Cost–Benefit Analysis of Real-Time Influenza Testing for Patients in German Emergency Rooms
Abstract
1. Introduction
1.1. Test System
1.2. Ethical Considerations
2. Materials and Methods
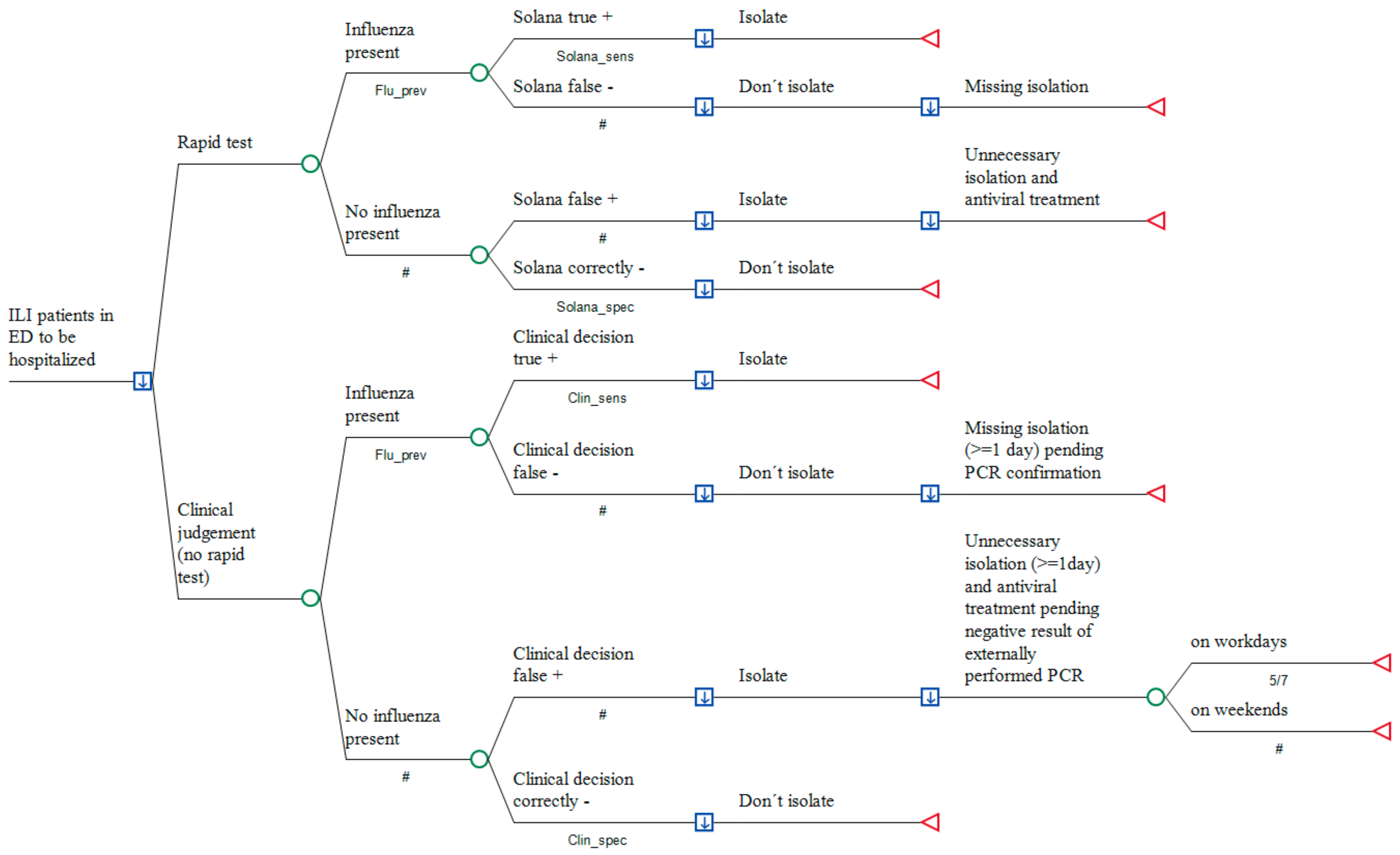
2.1. Model Approach
2.2. Model Structure
2.3. Model Input
2.3.1. Epidemiological and Laboratory Parameters
Prevalence of Influence in Season 2017/2018
Sensitivity and Specificity of Clinical Judgement
Sensitivity and Specificity of the Real-Time Influenza Test (Solana®)
Rate of Hospitalizations
Vaccination rate and its effectiveness among HCW
Secondary Attack Rate after Influenza Virus Transmission
2.3.2. Economic Parameters
Costs of the Rapid Influenza Test
Costs of External PCR
Hospital Opportunity Costs
Costs of Initial Treatment with Neuraminidase Inhibitors
Revenue from Administering a Neuraminidase Inhibitor
Costs of Intrahospital Transmission
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Report on the Epidemiology of Influenza in Germany in the Season 2017/2018. Robert Koch-Institut, 2018. Available online: https://www.rki.de (accessed on 2 July 2019).
