Does Cardiorespiratory Fitness Moderate the Association between Occupational Stress, Cardiovascular Risk, and Mental Health in Police Officers?
Abstract
1. Introduction
- Our first hypothesis was that higher levels of occupational stress will be associated with an increased cardiovascular risk, and more frequent mental health complaints, independent of the model used to operationalize occupational stress.
- Our second hypothesis was that higher levels of cardiorespiratory fitness will be associated with a lower cardiovascular risk, and fewer mental health complaints.
- Our third hypothesis was that cardiorespiratory fitness will moderate the relationship between occupational stress and physical/mental health indicators: Thus, the relationship will become smaller as a function of increasing fitness levels, independent on whether occupational stress is operationalized via the JDC or ERI model.
2. Materials and Methods
2.1. Participants and Procedures
2.2. Measures
2.2.1. Occupational Stress
2.2.2. Cardiovascular Risk Markers
2.2.3. Mental Health
2.2.4. Cardiorespiratory Fitness
2.3. Statistical Analyses
3. Results
3.1. Sample Description
3.2. Descriptive Statistics and Correlations between Independent and Dependent Variables
3.3. Main and Interaction Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chrousos, G.P. Stress and Disorders of the Stress System. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.; Schilling, R. Stress als Risikofaktor für körperliche und psychische Gesundheitsbeeinträchtigungen. In Handbuch Stressregulation und Sport; Fuchs, R., Gerber, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 93–122. [Google Scholar]
- Kaltsas, G.A.; Chrousos, G.P. The Neuroendocrinology of Stress. In Handbook of Psychophysiology, 3rd ed.; Cambridge University Press: New York, NY, USA, 2007; pp. 303–318. [Google Scholar]
- Russ, T.C.; Stamatakis, E.; Hamer, M.; Starr, J.M.; Kivimäki, M.; Batty, G.D. Association between Psychological Distress and Mortality: Individual Participant Pooled Analysis of 10 Prospective Cohort Studies. BMJ 2012, 345. [Google Scholar] [CrossRef] [PubMed]
- American Psychological Association (APA). Stress in America: The State of Our Nation (1 November 2017); APA: Washington, DC, USA, 2017. [Google Scholar]
- Parent-Thirion, A.; Vermeylen, G.; Cabrita, J.; Wilkens, M.; Biletta, I.; Vargas, O.; Wilczyńska, A. 6th European Working Conditions Survey—Overview Report; European Foundation for the Improvement of Living and Working Conditions: Luxembourg, 2016.
- Guazzi, M.; Faggiano, P.; Mureddu, G.F.; Faden, G.; Niebauer, J.; Temporelli, P.L. Worksite Health and Wellness in the European Union. Prog. Cardiovasc. Dis. 2014, 56, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Karasek, R.A., Jr. Job Demands, Job Decision Latitude, and Mental Strain: Implications for Job Redesign. Adm. Sci. Q. 1979, 24, 285–308. [Google Scholar] [CrossRef]
- Siegrist, J.; Siegrist, K.; Weber, I. Sociological Concepts in the Etiology of Chronic Disease: The Case of Ischemic Heart Disease. Soc. Sci. Med. 1986, 22, 247–253. [Google Scholar] [PubMed]
- Kivimäki, M.; Leino-Arjas, P.; Luukkonen, R.; Riihimaki, H.; Vahtera, J.; Kirjonen, J. Work Stress and Risk of Cardiovascular Mortality: Prospective Cohort Study of Industrial Employees. BMJ 2002, 325, 857. [Google Scholar]
