Effects of Air Pollution on Lung Innate Lymphoid Cells: Review of In Vitro and In Vivo Experimental Studies
Abstract
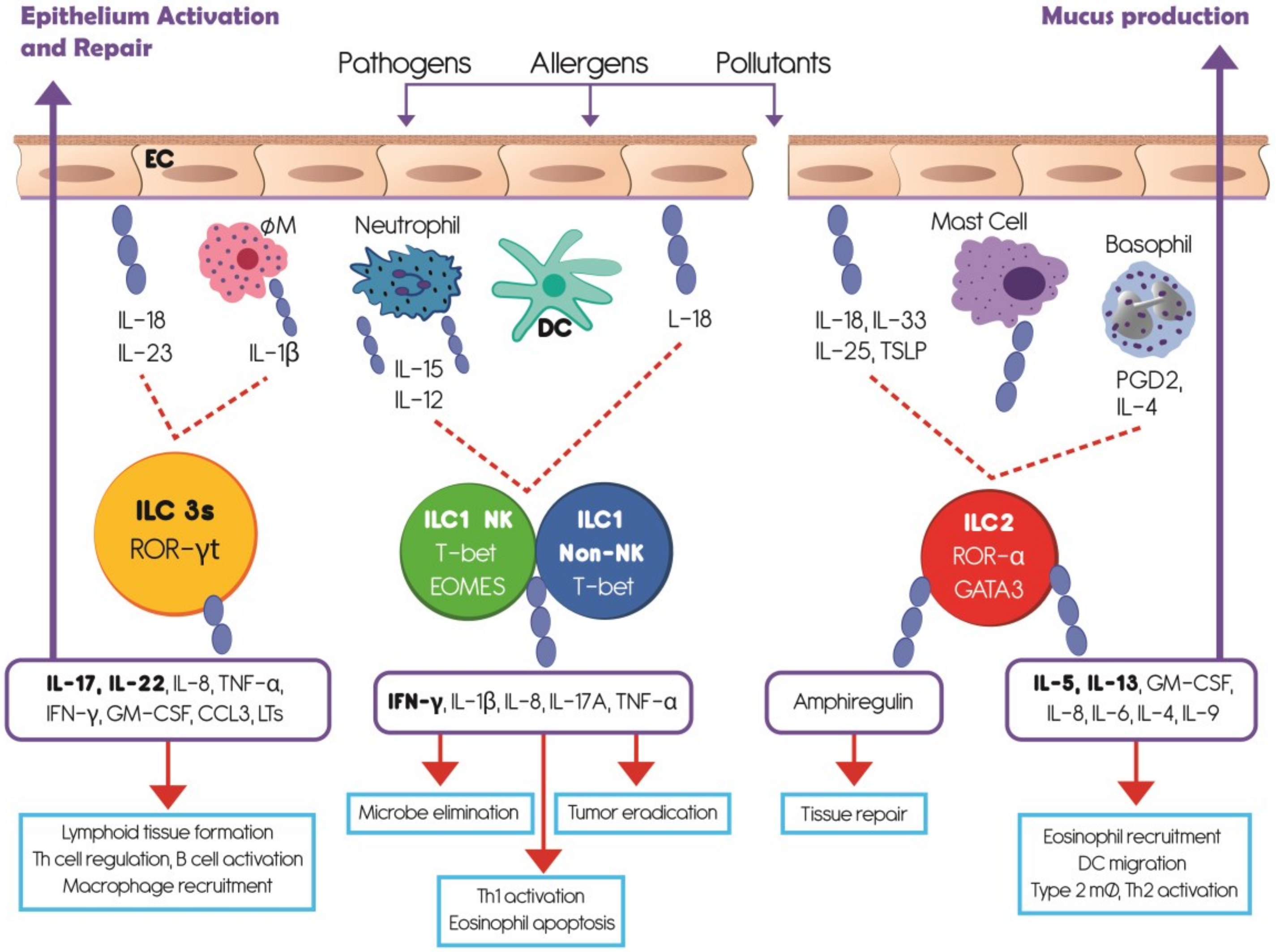
1. Introduction
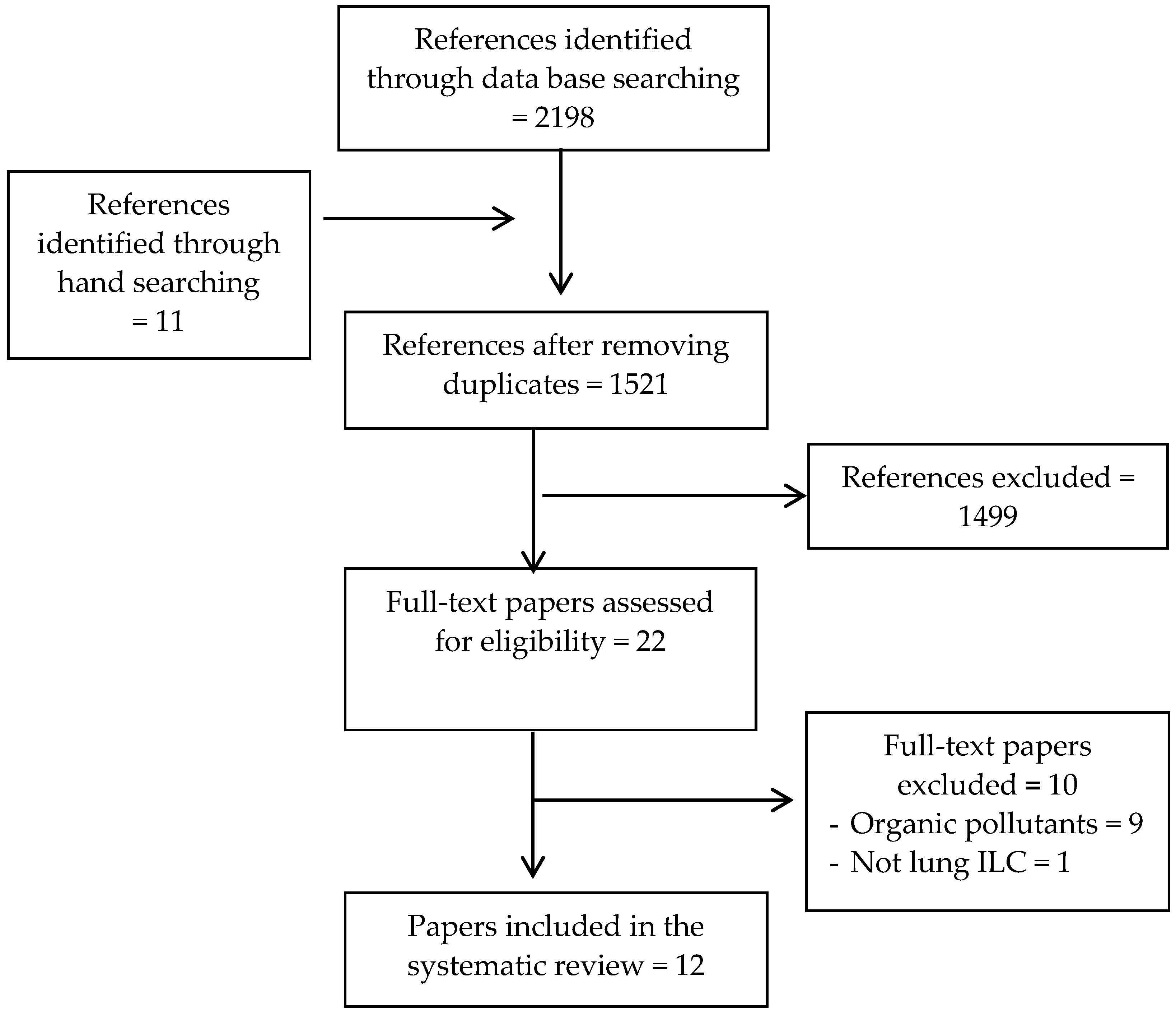
2. Materials and Methods
2.1. Search Strategy
2.2. Screening and Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment of The Evidence
2.6. Synthesis of the Evidence
3. Results
3.1. Effects of Air Pollutants on ILC1-NK Cells
3.1.1. Cell Number and Viability
3.1.2. Cytokine Production
3.1.3. Activity
3.2. Effects of Air Pollutants on ILC2
3.2.1. Cell Number and Viability
3.2.2. Cytokine Production and Activity
3.2.3. Allergen-Induced Response and Airway Hyperresponsiveness (AHR)
3.3. Effects of Air Pollutants on ILC3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bateson, T.F.; Schwartz, J. Children’s response to air pollutants. J. Toxicol. Environ. Health. A 2008, 71, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Bennett, W.D.; Zeman, K.L.; Jarabek, A.M. Nasal contribution to breathing and fine particle deposition in children versus adults. J. Toxicol. Environ. Health A 2008, 71, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Brugha, R.; Grigg, J. Urban air pollution and respiratory infections Paediatr. Respir. Rev. 2014, 15, 194–199. [Google Scholar] [CrossRef]
- Korten, I.; Ramsey, K.; Latzin, P. Air pollution during pregnancy and lung development in the child. Paediatr. Respir. Rev. 2017, 21, 38–46. [Google Scholar] [CrossRef]
- Darrow, L.A.; Klein, M.; Flanders, W.D.; Mulholland, J.A.; Tolbert, P.E.; Strickland, M.J. Air pollution and acute respiratory infections among children 0-4 years of age: an 18-year time-series study. Am. J. Epidemiol. 2014, 180, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Galeone, C.; Lelii, M.; Longhi, B.; Ascolese, B.; Senatore, L.; Prada, E.; Montinaro, V.; Malerba, S.; Patria, M.F.; et al. Impact of air pollution on respiratory diseases in children with recurrent wheezing or asthma. BMC Pulm. Med. 2014, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Estrella, B.; Estrella, R.; Oviedo, J.; Narvaez, X.; Reyes, M.T.; Gutierrez, M.; Naumova, E.N. Acute respiratory diseases and carboxyhemoglobin status in school children of Quito, Ecuador. Environ. Health. Perspect. 2005, 113, 607–611. [Google Scholar] [CrossRef]