- The Respiratory Virus Network (RespVir). Available online: https://clinical-virology.net/de (accessed on 2 July 2019).
- Haas, J.; Braun, S.; Wutzler, P. Burden of influenza in Germany: A retrospective claims database analysis for the influenza season 2012/2013. Eur. J. Health Econ. 2016, 17, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Vogl, M. Assessing the DRG cost accounting with respect to resource allocation and tariff calculation: The case of Germany. Health Econ. Rev. 2012, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Bruning, A.H.L.; Leeflang, M.M.G.; Vos, J.M.B.W.; Spijker, R.; de Jong, M.D.; Wolthers, K.C.; Pajkrt, D. Rapid Tests for Influenza, Respiratory Syncytial Virus, and Other Respiratory Viruses: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2017, 65, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Federal Statistical Office of Germany (Statistisches Bundesamt, Wiesbaden)—DESTATIS. Gesundheit. Grunddaten der Krankenhäuser; Fachserie 12, Reihe 6.1.1. Krankenhäuser 2017, 2.8; Bettenführende Fachabteilungen: Wiesbaden, Germany, 2017. [Google Scholar]
- Van, J.N.; Caméléna, F.; Dahoun, M.; Pilmis, B.; Mizrahi, A.; Lourtet, J.; Behillil, S.; Enouf, V.; Le Monnier, A. Prospective evaluation of the Alere i Influenza nucleic acid amplification versus Xpert Flu/RSV. Diagn. Microbiol. Infect. Dis. 2016, 85, 19–22. [Google Scholar]
- Barreda-García, S.; Miranda-Castro, R.; de-Los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Helicase-dependent isothermal amplification: A novel tool in the development of molecular-based analytical systems for rapid pathogen detection. Anal. Bioanal. Chem. 2018, 410, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Junker, R.; Schlebusch, H.; Luppa, P.B. Point-of-care testing in hospitals and primary care. Dtsch. Arztebl. Int. 2010, 107, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Prevention Strategies for Seasonal Influenza in Healthcare Settings. Available online: hppts://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm (accessed on 3 May 2019).
- Robert Koch Institute. Recommendation Influenza (Part 1): Diseases Caused by Seasonal Influenza Viruses. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Merkblaetter/Ratgeber_Influenza_saisonal.html (accessed on 3 May 2019).
- World Health Organization. WHO Guidelines for Pharmacological Management of Pandemic (H1N1) 2009 Influenza and Other Influenza Viruses. Available online: https://www.who.int/csr/resources/publications/swineflu/h1n1_use_antivirals_20090820/en/ (accessed on 3 May 2019).