- Siegrist, J.; Dragano, N. Psychosocial Stress and Disease Risks in Occupational Life. Results of International Studies on the Demand-Control and the Effort-Reward Imbalance Models. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2008, 51, 305–312. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Noncommunicable Diseases Country Profiles 2018; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Nyberg, S.T.; Fransson, E.I.; Heikkilä, K.; Alfredsson, L.; Casini, A.; Clays, E.; De Bacquer, D.; Dragano, N.; Erbel, R.; Hamer, M.; et al. Job Strain and Cardiovascular Disease Risk Factors: Meta-Analysis of Individual-Participant Data from 47,000 Men and Women. PLoS ONE 2013, 8, e67323. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction: The American Heart Association’s Strategic Impact Goal Through 2020 and Beyond. Circulation 2010, 121, 586–613. [Google Scholar]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The Metabolic Syndrome and Cardiovascular Risk a Systematic Review and Meta-Analysis. J. Am. Coll. Cardiolo. 2010, 56, 1113–1132. [Google Scholar]
- Quick, J.C.; Henderson, D.F. Occupational Stress: Preventing Suffering, Enhancing Wellbeing. Int. J. Environ. Res. Public Health 2016, 13, 459. [Google Scholar] [CrossRef] [PubMed]
- Marquez, P.V.; Saxena, S. Making Mental Health a Global Priority. Cerebrum Dana Forum Brain Sci. 2016, 2016, cer-10-16. [Google Scholar]
- Maslach, C.; Schaufeli, W.B.; Leiter, M.P. Job Burnout. Annu. Rev. Psychol. 2001, 52, 397–422. [Google Scholar] [CrossRef] [PubMed]
- Akerstedt, T.; Knutsson, A.; Westerholm, P.; Theorell, T.; Alfredsson, L.; Kecklund, G. Sleep Disturbances, Work Stress and Work Hours: A Cross-Sectional Study. J. Psychosom. Res. 2002, 53, 741–748. [Google Scholar] [CrossRef]
- Gerber, M.; Hartmann, T.; Brand, S.; Holsboer-Trachsler, E.; Pühse, U. The relationship between shift work, perceived stress, sleep and health in Swiss police officers. J. Crim. Justice 2010, 38, 1167–1175. [Google Scholar] [CrossRef]
- Shane, J.M. Organizational stressors and police performance. J. Crim. Justice 2010, 38, 807–818. [Google Scholar] [CrossRef]
- Tadje, K. Demographischer Wandel und Gesundheitsmanagement am Beispiel der Polizei: Kultur als Determinante organisationaler Veränderung; disserta Verlag: Hamburg, Germany, 2014. [Google Scholar]
- Waters, J.A.; Ussery, W. Police stress: History, contributing factors, symptoms, and interventions. Polic. Int. J. 2007, 30, 169–188. [Google Scholar] [CrossRef]
- Habersaat, S.A.; Geiger, A.M.; Abdellaoui, S.; Wolf, J.M. Health in police officers: Role of risk factor clusters and police divisions. Soc. Sci. Med. 2015, 143, 213–222. [Google Scholar] [CrossRef]
- Wang, X.S.; Armstrong, M.E.; Cairns, B.J.; Key, T.J.; Travis, R.C. Shift work and chronic disease: The epidemiological evidence. Occup. Med. (Oxf. Engl.) 2011, 61, 78–89. [Google Scholar] [CrossRef]
- Hartley, T.A.; Violanti, J.M.; Fekedulegn, D.; Andrew, M.E.; Burchfiel, C.M. Associations between major life events, traumatic incidents, and depression among Buffalo police officers. Int. J. Emerg. Ment. Health 2007, 9, 25–35. [Google Scholar]
- Hartley, T.A.; Burchfiel, C.M.; Fekedulegn, D.; Andrew, M.E.; Knox, S.S.; Violanti, J.M. Associations between Police Officer Stress and the Metabolic Syndrome. Int. J. Emerg. Ment. Health 2011, 13, 243–256. [Google Scholar] [PubMed]
- Magnavita, N.; Capitanelli, I.; Garbarino, S.; Pira, E. Work-Related Stress as a Cardiovascular Risk Factor in Police Officers: A Systematic Review of Evidence. Int. Arch. Occup. Environ. Health 2018, 91, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Collingwood, T.; Hoffmann, R.; Smith, J. Why Officers Need to Be Fit. Fit. Force Administrators Guide; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Hartley, T.A.; Burchfiel, C.M.; Fekedulegn, D.; Andrew, M.E.; Violanti, J.M. Health disparities in police officers: Comparisons to the U.S. general population. Int. J. Emerg. Ment. Health 2011, 13, 211–220. [Google Scholar] [PubMed]