- Harris, A.M.; Sempertegui, F.; Estrella, B.; Narvaez, X.; Egas, J.; Woodin, M.; Durant, J.L.; Naumova, E.N.; Griffiths, J.K. Air pollution and anemia as risk factors for pneumonia in Ecuadorian children: a retrospective cohort analysis. Environ. Health 2011, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Naumova, E.N.; Yepes, H.; Griffiths, J.K.; Sempertegui, F.; Khurana, G.; Jagai, J.S.; Jativa, E.; Estrella, B. Emergency room visits for respiratory conditions in children increased after Guagua Pichincha volcanic eruptions in April 2000 in Quito, Ecuador observational study: Time series analysis. Environ. Health 2007, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Goldizen, F.C.; Sly, P.D.; Knibbs, L.D. Respiratory effects of air pollution on children. Pediatr. Pulmonol. 2016, 51, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Tuan, T.S.; Venancio, T.S.; Nascimento, L.F. Air pollutants and hospitalization due to pneumonia among children. An ecological time series study. Sao Paulo Med. J. 2015, 133, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chan, C.C.; Chen, B.Y.; Cheng, T.J.; Guo, Y.L. Effects of particulate air pollution and ozone on lung function in non-asthmatic children. Environ. Res. 2015, 137, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Zheng, X.Y.; Chung, K.F.; Zhong, N.S. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet 2016, 388, 1939–1951. [Google Scholar] [CrossRef]
- Nightingale, U.A.; Maggs, R.; Cullinan, P.; Donnelly, L.E.; Rogers, D.F.; Kinnersley, R.; Chung, K.F.; Barnes, P.J.; Ashmore, M.; Newman-Taylor, A. Airway inflammation after controlled exposure to diesel exhaust particulates. Am. J. Respir. Crit. Care. Med. 2000, 162, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Paulin, L.; Hansel, N. Particulate air pollution and impaired lung function. F1000 Res. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Churg, A.; Brauer, M.; del Carmen Avila-Casado, M.; Fortoul, T.I.; Wright, J.L. Chronic exposure to high levels of particulate air pollution and small airway remodeling. Environ. Health Perspect. 2002, 111, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Gawda, A.; Majka, G.; Nowak, B.; Marcinkiewicz, J. Air pollution, oxidative stress, and exacerbation of autoimmune diseases. Cent. Eur. J. Immunol. 2011, 42, 305–312. [Google Scholar] [CrossRef]
- Lodovici, M.; Bigagli, E. Oxidative stress and air pollution exposure. J. Toxicol. 2011, 487074. [Google Scholar] [CrossRef]
- Losacco, C.; Perillo, A. Particulate matter air pollution and respiratory impact on humans and animals. Environ. Sci. Pollut. Res. Int. 2018, 25, 33901–33910. [Google Scholar] [CrossRef]