- European Centers for Disease Control: Draft Scientific Advice for Consultation. ECDC Preliminary Scientific Advice. Expert Opinion on Neuraminidase Inhibitors for Prevention and Treatment of Influenza. Review of Recent Systematic Reviews and Meta-Analyses. Available online: http://ecdc.europa.eu/en/publications/publications/neuraminidaseinhibitors-flu-consultation.pdf (accessed on 3 May 2019).
- Public Health England: Guidance. Influenza: Treatment and Prophylaxis Using Anti-Viral Agents. Available online: https://www.gov.uk/government/publications/influenza-treatment-and-prophylaxis-using-anti-viral-agents (accessed on 3 May 2019).
- Centers for Disease Control: Influenza Antiviral Medications: Summary for Clinicians. Available online: https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm (accessed on 3 May 2019).
- Lehnert RPletz, M.; Reuss, A.; Schaberg, T. Antivirale Arzneimittel bei saisonaler und pandemischer Influenza. Dtsch. Ärztebl. Int. 2016, 113, 799–807. [Google Scholar]
- Dobson, J.; Whitley, R.J.; Pocock, S.; Monot, A.S. Oseltamivir treatment for influenza in adults: A meta-analysis of randomized controlled trials. Lancet 2015, 385, 1729–1737. [Google Scholar] [CrossRef]
- Multiple Choices. Available online: http://drg.unimuenster.de/index.php?option=com_webgrouper&Itemid=112&view=webgrouper (accessed on 3 May 2019).
- Kong, H.; Pack, T.; Lollar, R.; Ranalli, T. Solana: An isothermal, helicase dependent, molecular platform for the rapid, cost effective detection of Influenza A and B in human specimens. In Proceedings of the 16th Conference of the European Scientific Working Groupe on Influenza (ESWI), Riga, Latvia, 10–13 September 2017. [Google Scholar]
- Yang, T.U.; Cheong, H.J.; Song, J.Y.; Lee, J.S.; Wie, S.H.; Kim, Y.K.; Choi, W.S.; Lee, J.; Jeong, H.W.; Kim, W.J. Age-and influenza activity-stratified case definitions of influenza-like illness: Experience from hospital-based influenza surveillance in South Korea. PLoS ONE 2014, 24, e8487. [Google Scholar] [CrossRef]
- Cauchemez, S.; Carrat, F.; Viboud, C.; Valleron, A.J.; Boelle, P.Y. A Bayesian MCMC approach to study transmission of influenza. Stat. Med. 2004, 23, 3469–3487. [Google Scholar] [CrossRef]
- Federal Statistical Office of Germany (Statistisches Bundesamt, Wiesbaden)—DESTATIS. Fachserie 16, Reihe 2.3: Verdienste und Arbeitskosten—Arbeitnehmerverdienste. In 4.5.1 Deutschland Durchschnittliche Bruttojahresverdienste und Sonderzahlungen nach Wirtschaftszweigen Arbeitnehmer im Krankenhaus (Q861); Statistisches Bundesamt: Wiesbaden, Germany, 2017. [Google Scholar]
- Allgemeine Ortskrankenkasse (AOK, München). Arbeitsunfähigkeit bei AOK-Pflichtmitgliedern ohne Rentner (Arbeitsunfähigkeitsfälle, Arbeitsunfähigkeitsfälle je 100.000 Pflichtmitglieder, Arbeitsunfähigkeitstage, Arbeitsunfähigkeitstage je 100.000 Pflichtmitglieder, Tage je Fall); Gliederungsmerkmale: München, Germany, Jahre, Geschlecht, ICD-10.
- Xue, Y.; Kristiansen, I.S.; de Blasio, B.F. Modeling the cost of influenza: The impact of missing costs of unreported complications and sick leave. BMC Public Health 2010, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Maybaum, T. Ärzte und Pfleger viel zu selten gegen Influenza geimpft. Deutsches Ärzteblatt, 10 August 2018. [Google Scholar]
- Report on the Epidemiology of Influenza in Germany in the season 2011/2012. Robert Koch-Institut. 2012. Available online: https://www.rki.de (accessed on 2 July 2019).
