Comparison of Cardiovascular Risk Factors among Somalis Living in Norway and Somaliland
Abstract
1. Introduction
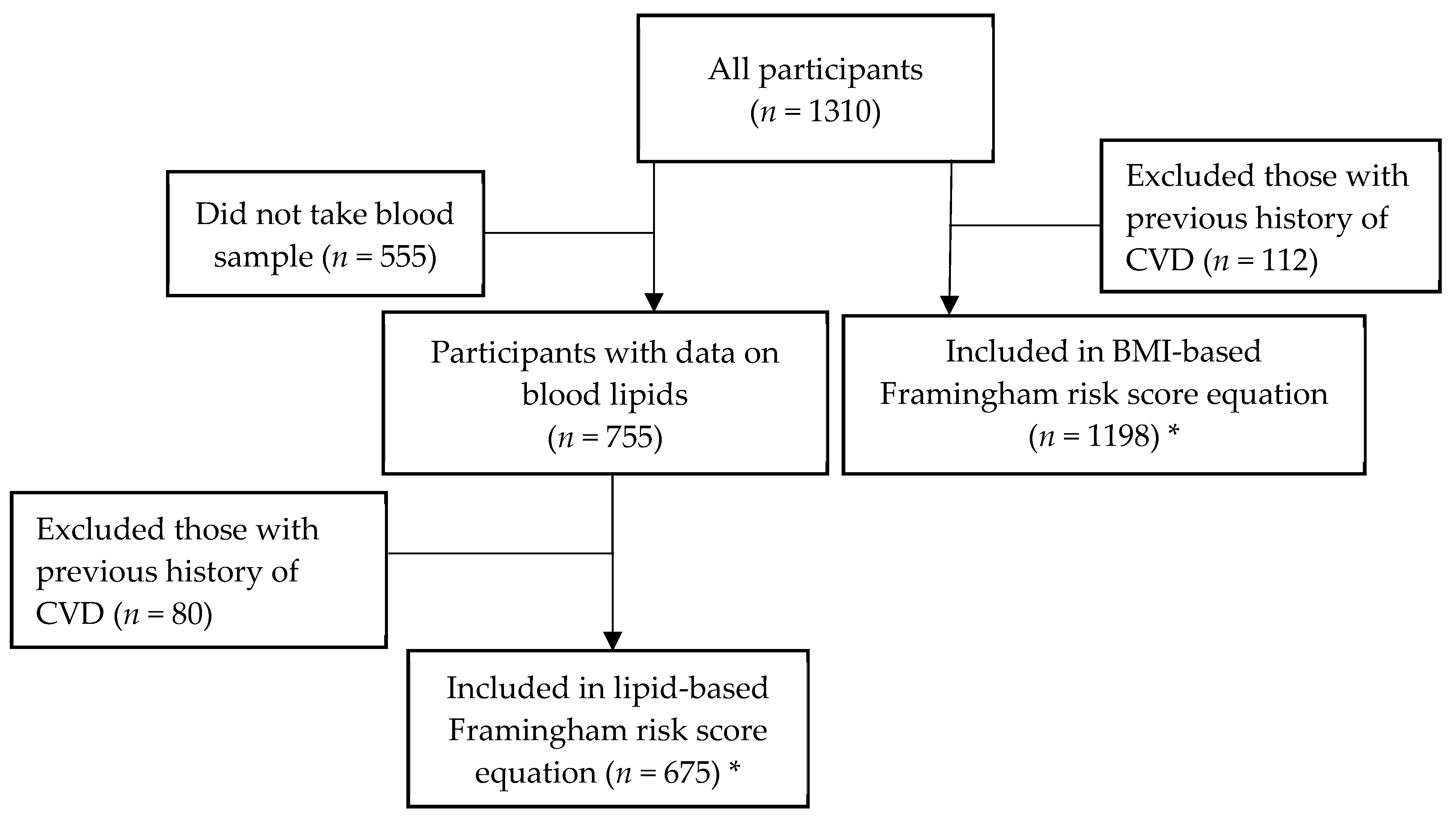
2. Materials and Methods
2.1. Oslo Study
2.2. Somaliland Study
2.3. Data Collection
2.4. Blood Sampling and Laboratory Assays
2.5. Definitions of Risk Factor Variables
2.6. Statistical Analysis
2.7. Estimating the Framingham Risk Score
3. Results
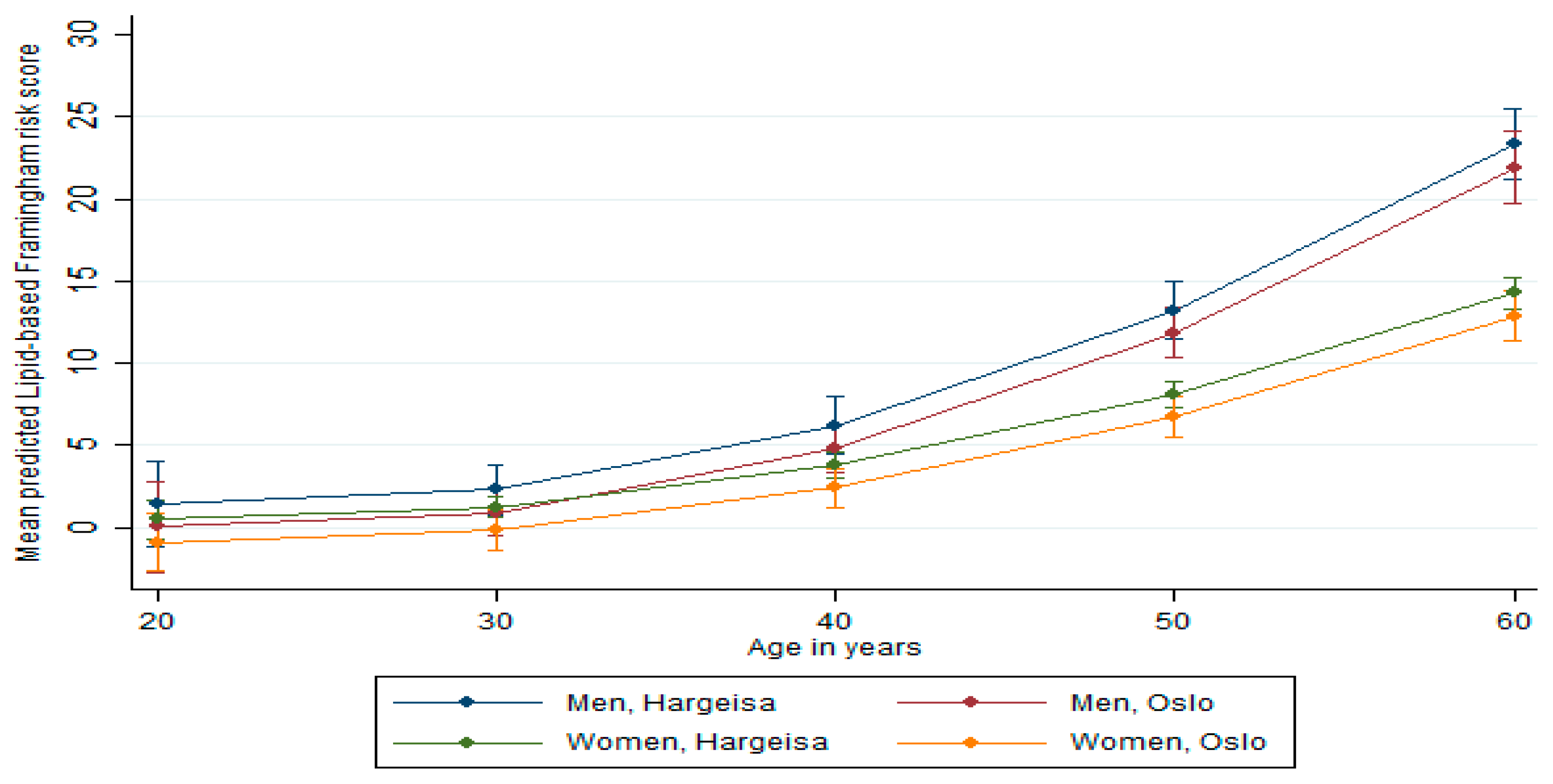
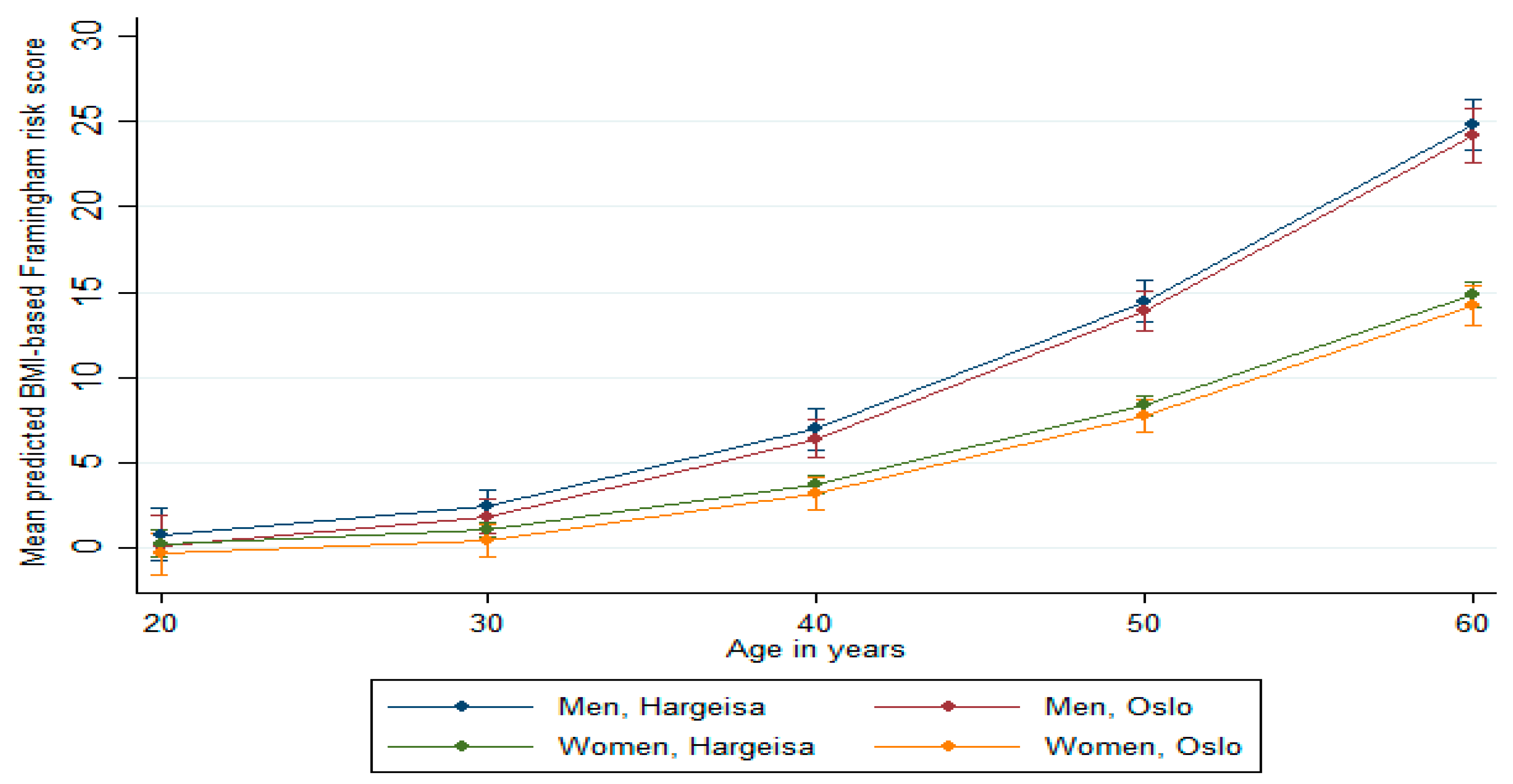
Predicted Framingham Risk Score
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dalal, S.; Beunza, J.J.; Volmink, J.; Adebamowo, C.; Bajunirwe, F.; Njelekela, M.; Mozaffarian, D.; Fawzi, W.; Willett, W.; Adami, H.O.; et al. Non-communicable diseases in sub-Saharan Africa: What we know now. Int. J. Epidemiol. 2011, 40, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Ziraba, A.K.; Fotso, J.C.; Ochako, R. Overweight and obesity in urban Africa: A problem of the rich or the poor? BMC Public Health 2009, 9, 465. [Google Scholar] [CrossRef] [PubMed]
- Keates, A.K.; Mocumbi, A.O.; Mpiko, N.; Karen, S.; Simon, S. Cardiovascular disease in Africa: Epidemiological profile and challenges. Nat. Rev. Cardiol. 2017, 14, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Global Atlas on Cardiovascular Disease Prevention and Control. 2011. Available online: https://www.who.int/cardiovascular diseases/publications/atlas_cvd/en/ (accessed on 26 May 2019).