- Bauer, R.N.; Muller, L.; Brighton, L.E.; Duncan, K.E.; Jaspers, I. Interaction with epithelial cells modifies airway macrophage response to ozone. Am. J. Respir. Cell. Mol. Biol. 2015, 52, 285–294. [Google Scholar] [CrossRef]
- Bromberg, P.A. Mechanisms of the acute effects of inhaled ozone in humans. Biochim. Biophys. Acta 2016, 1860, 2771–2781. [Google Scholar] [CrossRef] [PubMed]
- Jaguin, M.; Fardel, O.; Lecureur, V. Exposure to diesel exhaust particle extracts (DEPe) impairs some polarization markers and functions of human macrophages through activation of AhR and Nrf2. PLoS ONE 2015, 10, e0116560. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Kobzik, L. Effect of concentrated ambient particles on macrophage phagocytosis and killing of Streptococcus pneumoniae. Am. J. Respir. Cell. Mol. Biol. 2007, 36, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, J.; Zhou, J.; Wang, J.; Chen, C.; Song, Y.; Pan, J. Urban particulate matter (pm) suppresses airway antibacterial defence. Respir. Res. 2018, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Hiraiwa, K.; van Eeden, S.F. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediators Inflamm. 2013, 2013, 619523. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli-Rocha, F.; Fernandes, S.; Einicker-Lamas, M.; Zin, W.A. Roles of oxidative stress in signaling and inflammation induced by particulate matter. Cell. Biol. Toxicol. 2010, 26, 481–498. [Google Scholar] [CrossRef] [PubMed]
- Miyata, R.; van Eeden, S.F. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol. Appl. Pharmacol. 2011, 257, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Thakur, A.K. The toxicological mechanisms of environmental soot (black carbon) and carbon black: Focus on oxidative stress and inflammatory pathways. Front. Immunol. 2017, 8, 763. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Santiago, C.E.; Sarkar, S.; Cantarella, P.T.; Osornio-Vargas, A.; Quintana-Belmares, R.; Meng, Q.; Kirn, T.J.; Ohman Strickland, P.; Chow, J.C.; Watson, J.G.; et al. Air pollution particulate matter alters antimycobacterial respiratory epithelium innate immunity. Infect. Immun. 2015, 83, 2507–2517. [Google Scholar] [CrossRef]
- Sarkar, S.; Song, Y.; Sarkar, S.; Kipen, H.M.; Laumbach, R.J.; Zhang, J.; Strickland, P.A.; Gardner, C.R.; Schwander, S. Suppression of the NF-kB pathway by diesel exhaust particles impairs human antimycobacterial immunity. J. Immunol. 2012, 188, 2778–2793. [Google Scholar] [CrossRef]