- Monto, A.S.; Gravenstein, S.; Elliott, M.; Colopy, M.; Schweinle, J. Clinical signs and symptoms predicting influenza infection. Arch. Intern. Med. 2000, 27, 3243–3247. [Google Scholar] [CrossRef]
- vd Hoeven, A.M.; Scholing, M.; Wever, P.C.; Fijnheer, R.; Hermans, M.; Schneeberger, P.M. Lack of discriminating signs and symptoms in clinical diagnosis of influenza of patients admitted to the hospital. Infection 2007, 35, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Report on the Epidemiology of Influenza in Germany in the Season 2016/2017. Robert Koch-Institut. 2017. Available online: https://www.rki.de (accessed on 2 July 2019).
- Report on the Epidemiology of Influenza in Germany in the Season 2014/2015. Robert Koch-Institut. 2015. Available online: https://www.rki.de (accessed on 2 July 2019).
- Babcock, H.M.; Merz, L.R.; Fraser, V.J. Is influenza an influenza-like illness? Clinical presentation of influenza in hospitalized patients. Infect. Control. Hosp. Epidemiol. 2006, 27, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Aoki, F.Y.; Macleod, M.D.; Paggiaro, P.; Carewicz, O.; El Sawy, A.; Wat, C.; Griffiths, M.; Waalberg, E.; Ward, P. IMPACT Study Group. Early administration of oral Oseltamivir increases the benefits of influenza treatment. J. Antimicrob. Chemother. 2003, 51, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Savage, R. Assessing secondary attack rates among household contacts. BMC Public Health 2011, 11, 234. [Google Scholar]
- Allgemeine Ortskrankenkassen (AOK) Bundesbasisfallwert und Einheitlicher Basisfallwertkorridor. 2019. Available online: https://www.aok-gesundheitspartner.de/bund/krankenhaus/lbfw/bfw/index.html (accessed on 3 May 2016).
- Haubrock, M. Krankenhausfinanzwirtschaft. In Betriebswirtschaft und Management im Krankenhaus; Haubrock, M., Schär, W., Eds.; Huber: Bern, Switzerland, 2007; pp. 394–453. [Google Scholar]
- Brachmann, M.; Kikull, K.; Kill, C.; Betz, S. Economic and operational impact of an improved pathway using rapid molecular diagnostic testing for patients with influenza-like illness in a German emergency department. J. Clin. Monit. Comput. 2019. [Google Scholar] [CrossRef]
- Falsey, A.R.; Murata, Y.; Walsh, E.E. Impact of rapid diagnosis on management of adults hospitalized with influenza. Arch. Intern. Med. 2007, 167, 354–360. [Google Scholar] [PubMed]
- Bonner, A.B.; Monroe, K.W.; Talley, L.I.; Klasner, A.E.; Kimberlin, D.W. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: Results of a randomized, prospective, controlled trial. Pediatrics 2003, 112, 363–367. [Google Scholar] [CrossRef]
- Egilmezer, E.; Walker, G.J.; Bakthavathsalam, P.; Peterson, J.R.; Gooding, J.J.; Rawlinson, W.; Stelzer-Braid, S. Systematic review of the impact of point-of-care testing for influenza on the outcomes of patients with acute respiratory tract infection. Rev. Med. Virol. 2018, 28, e1995. [Google Scholar] [CrossRef]