- Scott, A.; Ejikeme, C.S.; Clottey, E.N.; Thomas, J.G. Obesity in sub-Saharan Africa: Development of an Ecological Theoretical Framework. Health Promot. Int. 2013, 28, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Unrepresented Nations & People’s Organization (UNPO). Member Profile: Somaliland in UNPO. Brussels, Belgium 2017. Available online: http://unpo.org/downloads/2343.pdf (accessed on 28 September 2018).
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Somalia’s Missing Million; The Somali Diaspora and Its Role in Development 2009. Available online: http://www.so.undp.org/content/somalia/en/home/library/poverty/publication3.html (accessed on 26 May 2019).
- Table: 09817: Immigrants and Norwegian-Born to Immigrant Parents, by Immigration Category, Country Background and Percentages of the Population. Available online: https://www.ssb.no/en/statbank/table/09817/ (accessed on 28 June 2019).
- Public Health Challenges of Immigrants in Norway: A Research Review. Available online: https://www.fhi.no/globalassets/dokumenterfiler/rapporter/2010/public-health-challenges-of-immigrants-in-norway-nakmireport-2-2010.pdf (accessed on 28 June 2019).
- Kumar, B.N.; Selmer, R.; Lindman, A.S.; Tverdal, A.; Falster, K.; Meyer, H.E. Ethnic differences in SCORE cardiovascular risk in Oslo, Norway. Eur. J. Cardiovasc. Prev. Rehabil. 2009, 16, 229–234. [Google Scholar] [CrossRef]
- Glenday, K.; Kumar, B.N.; Tverdal, A.; Meyer, H.E. Cardiovascular disease risk factors among five major ethnic groups in Oslo, Norway: The Oslo Immigrant Health Study. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 348–355. [Google Scholar] [CrossRef]
- Rabanal, K.S.; Selmer, R.M.; Igland, J.; Tell, G.S.; Meyer, H.E. Ethnic inequalities in acute myocardial infarction and stroke rates in Norway 1994–2009: A nationwide cohort study (CVDNOR). BMC Public Health 2015, 15, 1073. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B.; Grundy, S.; Sullivan, L.M.; Wilson, P.; the CHD Risk Prediction Group. Validation of the framingham coronary heart disease prediction scores: Results of a multiple ethnic groups investigation. JAMA 2001, 286, 180–187. [Google Scholar] [CrossRef]
- D’Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General Cardiovascular Risk Profile for Use in Primary Care. J. Am. Heart Assoc. 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Eichler, K.; Puhan, M.A.; Steurer, J.; Bachmann, L.M. Prediction of first coronary events with the Framingham score: A systematic review. Am. Heart J. 2007, 153, 722–731. [Google Scholar] [CrossRef]
- Schofield, P.; Chen, R.; Crichton, N. Methods for assessing cardiovascular disease risk in a UK black population. Heart 2012, 98, 1373–1377. [Google Scholar] [CrossRef]
- Brindle, P.M.; McConnachie, A.; Upton, M.N.; Hart, C.L.; Smith, G.D.; Watt, G.C. The accuracy of the Framingham risk-score in different socioeconomic groups: A prospective study. Br. J. Gen. Pract. 2005, 55, 838–845. [Google Scholar] [PubMed]
- Hurley, L.P.; Dickinson, L.M.; Estacio, R.O.; Steiner, J.F.; Havranek, E.P. Prediction of cardiovascular death in racial/ethnic minorities using Framingham risk factors. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Dugas, L.R.; Forrester, T.E.; Plange-Rhule, J.; Bovet, P.; Lambert, E.V.; Durazo-Arvizu, R.A.; Cao, G.; Cooper, R.S.; Khatib, R.; Tonino, L.; et al. Cardiovascular risk status of Afro-origin populations across the spectrum of economic development: Findings from the Modeling the Epidemiologic Transition Study. BMC Public Health 2017, 17, 438. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Ahmed, S.H.; Meyer, H.E.; Kjøllesdal, M.K.; Madar, A.A. Prevalence and Predictors of Overweight and Obesity among Somalis in Norway and Somaliland: A Comparative Study. J. Obes. 2018. [Google Scholar] [CrossRef]