- Sigaud, S.; Goldsmith, C.A.; Zhou, H.; Yang, Z.; Fedulov, A.; Imrich, A.; Kobsik, L. Air pollution particles diminish bacterial clearance in the primed lungs of mice. Toxicol. Appl. Pharmacol. 2007, 223, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.B.; Betteridge, B.C.; Earley, T.D.; Curtis, C.S.; Robinson, A.B.; Reynolds, P.R. Primary alveolar macrophages exposed to diesel particulate matter increase rage expression and activate rage signaling. Cell. Tissue Res. 2014, 358, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, L.A.; Sonnenberg, G.F.; Abt, M.C.; Alenghat, T.; Ziegler, C.G.; Doering, T.A.; Angelosanto, J.M.; Laidlaw, B.J.; Yang, C.Y.; Sathaliyawala, T.; et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011, 12, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.S.; Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016, 17, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, I.; Steer, C.A.; Takei, F. Lung ILC2s link innate and adaptive responses in allergic inflammation. Trends Immunol. 2015, 36, 189–195. [Google Scholar] [CrossRef]
- Lai, D.M.; Shu, Q.; Fan, J. The origin and role of innate lymphoid cells in the lung. Mil. Med. Res. 2016, 3, 25. [Google Scholar] [CrossRef]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Zook, E.C.; Kee, B.L. Development of innate lymphoid cells. Nat. Immunol. 2016, 17, 775–782. [Google Scholar] [CrossRef]
- Zhong, C.; Zhu, J. Transcriptional regulators dictate innate lymphoid cell fates. Protein Cell. 2017, 8, 242–254. [Google Scholar] [CrossRef]
- Cortez, V.S.; Colonna, M. Diversity and function of group 1 innate lymphoid cells. Immunol. Lett. 2016, 179, 19–24. [Google Scholar] [CrossRef]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E.; et al. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.A.; Barlow, J.L.; McKenzie, A.N. Innate lymphoid cells--how did we miss them? Nat. Rev. Immunol. 2013, 13, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Ealey, K.N.; Koyasu, S. How many subsets of innate lymphoid cells do we need? Immunity 2017, 46, 10–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simoni, Y.; Fehlings, M.; Kloverpris, H.N.; McGovern, N.; Koo, S.L.; Loh, C.Y.; Lim, S.; Kurioka, A.; Fergusson, J.R.; Tang, C.L.; et al. Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity 2017, 46, 148–161. [Google Scholar] [CrossRef] [PubMed]
- PRISMA. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Website. 2009. Available online: http://www.prisma-statement.org/ (accessed on 28 June 2019).