- van der Velden, P.G.; Rademaker, A.R.; Vermetten, E.; Portengen, M.-A.; Yzermans, J.C.; Grievink, L. Police officers: A high-risk group for the development of mental health disturbances? A cohort study. BMJ Open 2013, 3, e001720. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Kivimaki, M. Stress and cardiovascular disease. Nat. Rev. Cardiol. 2012, 9, 360–370. [Google Scholar] [CrossRef] [PubMed]
- von Haaren, B.; Ottenbacher, J.; Muenz, J.; Neumann, R.; Boes, K.; Ebner-Priemer, U. Does a 20-Week Aerobic Exercise Training Programme Increase Our Capabilities to Buffer Real-Life Stressors? A Randomized, Controlled Trial Using Ambulatory Assessment. Eur. J. Appl. Physiol. 2016, 116, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Paton, D.; Violanti, J.M.; Johnston, P.; Burke, K.J.; Clarke, J.; Keenan, D. Stress shield: A model of police resiliency. Int. J. Emerg. Ment. Health 2008, 10, 95–107. [Google Scholar]
- Kobasa, S.C.; Maddi, S.R.; Puccetti, M.C. Personality and Exercise as Buffers in the Stress-Illness-Relationship. J. Behav. Med. 1982, 5, 391–404. [Google Scholar] [CrossRef]
- Gerber, M.; Puhse, U. Review Article: Do Exercise and Fitness Protect Against Stress-Induced Health Complaints? A Review of the Literature. Scand. J. Public Health 2009, 37, 801–819. [Google Scholar] [CrossRef]
- Gerber, M.; Börjesson, M.; Ljung, T.; Lindwall, M.; Jonsdottir, I.H. Fitness Moderates the Relationship between Stress and Cardiovascular Risk Factors. Med. Sci. Sports Exerc. 2016, 48, 2075–2081. [Google Scholar] [CrossRef]
- Gerber, M.; Kellmann, M.; Hartmann, T.; Pühse, U. Do Exercise and Fitness Buffer Against Stress among Swiss Police and Emergency Response Service Officers? Psychol. Sport Exerc. 2010, 11, 286–294. [Google Scholar] [CrossRef]
- Holtermann, A.; Mortensen, O.S.; Burr, H.; Søgaard, K.; Gyntelberg, F.; Suadicani, P. Physical Demands at Work, Physical Fitness, and 30-Year Ischaemic Heart Disease and All-Cause Mortality in the Copenhagen Male Study. Scand. J. Work Environ. Health 2010, 36, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.; Jonsdottir, I.H.; Lindwall, M.; Ahlborg, G. Physical Activity in Employees with Differing Occupational Stress and Mental Health Profiles: A Latent Profile Analysis. Psychol. Sport Exerc. 2014, 15, 649–658. [Google Scholar] [CrossRef]
- Gerber, M.; Isoard-Gautheur, S.; Schilling, R.; Ludyga, S.; Brand, S.; Colledge, F. When Low Leisure-Time Physical Activity Meets Unsatisfied Psychological Needs: Insights from a Stress-Buffer Perspective. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Isoard-Gautheur, S.; Ginoux, C.; Gerber, M.; Sarrazin, P. The Stress-Burnout Relationship: Examining the Moderating Effect of Physical Activity and Intrinsic Motivation for Off-Job Physical Activity. Workplace Health Saf. 2019. [Google Scholar] [CrossRef] [PubMed]
- Siu, O.; Cooper, C.L.; Leung, T. Three-Wave Trend Study of Managerial Stress in Hong Kong: The Role of Type A Behavior and Exercise. Int. J. Stress Manag. 2000, 7, 153–157. [Google Scholar] [CrossRef]
- Schmidt, K.-H.; Beck, R.; Rivkin, W.; Diestel, S. Self-Control Demands at Work and Psychological Strain: The Moderating Role of Physical Fitness. Int. J. Stress Manag. 2016, 23, 255–275. [Google Scholar] [CrossRef]
- Ekelund, U.; Anderssen, S.A.; Froberg, K.; Sardinha, L.B.; Andersen, L.B.; Brage, S. Independent Associations of Physical Activity and Cardiorespiratory Fitness with Metabolic Risk Factors in Children: The European Youth Heart Study. Diabetologia 2007, 50, 1832–1840. [Google Scholar] [CrossRef]
- Semmer, N.; Zapf, D. Theorien der Stressentstehung und bewältigung; Springer: Berlin, Germany, 2018; pp. 23–50. [Google Scholar]
- Karasek, R.; Baker, D.; Marxer, F.; Ahlbom, A.; Theorell, T. Job decision latitude, job demands, and cardiovascular disease: A prospective study of Swedish men. Am. J. Public Health 1981, 71, 694–705. [Google Scholar] [CrossRef]
