2.24, 3.12) |
| 10–12 years old | 1796 (15.5) | 1.20 (0.83, 1.57) | 2.75 (2.30, 3.20) |
| 13–15 years old | 944 (8.0) | 1.12 (0.80, 1.45) | 2.93 (2.48, 3.37) |
| 16–18 years old | 417 (3.5) | 1.16 (0.80, 1.52) | 3.14 (2.73, 3.55) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zou, Z.; Dong, B.; Dong, Y.; Ma, Y.; Gao, D.; Yang, Z.; Wu, S.; Ma, J. Association of School Residential PM2.5 with Childhood High Blood Pressure: Results from an Observational Study in 6 Cities in China. Int. J. Environ. Res. Public Health 2019, 16, 2515. https://doi.org/10.3390/ijerph16142515
Wang X, Zou Z, Dong B, Dong Y, Ma Y, Gao D, Yang Z, Wu S, Ma J. Association of School Residential PM2.5 with Childhood High Blood Pressure: Results from an Observational Study in 6 Cities in China. International Journal of Environmental Research and Public Health. 2019; 16(14):2515. https://doi.org/10.3390/ijerph16142515
Chicago/Turabian StyleWang, Xijie, Zhiyong Zou, Bin Dong, Yanhui Dong, Yinghua Ma, Di Gao, Zhaogeng Yang, Shaowei Wu, and Jun Ma. 2019. "Association of School Residential PM2.5 with Childhood High Blood Pressure: Results from an Observational Study in 6 Cities in China" International Journal of Environmental Research and Public Health 16, no. 14: 2515. https://doi.org/10.3390/ijerph16142515
APA StyleWang, X., Zou, Z., Dong, B., Dong, Y., Ma, Y., Gao, D., Yang, Z., Wu, S., & Ma, J. (2019). Association of School Residential PM2.5 with Childhood High Blood Pressure: Results from an Observational Study in 6 Cities in China. International Journal of Environmental Research and Public Health, 16(14), 2515. https://doi.org/10.3390/ijerph16142515