- Estrella, B.; Cepda, M.; Naumova, E.N.; Katsikis, P.D.; Drexhage, H.A. The Role of Air Pollution in Lung Innate Lymphoid Cells. Quito: Universidad Central del Ecuador Website 2018. Available online: http://repositorio.uce.edu.ec/archivos/mrpallasco/PDF/PROTOCOL SYSTEMATIC REVIEW AIR POLLUTION-ILCs -ESTRELLA B.pdf. (accessed on 14 April 2019).
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The Arrive Guidelines For Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Beamer, C.A.; Girtsman, T.A.; Seaver, B.P.; Finsaas, K.J.; Migliaccio, C.T.; Perry, V.K.; Rottman, J.B.; Smith, D.E.; Holian, A. IL-33 mediates multi-walled carbon nanotube (MWCNT)-induced airway hyper-reactivity via the mobilization of innate helper cells in the lung. Nanotoxicology 2013, 7, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- De Grove, K.C.; Provoost, S.; Hendriks, R.W.; McKenzie, A.N.J.; Seys, L.J.M.; Kumar, S.; Maes, T.; Brusselle, G.G.; Joos, G.F. Dysregulation of type 2 innate lymphoid cells and Th2 cells impairs pollutant-induced allergic airway responses. J. Allergy Clin. Immunol. 2017, 139, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Finkelman, F.D.; Yang, M.; Orekhova, T.; Clyne, E.; Bernstein, J.; Whitekus, M.; Diaz-Sanchez, D.; Morris, S.C. Diesel exhaust particles suppress in vivo IFN- production by inhibiting cytokine effects on NK and NKt cells. J. Immunol. 2004, 172, 3808–3813. [Google Scholar] [CrossRef]
- Mathews, J.A.; Krishnamoorthy, N.; Kasahara, D.I.; Cho, Y.; Wurmbrand, A.P.; Ribeiro, L.; Smith, D.; Umetsu, D.; Levy, B.D.; Shore, S.A. IL-33 drives augmented responses to ozone in obese mice. Environ. Health Perspect. 2017, 125, 246–253. [Google Scholar] [CrossRef]
- Yang, Q.; Ge, M.Q.; Kokalari, B.; Redai, I.G.; Wang, X.; Kemeny, D.M.; Bhandoola, A.; Haczku, A. Group 2 innate lymphoid cells mediate ozone-induced airway inflammation and hyperresponsiveness in mice. J. Allergy. Clin. Immunol. 2016, 137, 571–578. [Google Scholar] [CrossRef]
- Zhao, H.; Li, W.; Gao, Y.; Li, J.; Wang, H. Exposure to particular matter increases susceptibility to respiratory staphylococcus aureus infection in rats via reducing pulmonary natural killer cells. Toxicology 2014, 325, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Burleson, G.R.; Keyes, L.L.; Stutzman, J.D. Immunosuppression of pulmonary natural killer activity by exposure to ozone. Immunopharmacol. Immunotoxicol. 1989, 11, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Lewandowski, R.P.; Jackson-Humbles, D.N.; Buglak, N.; Li, N.; White, K.; Van Dyken, S.J.; Wagner, J.G.; Harkema, J.R. Innate lymphoid cells mediate pulmonary eosinophilic inflammation, airway mucous cell metaplasia, and type 2 immunity in mice exposed to ozone. Toxicol. Pathol. 2017, 45, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Fu, H.; Han, F.; Fang, Y.; Xu, J.; Zhang, L.; Du, Q. Lipoxin A4 regulates PM2.5-induced severe allergic asthma in mice via the Th1/Th2 balance of group 2 innate lymphoid cells. J. Thorac. Dis. 2018, 10, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Chehrazi, C.V.E.; Henderson, M.W.; Noah, T.R.; Jasper, I. Diesel exhaust particles modify natural killer cell function and cytokine release. Part. Fibre Toxicol. 2013, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Kucuksezer, U.C.; Zekiroglu, E.; Kasapoglu, P.; Adin-Cinar, S.; Aktas-Cetin, E.; Deniz, G. A stimulatory role of ozone exposure on human natural killer cells. Immunol. Invest. 2014, 43, 1–12. [Google Scholar] [CrossRef]