- Siegrist, J. Adverse Health Effects of High Effort—Low Reward Conditions at Work. J. Occup. Psychol. 1996, 1, 27–43. [Google Scholar] [CrossRef]
- Karasek, R.; Brisson, C.; Kawakami, N.; Houtman, I.; Bongers, P.; Amick, B. The Job Content Questionnaire (JCQ): An instrument for internationally comparative assessments of psychosocial job characteristics. J. Occup. Health Psychol. 1998, 3, 322–355. [Google Scholar] [CrossRef] [PubMed]
- Van der Doef, M.; Maes, S. The Job Demand-Control (-Support) Model and Psychological Well-Being: A Review of 20 Years of Empirical Research. Work Stress 1999, 13, 87–114. [Google Scholar] [CrossRef]
- Siegrist, J.; Starke, D.; Chandola, T.; Godin, I.; Marmot, M.; Niedhammer, I.; Peter, R. The measurement of effort-reward imbalance at work: European comparisons. Soc. Sci. Med. 2004, 58, 1483–1499. [Google Scholar] [CrossRef]
- Bergenstal, R.M. Evaluating the Accuracy of Modern Glucose Meters. Insulin 2008, 3, 5–14. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy; WHO Document Production Services; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Parikh, P.; Mochari, H.; Mosca, L. Clinical Utility of a Fingerstick Technology to Identify Individuals with Abnormal Blood Lipids and High-Sensitivity C-Reactive Protein Levels. Am. J. Health Promot. 2009, 23, 279–282. [Google Scholar] [CrossRef]
- Jain, A.; Rao, N.; Sharifi, M.; Bhatt, N.; Patel, P.; Nirmal, D.; Persaud, J.W.; Nair, D.R. Evaluation of the point of care Afinion AS100 analyser in a community settin. Ann. Clin. Biochem. 2017, 54, 331–341. [Google Scholar] [CrossRef]
- Moebus, S.; Göres, L.; Lösch, C.; Jöckel, K.-H. Impact of time since last caloric intake on blood glucose levels. Eur. J. Epidemiol. 2011, 26, 719–728. [Google Scholar] [CrossRef]
- Mora, S. Nonfasting for Routine Lipid Testing: From Evidence to Action. JAMA Int. Med. 2016, 176, 1005–1006. [Google Scholar] [CrossRef]
- Shirom, A.; Melamed, S. A Comparison of the Construct Validity of Two Burnout Measures in Two Groups of Professionals. Int. J. Stress Manag. 2006, 13, 176–200. [Google Scholar] [CrossRef]
- Lundgren-Nilsson, A.; Jonsdottir, I.H.; Pallant, J.; Ahlborg, G., Jr. Internal construct validity of the Shirom-Melamed Burnout Questionnaire (SMBQ). BMC Public Health 2012, 12, 1. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index (ISI) as an Outcome Measure for Insomnia Research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Belanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.; Lang, C.; Lemola, S.; Colledge, F.; Kalak, N.; Holsboer-Trachsler, E.; Pühse, U.; Brand, S. Validation of the German Version of the Insomnia Severity Index in Adolescents, Young Adults and Adult Workers: Results from Three Cross-Sectional Studies. BMC Psychiatry 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Romppel, M.; Braehler, E.; Roth, M.; Glaesmer, H. What is the General Health Questionnaire-12 assessing?: Dimensionality and Psychometric Properties of the General Health Questionnaire-12 in a Large Scale German Population Sample. Compr. Psychiatry 2013, 54, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Puustinen, P.J.; Koponen, H.; Kautiainen, H.; Mäntyselkä, P.; Vanhala, M. Psychological Distress Measured by the GHQ-12 and Mortality: A Prospective Population-Based Study. Scand. J. Public Health 2011, 39, 577–581. [Google Scholar] [CrossRef]
- Åstrand, P.-O.; Rodahl, K. Textbook of Work Physiology: Physiological Bases of Exercise; Human Kinetics: Champaign, IL, USA, 2003. [Google Scholar]
- Foss, M.L.; Keteyian, S.J.; Fox, E.L. Fox’s Physiological Basis for Exercise and Sport, 6th ed.; McGraw-Hill: Boston, MA, USA, 1998. [Google Scholar]