- Lee, J.J.; Verbakel, J.Y.; Goyder, C.R.; Ananthakumar, T.; Tan, P.S.; Turner, P.J.; Hayward, G.; Van den Bruel, A. The clinical utility of point-of-care tests for influenza in ambulatory care: A systematic review and meta-analysis. Clin. Infect. Dis. 2018, 69, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Gianino, M.M.; Politano, G.; Scarmozzino, A.; Stillo, M.; Amprino, V.; Di Carlo, S.; Benso, A.; Zotti, C.M. Cost of Sickness Absenteeism during Seasonal Influenza Outbreaks of Medium Intensity among Health Care Workers. Int. J. Environ. Res. Public Health 2019, 16, E747. [Google Scholar] [CrossRef] [PubMed]
- Rahamat-Langendoen, J.; Groenewoud, H.; Kuijpers, J.; Melchers, W.J.G.; van der Wilt, G.J. Impact of molecular point-of-care testing on clinical management and in-hospital costs of patients suspected of influenza or RSV infection: A modeling study. J. Med. Virol. 2019, 91, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
| Variables Category | Variable Name | Distribution * | Value (Base Case) | Relative Change (Range) | Reference |
|---|---|---|---|---|---|
| Prevalence of influenza | Flu_prev | triangular | 0.426 | 0.2–0.426 | Calculated from [2] |
| Additional revenue per day due to NI | cRev_day | uniform | €233.46 | ±20% (€186.77–280.15 | Calculated from InEK data [18] |
| Combined Solana® specificity | Solana_spec | uniform | 0.96.7 | (95% CI) 0.954–0.977 | [19] |
| Opportunity costs due to blocking twin bed | cOpp | triangular | €350.19 | ±20% (€280.15–€420.23) | Calculated from InEK data [18] |
| Probability of correctly excluding non-influenza | Clin_spec | uniform | 0.601 | 95% CI (65.4–76.7) | [20] |
| Sensitivity of diagnosing influenza if present | Clin_sens | uniform | 0.713 | 95% CI (0.65–76.7) | [20] |
| Costs of virustatics per day | cAntivir_day | triangular | €12.37 | ±20% (€9.89–€14.83) | Adapted from Rote Liste 2019 |
| Costs of Solana® | cSolana | triangular | €14 | ±20% (€11.20–€16.80) | As declared by manufacturer |
| Combined sensitivity of the Solana® test | Solana_sens | triangular | 0.972 | 95% CI (0.954–0.984) | [19] |
| Secondary cases due to one unknown influenza case | sec_flu | normal | 0.202 | 95% CI (0154–0.256) | [21] |
| Costs of productivity loss per day | cPL_day | triangular | €156. | ±20% (€125.59–€188.39) | calculated from Federal Statistical Office data [22] |
| Number of days of HCW out of work due to influenza | sick_days | normal | 7.2 10.32 days | ±SD 8.9 days (5.76–8.64) | [23,24] |
| Probability of vaccinated HCW | pVacc_HCW | normal | 0.401 | ±20% (0.33–0.49) | [25] |
| Probability that hospitalization is required | pHosp | uniform | 0.18 | 0.157–0.23 | Adapted from [1] |
| Costs of PCR in external laboratory | cPCR | uniform | €44.88 | €30-65–€7.56 | Nationwide laboratory inquiry |
| Effectiveness of influenza vaccination | Vacc_eff | uniform | 0.15 | 0.15–0.49 | [1,26] |
| Base-Case Analysis | Comparators | Mean Cost Per Patient (€) | Incremental Cost (€) * | Absolute Cost Savings (€) |
|---|---|---|---|---|
| ILI patients prior to hospitalisation followed by immediate intake of NI | Solana® as an add-on | −6.90 | 0 | −6.90 |
| Conventional approach | 125.71 | 132.61 |
| Variable Name | Variable Description | Variable Lowest Bound | Variable Highest Bound | Lowest cost Value | Highest Costs Value | Spread Ƭ | Threshold Value µ | Risk % ¥ | Cumulative Risk% |