- Ahmed, H.A. Prevalence of Cardiovascular Disease Risk Factors in Urban Garissa Residents; University of Nairobi: Nairobi, Kenya, 2012. [Google Scholar]
- Kish, L. A Procedure for Objective Respondent Selection within the Household. J. Am. Stat. Assoc. 1949, 44, 380–387. [Google Scholar] [CrossRef]
- World Health Organization (WHO). STEPS: A Framework for Surveillance, in the WHO STEPwise Approach to Surveillance of Noncommunicable Diseases (STEPS); World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Takahashi, H.; Yoshika, M.; Yokoi, T. Validation of Omron RS8, RS6, and RS3 Home Blood Pressure Monitoring Devices, in Accordance with the European Society of Hypertension International Protocol Revision 2010. Vasc. Health Risk Manag. 2013, 9, 265–272. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Waist Circumference and Waist-Hip Ratio: Report of A WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2011; p. 39. [Google Scholar]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Hjerte-/Karsykdom Risikofaktorer Tolkning Fürst Medisinsk Laboratorium. Available online: http://www.furst.no/analyse-og-klinikk/kliniske-problemstillinger/hjerte-karsykdom-risikofaktorer/tolkning/ (accessed on 1 August 2018).
- Framingham Heart Study Primary Risk Functions. Available online: https://www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/ (accessed on 26 May 2019).
- Tennakoon, S.U.B.; Kumar, B.N.; Nugegoda, D.B.; Meyer, H.E. Comparison of cardiovascular risk factors between Sri Lankans living in Kandy and Oslo. BMC Public Health 2010, 10, 654. [Google Scholar] [CrossRef]
- Tennakoon, S.U.; Kumar, B.N.; Meyer, H.E. Differences in selected lifestyle risk factors for cardiovascular disease between Sri Lankans in Oslo, Norway, and in Kandy, Sri Lanka. Asia Pac. J. Public Health 2015, 27, 616–625. [Google Scholar] [CrossRef]
- Mensink, R.P.; Katan, M.B. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler. Thromb. 1992, 12, 911–919. [Google Scholar] [CrossRef]
- Hajar, R. Framingham Contribution to Cardiovascular Disease. Heart Views 2016, 17, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Gele, A.A.; Pettersen, K.S.; Kumar, B.; Torheim, L.E. Diabetes Risk by Length of Residence among Somali Women in Oslo Area. J. Diabetes Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Chapman, R.H.; Grandy, S.; the SHIELD Investigators’ Group. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: Comparison of data from two national surveys. Int. J. Clin. Pract. 2007, 61, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; Miller, M.A. Cardiovascular disease and hypertension in sub-Saharan Africa: Burden, risk and interventions. Intern. Emerg. Med. 2016, 11, 299–305. [Google Scholar] [CrossRef]
- Tennakoon, S.U.; Kumar, B.N.; Selmer, R.; Mikram, M.J.; Meye, H.E. Differences in predicted cardiovascular risk in Sinhalese and Tamils in Sri Lanka compared with Sri Lankans in Norway. Asia Pac. J. Public Health 2013, 25, 452–462. [Google Scholar] [CrossRef]
- Respondent Selection within the Household—A Modification of the Kish Grid. Available online: https://www.stat.aau.at/Tagungen/Ossiach/Nemeth.pdf (accessed on 28 June 2019).