- Nordgren, B.; Fridén, C.; Jansson, E.; Österlund, T.; Grooten, W.J.; Opava, C.H.; Rickenlund, A. Criterion Validation of Two Submaximal Aerobic Fitness Tests, the Self-Monitoring Fox-Walk Test and the Åstrand Cycle Test in People with Rheumatoid Arthritis. BMC Musculoskelet. Disord. 2014, 15, 305. [Google Scholar] [CrossRef][Green Version]
- Ferguson, B. ACSM’s Guidelines for Exercise Testing and Prescription 9th Ed. J. Can. Chiropr. Assoc. 2014, 58, 328. [Google Scholar]
- McLachlan, G.J.; Krishnam, T. The EM Algorithm and Extensions; Wiley Interscience Publishers: New York, NY, USA, 2007. [Google Scholar]
- Cifkova, R.; Erdine, S.; Fagard, R.; Farsang, C.; Heagerty, A.M.; Kiowski, W. Hypertension Guidelines Committee. Practice Guidelines for Primary Care Physicians: 2003 ESH/ESC Hypertension Guidelines. J. Hypertens. 2003, 21, 1779–1786. [Google Scholar]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; Technical Report Series No. 894; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Rodondi, N.; Gencer, B.; Collet, T.H.; Battegay, E. Ab welchem Cholesterinwert Soll in der Schweiz eine Behandlung Erfolgen? Swiss Med. Forum 2011, 11, 467–472. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. 1), S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.; Williams, P.A. User’s Guide to the General Health Questionnaire; NFER-Nelson: Windsor, UK, 1988. [Google Scholar]
- West, S.G.; Finch, J.F.; Curran, P.J. Structural Equation Models with Nonnormal Variables: Problems and Remedies. In Structural Equation Modeling Concepts, Issues, and Applications; Hoyle, R.H., Ed.; Sage: Thousand Oaks, CA, USA, 1995; pp. 56–75. [Google Scholar]
- Ahola, K.; Hakanen, J. Job Strain, Burnout, and Depressive Symptoms: A Prospective Study Among Dentists. J. Affect. Disord. 2007, 104, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Vahtera, J.; Elovainio, M.; Virtanen, M.; Siegrist, J. Effort-Reward Imbalance, Procedural Injustice and Relational Injustice as Psychosocial Predictors of Health: Complementary or Redundant Models? Occup. Environ. Med. 2007, 64, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, L.; Stephens, C.; Paton, D. An evaluation of traumatic and organizational experiences on the psychological health of New Zealand police recruits. Work (Read. Mass) 2007, 28, 199–207. [Google Scholar]
- Violanti, J.M.; Ma, C.C.; Fekedulegn, D.; Andrew, M.E.; Gu, J.K.; Hartley, T.A.; Charles, L.E.; Burchfiel, C.M. Associations Between Body Fat Percentage and Fitness among Police Officers: A Statewide Study. Saf. Health Work 2017, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health Benefits of Physical Activity: The Evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Despres, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef]
- Aspenes, S.T.; Nilsen, T.I.; Skaug, E.A.; Bertheussen, G.F.; Ellingsen, O.; Vatten, L.; Wisløff, U. Peak Oxygen Uptake and Cardiovascular Risk Factors in 4631 Healthy Women and Men. Med. Sci. Sports Exerc. 2011, 43, 1465–1473. [Google Scholar] [CrossRef]
- Fernström, M.; Fernberg, U.; Eliason, G.; Hurtig-Wennlöf, A. Aerobic Fitness is Associated with Low Cardiovascular Disease Risk: The Impact of Lifestyle on Early Risk Factors for Atherosclerosis in Young Healthy Swedish Individuals—The Lifestyle, Biomarker, and Atherosclerosis Study. Vasc. Health Risk Manag. 2017, 13, 91–99. [Google Scholar] [CrossRef]
- Vancampfort, D.; Rosenbaum, S.; Schuch, F.; Ward, P.B.; Richards, J.; Mugisha, J.; Probst, M.; Stubbs, B. Cardiorespiratory Fitness in Severe Mental Illness: A Systematic Review and Meta-Analysis. Sports Med. (Auckl. NZ) 2017, 47, 343–352. [Google Scholar] [CrossRef]