- Müller, L.; Brighton, L.E.; Jaspers, I. Ozone exposed epithelial cells modify cocultured natural killer cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L332–L341. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Su, X.; Yu, Y.; Zhong, Y.; Giannopoulou, E.G.; Hu, X.; Liu, H.; Cross, J.R.; Ratsch, G.; Rice, C.M.; Ivashkiv, L.B. Interferon-gamma regulates cellular metabolism and mRNA translation to potentiate macrophage activation. Nat. Immunol. 2015, 16, 838–849. [Google Scholar] [CrossRef]
- Trost, M.; English, L.; Lemieux, S.; Courcelles, M.; Desjardins, M.; Thibault, P. The phagosomal proteome in interferon-gamma-activated macrophages. Immunity 2009, 30, 143–154. [Google Scholar] [CrossRef]
- Billiau, A.; Matthys, P. Interferon-gamma: A historical perspective. Cytokine Growth Factor Rev. 2009, 20, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4(+)Tcells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Paul, W.E. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immuno. Rev. 2010, 238, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Corren, J. Role of interleukin-13 in asthma. Curr. Allergy Asthma Rep. 2013, 13, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Crestani, E.; Lohman, I.C.; Guerra, S.; Wright, A.L.; Halonen, M. Association of IL-5 cytokine production and in vivo IgE levels in infants and parents. J. Allergy Clin. Immunol. 2007, 120, 820–826. [Google Scholar] [CrossRef]
- Kouro, T.; Takatsu, K. IL-5- and eosinophil-mediated inflammation: From discovery to therapy. Int. Immunol. 2009, 21, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Sehmi, R.; Nair, P. Anti-il5 therapy for asthma and beyond. World Allergy Organ. J. 2014, 7, 32. [Google Scholar] [CrossRef]
- Estrella, B.; Sempertegui, F.; Franco, O.H.; Cepeda, M.; Naumova, E.N. Air pollution control and the occurrence of acute respiratory illness in school children of Quito, Ecuador. J. Public Health Policy 2019, 40, 17–34. [Google Scholar] [CrossRef]
| Authors, Year | Type of Exposure (Doses; Method of Administration) | Outcome | Summary of Findings/Observed Effects of Exposure on the Outcome |
|---|---|---|---|
| Type of Cell: Mice ILCs | |||
| Beamer, et al. 2013 [48] | Multi-walled carbon nanotubes (50 μg; oropharyngeal) | IL-33 function on ILC2 |
|
| Type of Cell: Mice ILC2 | |||
| De Grove, et al. 2016 [49] | DEP (25 mg on days 1, 8, and 15; intranasal) | Function and cytokine production |
|
| Mathews, et al. 2017 [51] | O3 (2 ppm for 3 h; inhaled) | IL-33 action on ILC2 and γδ T |
|
| Yang et al. 2016 [52] | O3 (3 ppm for 2 h on day 16; inhaled) | Il5 and Il13 RNA expression |
|
| Kumagai, et al. 2017 [55] | O3 (0.8 ppm on day 1 or for 9 consecutive weekdays; inhaled) | ILC2 in airway inflammation, mucus cell metaplasia, and Type 2 immunity |
|
| Lu, et al. 2018 [56] | PM2.5 (25 mL/kg of a suspension of 15 g/L on days 1, 8, 15, and 21; intranasal) | ILC2-related transcription factors |
|
| Type of Cell: Rats NK | |||
| Burleson, et al. 1989 [54] | O3 (1.0 ppm for 23.5 h /day on 1, 3, 7, or 10 consecutive days; ambient) | Number and function of NK, and function of adherent cells |