|---|---|---|---|---|---|---|---|---|---|
| Flu_prev | Prevalence of influenza | 0.20 | 0.43 | −6.90 | 48.19 | 55.09 | <0.398 | 0.49 | 0.49 |
| cRev_day | Additional revenue per day due to NI | 186.77 | 280.15 | −26.23 | 12.43 | 38.67 | <217.10 | 0.24 | 0.74 |
| Solana_spec | Combined Solana® specificity | 0.95 | 0.98 | −21.32 | 11.86 | 33.18 | <0.9622 | 0.18 | 0.92 |
| cOpp | Opportunity costs due to blocking twin bed | 280.15 | 420.23 | −16.19 | 2.39 | 18.57 | >402.37 | 0.06 | 0.97 |
| cAntivir_day | Costs of virustatics per day | 9.89 | 14.83 | −12.27 | −1.57 | 10.70 | - | 0.02 | 0.99 |
| cSolana | Costs of Solana® | 11.20 | 16.80 | −9.70 | −4.10 | 5.60 | - | 0.01 | 0.99 |
| Solana_sens | Combined sensitivity of the Solana® test | 0.95 | 0.98 | −8.87 | −3.28 | 5.59 | - | 0.01 | 1.00 |
| sec_flu | Secondary cases due to one unknown influenza case | 0.15 | 0.26 | −7.50 | −6.21 | 1.29 | - | 0.00 | 1.00 |
| cPL_day | Costs of productivity loss per day | 125.59 | 188.39 | −7.41 | −6.39 | 1.02 | - | 0.00 | 1.00 |
| sick_days | Number of days of HCW out of work due to influenza | 5.76 | 8.64 | −7.41 | −6.39 | 1.02 | - | 0.00 | 1.00 |
| Vacc_eff | Effectiveness of influenza vaccination | 0.15 | 0.49 | −7.28 | −6.90 | 0.38 | - | 0.00 | 1.0 |
| pVacc_HCW | Probability of HCW being vaccinated | 0.33 | 0.49 | −6.93 | −6.86 | 0.07 | - | 0.00 | 1.00 |
| pHosp | Probability that hospitalization is required | 0.16 | 0.23 | −6.90 | −6.90 | 0.00 | - | 0.00 | 1.00 |
| Clin_sens | Sensitivity of diagnosing influenza if present | 0.65 | 0.77 | −6.90 | −6.90 | 0.00 | - | 0.00 | 1.00 |
| Clin_spec | Probability of correctly excluding non-influenza | 0.57 | 0.63 | −6.90 | −6.90 | 0.00 | - | 0.00 | 1.00 |
| cPCR_ext | Costs of PCR in external laboratory | 30.00 | 65.00 | −6.90 | −6.90 | 0.00 | 0.00 | 1.00 |
| Probabilistic Sensitivity Analysis | Comparators | Mean Cost Per Patient (€) | Standard Deviation (± SD) | Incremental Cost (€) * |
|---|---|---|---|---|
| ILI patients prior to hospitalisation followed by immediate intake of NI | Solana® as an add-on | 12.92 | 24.66 | 0 |
| Conventional approach | 157.05 | 24.68 | 144.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diel, R.; Nienhaus, A. Cost–Benefit Analysis of Real-Time Influenza Testing for Patients in German Emergency Rooms. Int. J. Environ. Res. Public Health 2019, 16, 2368. https://doi.org/10.3390/ijerph16132368
Diel R, Nienhaus A. Cost–Benefit Analysis of Real-Time Influenza Testing for Patients in German Emergency Rooms. International Journal of Environmental Research and Public Health. 2019; 16(13):2368. https://doi.org/10.3390/ijerph16132368
Chicago/Turabian StyleDiel, Roland, and Albert Nienhaus. 2019. "Cost–Benefit Analysis of Real-Time Influenza Testing for Patients in German Emergency Rooms" International Journal of Environmental Research and Public Health 16, no. 13: 2368. https://doi.org/10.3390/ijerph16132368
APA StyleDiel, R., & Nienhaus, A. (2019). Cost–Benefit Analysis of Real-Time Influenza Testing for Patients in German Emergency Rooms. International Journal of Environmental Research and Public Health, 16(13), 2368. https://doi.org/10.3390/ijerph16132368