- Gerber, M.; Lindwall, M.; Lindegård, A.; Börjesson, M.; Jonsdottir, I.H. Cardiovascular Fitness Protects from Stress-Related Symptoms of Burnout and Depression. Patient Educ. Couns. 2013, 93, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.B.; Lee, D.-C.; Sui, X.; Kubzansky, L.D.; Ruiz, J.R.; Baruth, M.; Castillo, M.J.; Blair, S.N. Psychological Well-Being, Cardiorespiratory Fitness, and Long-Term Survival. Am. J. Prev. Med. 2010, 39, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Shah, D.; Payal, A.R. Healthy Worker Effect Phenomenon: Revisited with Emphasis on Statistical Methods—A Review. Indian J. Occup. Environ. Med. 2017, 21, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.; Endes, K.; Herrmann, C.; Colledge, F.; Brand, S.; Donath, L.; Faude, O.; Pühse, U.; Hanssen, H.; Zahner, L. Fitness, Stress, and Body Composition in Primary Schoolchildren. Med. Sci. Sports Exerc. 2017, 49, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Klaperski, S. Exercise, Stress and Health: The Stress-Buffering Effect of Exercise. In Handbuch Stressregulation und Sport; Fuchs, R., Gerber, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 227–249. [Google Scholar]
- Young, D.R. Can Cardiorespiratory Fitness Moderate the Negative Effects of Stress on Coronary Artery Disease Risk Factors? J. Psychosom. Res. 1994, 38, 451–459. [Google Scholar] [CrossRef]
- Ritvanen, T.; Louhevaara, V.; Helin, P.; Halonen, T.; Hanninen, O. Effect of Aerobic Fitness on the Physiological Stress Responses at Work. Int. J. Occup. Med. Environ. Health 2007, 20, 1–8. [Google Scholar] [CrossRef]
- Mücke, M.; Ludyga, S.; Colledge, F.; Gerber, M. Influence of Regular Physical Activity and Fitness on Stress Reactivity as Measured with the Trier Social Stress Test Protocol: A Systematic Review. Sports Med. 2018, 48, 2607–2622. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.; Schablon, A.; Latza, U.; Nienhaus, A. Musculoskeletal Pain and Effort-Reward Imbalance—A Systematic Review. BMC Public Health 2014, 14, 37. [Google Scholar] [CrossRef]
| Descriptive Statistics | n | M | SD | Range | Skew | Kurt | Bivariate Correlations between Independent and Dependent Variables | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. | 16. | |||||||
| Stress | ||||||||||||||||||||||
| 1. JDC ratio | 201 | 1.0 | 0.2 | 0.5 to 1.6 | 0.7 | 0.4 | - | |||||||||||||||
| 2. ERI ratio | 201 | 0.9 | 0.3 | 0.3 to 2.0 | 0.9 | 1.5 | 0.33 * | - | ||||||||||||||
| Cardiorespiratory fitness | ||||||||||||||||||||||
| 3. VO2max | 201 | 3.5 | 0.9 | 1.6 to 5.9 | 0.3 | −0.6 | 0.10 | 0.05 | - | |||||||||||||
| Cardiovascular risk factors | ||||||||||||||||||||||
| 4. SBP (mmHg) | 201 | 129 | 13 | 104 to 172 | 0.5 | 0.2 | −0.05 | 0.13 | 0.10 | - | ||||||||||||
| 5. DBP (mmHg) | 201 | 85 | 10 | 63 to 118 | 0.2 | −0.3 | −0.11 | 0.12 | 0.02 | 0.84 * | - | |||||||||||
| 6. BMI (kg·m−2) | 201 | 25.8 | 3.6 | 17.9 to 37.4 | 0.7 | 0.8 | 0.00 | 0.16 * | 0.25 * | 0.33 * | 0.37 * | - | ||||||||||
| 7. Waist circumference (cm) | 201 | 91.1 | 11.3 | 60.0 to 126.0 | 0.4 | 0.8 | −0.08 | 0.14 * | 0.20 * | 0.37 * | 0.43 * | 0.83 * | - | |||||||||
| 8. Body fat (%) | 201 | 21.8 | 7.3 | 5.6 to 42.6 | 0.4 | −0.2 | −0.01 | 0.02 | −0.38 * | 0.01 | 0.03 | 0.37 * | 0.30 * | - | ||||||||
| 9. TC (mmol·L−1) | 201 | 5.0 | 1.0 | 2.9 to 10.4 | 1.1 | 3.2 | −0.11 | 0.05 | 0.00 | 0.28 * | 0.34 * | 0.31 * | 0.32 * | 0.14 | - | |||||||
| 10. HDL-C (mmol·L−1) | 201 | 1.8 | 0.4 | 0.6 to 2.6 | 0.1 | −0.5 | −0.07 | −0.08 | −0.14 * | −0.13 | −0.15 * | −0.35 * | −0.35 * | 0.15 * | 0.20 * | - | ||||||
| 11. LDL-C (mmol·L−1) | 201 | 2.5 | 0.8 | 1.0 to 5.0 | 0.6 | 0.2 | −0.06 | 0.05 | 0.05 | 0.24 * | 0.33 * | 0.28 * | 0.29 * | 0.05 | 0.81 * | −0.06 | - | |||||