|
| Zhao, et al. 2014 [53] | PM2.5 (1, 5, or 10mg/kg body weight; intratracheal) | Number and bacterial response |
|
| Type of Cell: Mice NK | |||
| Finkelman, et al. 2004 [50] | DEP (2 mg once; injected i.p.) | INF gamma production |
|
| Type of Cell: Human NK | |||
| Müller, et al. 2013 [57] | DEP (10 μg/mL; direct exposure of cell) | Function and cytokine release |
|
| Kucuksezer, et al. 2014 [58] | O3 (1, 5, 10, and 50 mg/mL cRPMI; direct exposure of cell) | Number, function |
|
| Müller, et al. 2013 [59] | O3 (0.4 ppm; direct exposure of cell) | Effect of O3 exposed epithelial cells on natural killer cells function, cytokine release. |
|
| NK Cell Features a | ||||||
| Exposure | PM 2.5 | DEP | O3 | CN | ||
| Model | Rats | Mice | Human | Rats | Human | Mice |
| Number | ↓NK BALF ↓Influx into airways | ↓NK in spleen | ↓ % lung lymphocytes | Low doses: ↑ number | ||
| Cytokine | ↓IFN-γ | ↑IL-1β ↑ IL-8 ↑ TNF-α no changes in INF- γ | ↓ IFN-γ | |||
| Activity | ↑ Susceptibility to respiratory infection by S. aureus | ↓ Cytotoxicity ↓ CD16 expression ↓ Granzyme B levels ↓ Perforin levels | ↓ Pulmonary NK activity | ↓ Cytotoxicity ↓ Granzyme B levels ↓ Markers of cytotoxicity Low doses: ↑ cytotoxicity | ||
| ILC2 cell features b | ||||||
| Exposure | PM 2.5 | DEP | O3 | CN | ||
| Model | Rats | Mice | Human | Mice | Human | Mice |
| Number | ↑ in alveolar space but not in lungs | No effect in lean mice ↑IL-5+ and IL-13+ ILC2s in BAL in obese mice | ↑ in lung | |||
| Cytokine | ↑IL-5 ↑IL-13 | ↑IL-5 ↑IL-13 | ↑IL-13 | |||
| Air way hiperresponsiveness (AHR) | Enhances AHR: ↑ RORα and GATA3 transcription factors related to ILC2 | Enhances AHR: Accumulation of ILC2s and Th2 cells and type 2 cytokine production | Induces AHR: ↑ expression of lung mRNA transcripts associated with type 2 immunity | Induces AHR: ↑ IL-13 from ILC2 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrella, B.; Naumova, E.N.; Cepeda, M.; Voortman, T.; Katsikis, P.D.; Drexhage, H.A. Effects of Air Pollution on Lung Innate Lymphoid Cells: Review of In Vitro and In Vivo Experimental Studies. Int. J. Environ. Res. Public Health 2019, 16, 2347. https://doi.org/10.3390/ijerph16132347
Estrella B, Naumova EN, Cepeda M, Voortman T, Katsikis PD, Drexhage HA. Effects of Air Pollution on Lung Innate Lymphoid Cells: Review of In Vitro and In Vivo Experimental Studies. International Journal of Environmental Research and Public Health. 2019; 16(13):2347. https://doi.org/10.3390/ijerph16132347
Chicago/Turabian StyleEstrella, Bertha, Elena N. Naumova, Magda Cepeda, Trudy Voortman, Peter D. Katsikis, and Hemmo A. Drexhage. 2019. "Effects of Air Pollution on Lung Innate Lymphoid Cells: Review of In Vitro and In Vivo Experimental Studies" International Journal of Environmental Research and Public Health 16, no. 13: 2347. https://doi.org/10.3390/ijerph16132347
APA StyleEstrella, B., Naumova, E. N., Cepeda, M., Voortman, T., Katsikis, P. D., & Drexhage, H. A. (2019). Effects of Air Pollution on Lung Innate Lymphoid Cells: Review of In Vitro and In Vivo Experimental Studies. International Journal of Environmental Research and Public Health, 16(13), 2347. https://doi.org/10.3390/ijerph16132347