- Sogaard, A.J.; Selmer, R.; Bjertness, E.; Thelle, D. The Oslo Health Study: The impact of self-selection in a large, population-based survey. Int. J. Equity Health 2004, 3, 3. [Google Scholar] [CrossRef]
| Men | Oslo (n = 106) | Hargeisa (n = 145) | pValue |
| Age (years), Mean ± SD | 39.4 ± 11.0 | 38.2 ± 6.1 | 0.493 |
| Education (%) * | 89.4 | 52.4 | <0.001 |
| Employment (%) ** | 19.2 | 32.2 | 0.023 |
| Marital status (%) *** | 64.4 | 56.6 | 0.211 |
| Body mass index, Mean ± SD | 25.9 ± 3.4 | 22.1 ± 4.6 | <0.001 |
| SBP (mmHg), Mean ± SD | 126.0 ± 17.0 | 129.7 ± 27.8 | 0.185 |
| DBP (mmHg), Mean ± SD | 81.3 ± 10.3 | 82.7 ± 12.7 | 0.349 |
| Women | Oslo (n = 106) | Hargeisa (n = 953) | pValue |
| Age (years), Mean ± SD | 37.5 ± 9.6 | 39.1 ± 14.1 | 0.137 |
| Education (%) * | 48.6 | 14.3 | <0.001 |
| Employment (%) ** | 22.3 | 88.4 | <0.001 |
| Marital status (%) *** | 62.9 | 78.4 | <0.001 |
| Body mass index, Mean ± SD | 30.0 ± 6.7 | 27.2 ± 5.9 | <0.001 |
| SBP (mmHg), Mean ± SD | 118.1 ± 19.8 | 128.2 ± 24.6 | <0.001 |
| DBP (mmHg), Mean ± SD | 78.3 ± 9.7 | 83.8 ± 12.5 | <0.001 |
| Variables | Men | Women | ||||
|---|---|---|---|---|---|---|
| Oslo (n = 76) | Hargeisa (n = 60) | Mean Difference b (95% CI) | Oslo (n = 85) | Hargeisa (n = 534) | Mean Difference b (95% CI) | |
| Fasting serum glucose (mmol/L) | 5.3 | 5.4 | −0.1 (−0.6, 0.3) | 5.2 | 5.4 | −0.2 (−0.6, 0.3) |
| Triglycerides (mmol/L) | 1.1 | 1.6 | −0.5 (−0.8, −0.1) | 0.9 | 1.3 | −0.4 (−0.5, −0.2) |
| Total cholesterol (mmol/L) | 4.6 | 4.5 | 0.1 (−0.2, 0.3) | 4.5 | 4.7 | −0.2 (−0.3, 0.02) |
| HDL cholesterol (mmol/L) | 1.2 | 1.00 | 0.2 (0.1, 0.2) | 1.4 | 1.1 | 0.3 (0.2, 0.3) |
| LDL cholesterol (mmol/L) | 3.0 | 2.7 | 0.3 (0.1, 0.6) | 2.9 | 2.8 | 0.1 (−0.1, 0.3) |
| TC/HDL ratio | 3.9 | 4.4 | −0.5 (−0.8, −0.2) | 3.3 | 4.1 | −0.8 (−1.0, −0.6) |
| Risk Factors | Prevalence, % (95% CI) | ||
|---|---|---|---|
| Oslo | Hargeisa | p Value | |
| Men | |||
| Overweight/obese (BMI ≥ 25 kg/m2) | 61.5 (52.0, 71.1) | 22.9 (15.7, 30.0) | <0.001 |
| Hypertension (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg and/or medicated) | 34.1 (24.6, 43.5) | 32.9 (24.5, 41.3) | 0.860 |
| Diabetes (FSG ≥ 7.0 mmol/l and/or medicated) | 5.4 (6.8, 10.2) | 8.3 (4.3, 16.6) | 0.467 |
| High cholesterol (TC/HDL ratio ≥ 5 and/or medicated) | 19.1 (10.2, 28.0) | 35.0 (22.6, 47.4) | 0.040 |
| Current smokers | 20.1 (12.4, 27.9) | 27.1 (19.8, 34.4) | 0.209 |
| Alcohol consumption | 8.6 (3.2, 14.1) | 0 | n/a |
| Women | |||
| Overweight/obese (BMI ≥ 25 kg/m2) | 78.1 (70.4, 85.9) | 64.3 (61.1, 67.5) | 0.005 |
| Hypertension (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg and/or medicated) | 17.4 (9.9, 25.0) | 34.9 (31.5, 38.4) | 0.001 |
| Diabetes (FSG ≥ 7.0 mmol/l and/or medicated) | 9.4 (3.1, 15.7) | 11.1 (8.3, 13.9) | 0.649 |