| 12. TG (mmol·L−1) | 201 | 1.7 | 1.2 | 0.5 to 7.4 | 2.3 (0.6) | 6.5 (0.2) | −0.07 | 0.09 | 0.03 | 0.26 * | 0.27 * | 0.42 * | 0.41 * | 0.05 | 0.53 * | −0.29 * | 0.12 | - | ||||
| 13. HbA1c (%) | 201 | 5.4 | 0.3 | 4.9 to 7.5 | 2.4 (1.7) | 13.7 (6.9) | −0.15 * | 0.03 | −0.06 | 0.19 * | 0.26 * | 0.27 * | 0.35 * | 0.09 | 0.09 | −0.24 * | 0.14 * | 0.14 | - | |||
| 14. Total cardiometabolic risk | 201 | 0.0 | 4.7 | −10.6 to 16.7 | 0.7 | 0.8 | −0.10 | 0.15 * | 0.06 | 0.63 * | 0.69 * | 0.78 * | 0.80 * | 0.32 * | 0.62 * | −0.38 * | 0.57 * | 0.60 * | 0.47 * | - | ||
| Mental health indicators | ||||||||||||||||||||||
| 15. Burnout symptoms | 201 | 2.5 | 1.0 | 1 to 6 | 0.9 | 0.7 | 0.31 * | 0.31 * | −0.08 | −0.08 | −0.05 | 0.02 | −0.02 | 0.14 * | −0.08 | 0.03 | −0.09 | −0.04 | −0.03 | −0.05 | - | |
| 16. Insomnia symptoms | 201 | 7.9 | 4.3 | 0 to 22 | 0.5 | 0.0 | 0.23 * | 0.21 * | −0.06 | −0.06 | −0.05 | 0.00 | −0.02 | 0.05 | −0.01 | −0.08 | 0.00 | 0.03 | −0.04 | 0.00 | 0.46 * | - |
| 17. Overall mental wellbeing | 201 | 1.7 | 2.5 | 0 to 11 | 1.7 | 2.2 | 0.26 * | 0.25 * | −0.04 | 0.02 | 0.00 | 0.14 * | 0.09 | 0.18 * | −0.02 | 0.08 | −0.08 | 0.02 | −0.04 | 0.04 | −0.62 * | 0.31 * |
| Low Occupational Stress (JDC Score) | High Occupational Stress (JDC Score) | Stress (JDC) | CRF | Stress (JDC) × CRF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low CRF (n = 36) | Moderate CRF (n = 34) | High CRF (n = 51) | Low CRF (n = 17) | Moderate CRF (n = 25) | High CRF (n = 38) | |||||||
| M ± SD | M ± SD | M ± SD | M ± SD | M ± SD | M ± SD | F | η2 | F | η2 | F | η2 | |
| Cardiovascular risk factors | ||||||||||||
| SBP (mmHg) | 129 ± 12 | 130 ± 8 | 129 ± 15 | 132 ± 14 | 132 ± 15 | 127 ± 13 | 1.6 | 0.009 | 1.2 | 0.013 | 3.0 | 0.032 |
| DBP (mmHg) | 88 ± 11 | 86 ± 7 | 83 ± 10 | 86 ± 12 | 86 ± 10 | 83 ± 11 | 0.5 | 0.002 | 2.9 | 0.031 | 0.7 | 0.007 |
| BMI (kg·m−2) | 27.8 ± 4.7 | 26.3 ± 3.3 | 24.0 ± 2.7 | 26.6 ± 4.0 | 25.8 ± 3.4 | 25.8 ± 2.6 | 0.0 | 0.000 | 7.1 ** | 0.071 | 1.6 | 0.017 |
| Waist circumference (cm) | 97.6 ± 13.6 | 93.9 ± 10.9 | 85.6 ± 9.0 | 95.1 ± 12.3 | 90.4 ± 9.3 | 88.6 ± 8.9 | 0.0 | 0.000 | 11.8 *** | 0.114 | 0.9 | 0.010 |
| Body fat (%) | 24.9 ± 7.7 | 20.8 ± 5.5 | 20.4 ± 7.3 | 23.6 ± 6.2 | 23.4 ± 8.2 | 19.8 ± 7.0 | 0.5 | 0.003 | 22.1 *** | 0.194 | 1.4 | 0.015 |
| TC (mmol·L−1) | 5.1 ± 1.4 | 5.1 ± 1.1 | 5.1 ± 0.9 | 5.3 ± 1.2 | 4.9 ± 0.8 | 4.8 ± 0.8 | 0.2 | 0.000 | 0.7 | 0.007 | 1.1 | 0.012 |
| HDL-C (mmol·L−1) | 1.7 ± 0.4 | 1.8 ± 0.4 | 2.0 ± 0.4 | 1.6 ± 0.5 | 1.8 ± 0.5 | 1.9 ± 0.3 | 0.2 | 0.001 | 5.5 ** | 0.056 | 0.0 | 0.000 |
| LDL-C (mmol·L−1) | 2.5 ± 1.0 | 2.4 ± 0.8 | 2.5 ± 0.8 | 2.6 ± 0.8 | 2.4 ± 0.7 | 2.3 ± 0.6 | 0.0 | 0.000 | 0.6 | 0.007 | 0.7 | 0.007 |
| TG (mmol·L−1) | 1.9 ± 1.3 | 2.0 ± 1.5 | 1.4 ± 0.6 | 2.3 ± 1.9 | 1.6 ± 1.0 | 1.4 ± 0.7 | 0.1 | 0.001 | 3.7 * | 0.038 | 1.9 | 0.020 |
| HbA1c (%) | 5.5 ± 0.3 | 5.5 ± 0.4 | 5.4 ± 0.2 | 5.5 ± 0.3 | 5.5 ± 0.2 | 5.4 ± 0.2 | 0.0 | 0.000 | 3.6 * | 0.038 | 0.2 | 0.003 |
| Total cardiometabolic risk | 2.8 ± 7.7 | 0.8 ± 5.0 | −2.0 ± 4.9 | 2.4 ± 6.7 | 0.3 ± 5.2 | −2.0 ± 4.0 | 0.5 | 0.003 | 13.3 *** | 0.127 | 0.2 | 0.002 |
| Mental health indicators | ||||||||||||
| Burnout symptoms | 2.3 ± 0.9 | 2.4 ± 1.1 | 2.4 ± 0.8 | 2.6 ± 0.9 | 2.9 ± 1.4 | 2.9 ± 1.0 | 15.9 *** | 0.080 | 1.0 | 0.010 | 1.8 | 0.020 |
| Sleep complaints | 7.7 ± 4.1 | 7.2 ± 3.5 | 6.4 ± 3.6 | 8.8 ± 4.3 | 9.4 ± 5.2 | 9.1 ± 5.0 | 6.5 * | 0.034 | 0.6 | 0.007 | 1.6 | 0.017 |
| Overall mental distress | 1.4 ± 2.0 | 1.1 ± 2.0 | 0.9 ± 1.8 | 1.8 ± 2.1 | 2.9 ± 3.3 | 2.5 ± 3.2 | 14.1 *** | 0.071 | 1.0 | 0.010 | 1.2 | 0.013 |
| Low Occupational Stress (ERI Score) | High Occupational Stress (ERI Score) | Stress (ERI) | CRF | Stress (ERI) × CRF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low CRF (n = 34) | Moderate CRF (n = 44) | High CRF (n = 69) | Low CRF (n = 19) | Moderate CRF (n = 15) | High CRF (n = 20) | |||||||