| High cholesterol (TC/HDL ratio ≥ 5 and/or medicated) | 6.0 (1.0, 11.1) | 21.0 (17.4, 24.6) | 0.003 |
| Current smokers | 0 | 0 | |
| Alcohol consumption | 0 | 0 | |
| Predicted Risk | Men | Women | ||||
|---|---|---|---|---|---|---|
| Lipid-based | Oslo (n = 73) | Hargeisa (n = 50) | pValue | Oslo (n = 82) | Hargeisa (n = 470) | pValue |
| Predicted 10-year risk ≥ 10%, % (95% CI), at age 45 years | 22.8 (8.8, 36.8) | 49.6 (11.5, 87.7) | 0.118 | 8.5 (1.3, 15.6) | 8.4 (4.5, 12.5) | 0.987 |
| BMI-based | Oslo (n = 101) | Hargeisa (n = 134) | pValue | Oslo (n = 103) | Hargeisa (n = 860) | pValue |
| Predicted 10-year risk ≥ 10%, % (95% CI), at age 45 years | 41.4 (27.0, 55.7) | 54.4 (33.1, 75.8) | 0.335 | 9.1 (0.1, 16.3) | 8.6 (5.9, 11.3) | 0.895 |
| Level of Education | Lipid-Based Framingham Risk Score | BMI-Based Framingham Risk Score | ||||
|---|---|---|---|---|---|---|
| Men | N= 123 | Mean (95% CI) | pValue | N= 235 | Mean (95% CI) | pValue |
| Education | ||||||
| < Secondary school | 35 | 5.5 (3.0, 7.9) | 0.890 | 73 | 5.8 (4.3, 7.4) | 0.149 |
| ≥ Secondary school | 88 | 5.3 (3.0, 7.4) | 162 | 7.2 (5.6, 8.8) | ||
| Women | N= 552 | Mean (95% CI) | pValue | N= 963 | Mean (95% CI) | pValue |
| Education | ||||||
| < Secondary school | 445 | 3.6 (3.0, 4.3) | 0.078 | 781 | 3.7 (3.2, 4.2) | 0.897 |
| ≥ Secondary school | 107 | 3.1 (2.3, 3.9) | 182 | 3.8 (3.0, 4.5) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.H.; Marjerrison, N.; Kjøllesdal, M.K.R.; Stigum, H.; Htet, A.S.; Bjertness, E.; Meyer, H.E.; Madar, A.A. Comparison of Cardiovascular Risk Factors among Somalis Living in Norway and Somaliland. Int. J. Environ. Res. Public Health 2019, 16, 2353. https://doi.org/10.3390/ijerph16132353
Ahmed SH, Marjerrison N, Kjøllesdal MKR, Stigum H, Htet AS, Bjertness E, Meyer HE, Madar AA. Comparison of Cardiovascular Risk Factors among Somalis Living in Norway and Somaliland. International Journal of Environmental Research and Public Health. 2019; 16(13):2353. https://doi.org/10.3390/ijerph16132353
Chicago/Turabian StyleAhmed, Soheir H., Niki Marjerrison, Marte Karoline Råberg Kjøllesdal, Hein Stigum, Aung Soe Htet, Espen Bjertness, Haakon E. Meyer, and Ahmed A. Madar. 2019. "Comparison of Cardiovascular Risk Factors among Somalis Living in Norway and Somaliland" International Journal of Environmental Research and Public Health 16, no. 13: 2353. https://doi.org/10.3390/ijerph16132353
APA StyleAhmed, S. H., Marjerrison, N., Kjøllesdal, M. K. R., Stigum, H., Htet, A. S., Bjertness, E., Meyer, H. E., & Madar, A. A. (2019). Comparison of Cardiovascular Risk Factors among Somalis Living in Norway and Somaliland. International Journal of Environmental Research and Public Health, 16(13), 2353. https://doi.org/10.3390/ijerph16132353