| M ± SD | M ± SD | M ± SD | M ± SD | M ± SD | M ± SD | F | η2 | F | η2 | F | η2 | |
| Cardiovascular risk factors | ||||||||||||
| SBP (mmHg) | 130 ± 13 | 130 ± 11 | 129 ± 15 | 131 ± 12 | 132 ± 15 | 127 ± 13 | 0.1 | 0.001 | 1.9 | 0.020 | 1.8 | 0.019 |
| DBP (mmHg) | 87 ± 11 | 85 ± 8 | 83 ± 11 | 87 ± 12 | 87 ± 10 | 83 ± 7 | 0.2 | 00.001 | 4.1 * | 0.043 | 1.3 | 0.014 |
| BMI (kg·m−2) | 27.0 ± 3.8 | 25.9 ± 3.4 | 24.4 ± 2.8 | 28.1 ± 5.6 | 26.8 ± 3.0 | 25.2 ± 2.4 | 1.7 | 0.009 | 9.7 *** | 0.095 | 1.1 | 0.012 |
| Waist circumference (cm) | 95.5 ± 11.8 | 92.5 ± 10.5 | 86.2 ± 8.8 | 99.2 ± 15.3 | 92.2 ± 10.1 | 89.3 ± 9.3 | 0.2 | 0.001 | 12.6 *** | 0.121 | 0.4 | 0.004 |
| Body fat (%) | 24.4 ± 8.0 | 20.0 ± 6.1 | 20.7 ± 7.0 | 24.6 ± 6.0 | 24.4 ± 8.5 | 18.4 ± 8.2 | 0.7 | 0.004 | 21.2 *** | 0.187 | 0.1 | 0.002 |
| TC (mmol·L−1) | 4.9 ± 1.0 | 5.0 ± 1.0 | 5.0 ± 0.8 | 5.6 ± 1.7 | 5.2 ± 0.9 | 5.0 ± 0.9 | 2.4 | 0.013 | 1.3 | 0.014 | 3.0 * | 0.032 |
| HDL-C (mmol·L−1) | 1.7 ± 0.4 | 1.7 ± 0.4 | 1.9 ± 0.4 | 1.7 ± 0.5 | 1.9 ± 0.4 | 1.9 ± 0.4 | 0.7 | 0.004 | 5.5 ** | 0.057 | 0.1 | 0.001 |
| LDL-C (mmol·L−1) | 2.5 ± 0.9 | 2.4 ± 0.7 | 2.4 ± 0.7 | 2.7 ± 1.0 | 2.4 ± 0.8 | 2.5 ± 0.7 | 0.1 | 0.000 | 0.9 | 0.011 | 0.8 | 0.009 |
| TG (mmol·L−1) | 1.7 ± 0.9 | 1.7 ± 1.3 | 1.4 ± 0.6 | 2.5 ± 2.2 | 2.0 ± 1.4 | 1.4 ± 0.8 | 3.4 | 0.018 | 4.9 ** | 0.050 | 3.6 * | 0.038 |
| HbA1c (%) | 5.5 ± 0.3 | 5.5 ± 0.4 | 5.4 ± 0.2 | 5.5 ± 0.4 | 5.5 ± 0.2 | 5.4 ± 0.2 | 1.2 | 0.007 | 2.9 | 0.031 | 0.6 | 0.007 |
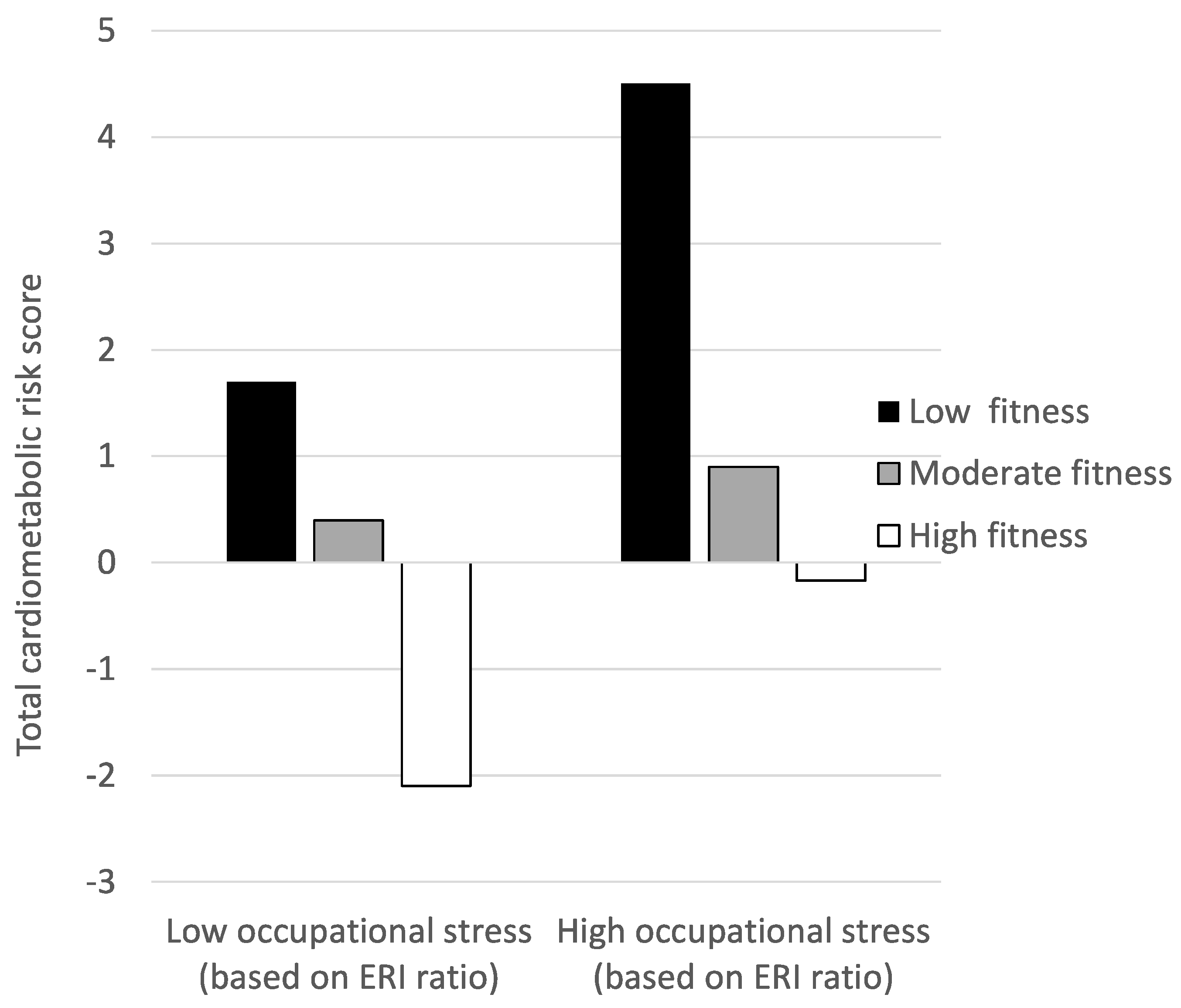
| Total cardiometabolic risk | 1.7 ± 5.9 | 0.4 ± 5.1 | −2.1 ± 4.5 | 4.5 ± 9.3 | 0.9 ± 5.2 | −1.7 ± 4.5 | 0.5 | 0.003 | 16.8 *** | 0.154 | 3.1 * | 0.033 |
| Mental health indicators | ||||||||||||
| Burnout symptoms | 2.3 ± 0.9 | 2.4 ± 1.1 | 2.4 ± 0.8 | 2.6 ± 0.9 | 3.1 ± 1.2 | 3.0 ± 1.2 | 14.1 *** | 0.071 | 2.2 | 0.023 | 1.5 | 0.016 |
| Sleep complaints | 7.7 ± 4.1 | 7.2 ± 3.5 | 6.4 ± 3.6 | 8.8 ± 4.3 | 9.4 ± 5.2 | 9.1 ± 5.0 | 10.0 ** | 0.049 | 0.6 | 0.007 | 1.0 | 0.010 |
| Overall mental distress | 1.4 ± 2.0 | 1.1 ± 2.0 | 0.9 ± 1.8 | 1.8 ± 2.1 | 2.9 ± 3.3 | 2.5 ± 3.2 | 17.1 *** | 0.081 | 1.1 | 0.011 | 1.0 | 0.011 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schilling, R.; Colledge, F.; Ludyga, S.; Pühse, U.; Brand, S.; Gerber, M. Does Cardiorespiratory Fitness Moderate the Association between Occupational Stress, Cardiovascular Risk, and Mental Health in Police Officers? Int. J. Environ. Res. Public Health 2019, 16, 2349. https://doi.org/10.3390/ijerph16132349
Schilling R, Colledge F, Ludyga S, Pühse U, Brand S, Gerber M. Does Cardiorespiratory Fitness Moderate the Association between Occupational Stress, Cardiovascular Risk, and Mental Health in Police Officers? International Journal of Environmental Research and Public Health. 2019; 16(13):2349. https://doi.org/10.3390/ijerph16132349
Chicago/Turabian StyleSchilling, René, Flora Colledge, Sebastian Ludyga, Uwe Pühse, Serge Brand, and Markus Gerber. 2019. "Does Cardiorespiratory Fitness Moderate the Association between Occupational Stress, Cardiovascular Risk, and Mental Health in Police Officers?" International Journal of Environmental Research and Public Health 16, no. 13: 2349. https://doi.org/10.3390/ijerph16132349
APA StyleSchilling, R., Colledge, F., Ludyga, S., Pühse, U., Brand, S., & Gerber, M. (2019). Does Cardiorespiratory Fitness Moderate the Association between Occupational Stress, Cardiovascular Risk, and Mental Health in Police Officers? International Journal of Environmental Research and Public Health, 16(13), 2349. https://doi.org/10.3390/ijerph16132349






