Seroprevalence of Leptospira spp. Infection in Cattle from Central and Northern Madagascar
Abstract
1. Introduction
2. Materials and Methods
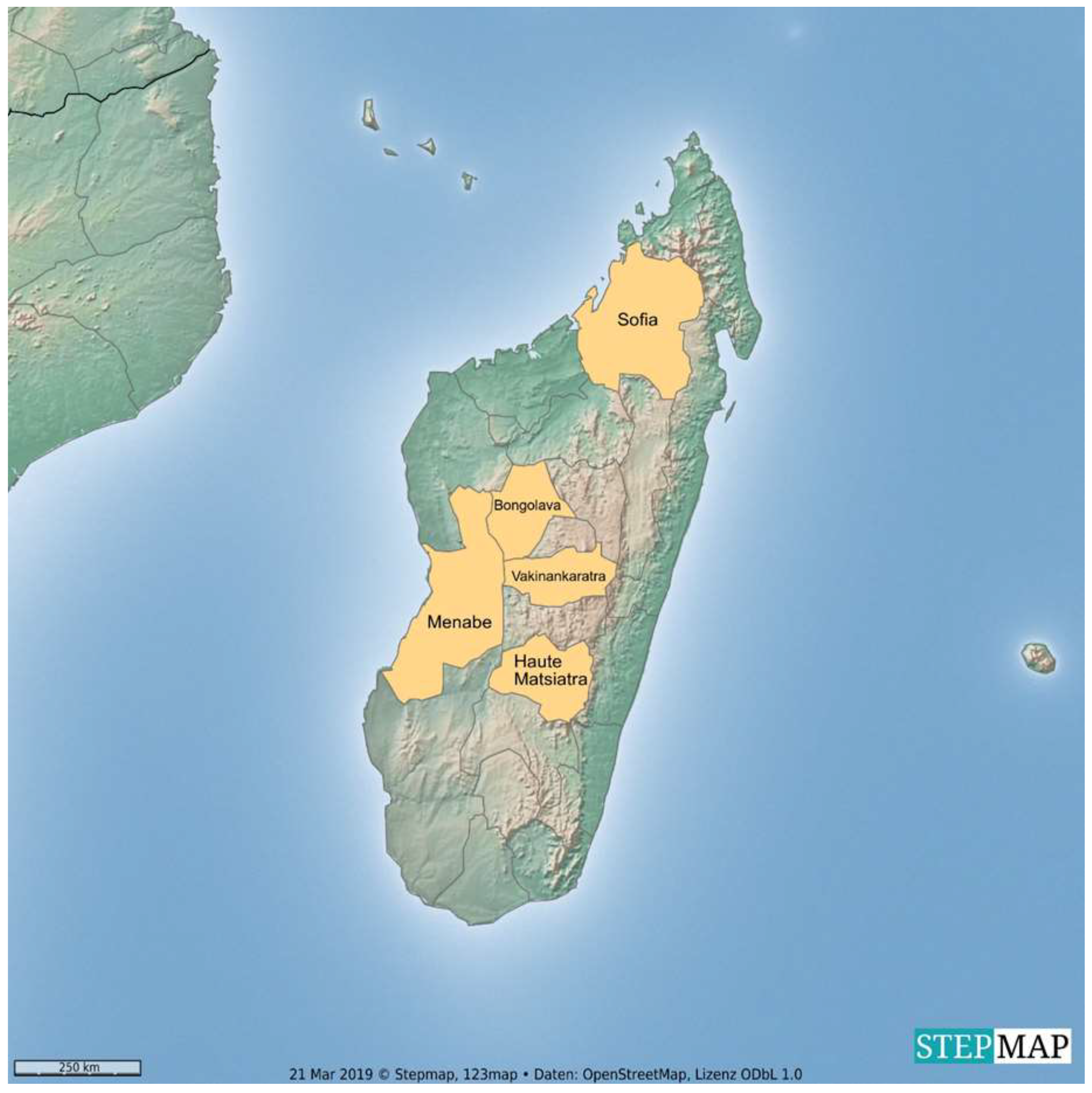
2.1. Animals and Sample Collection
2.2. Serological Analysis
2.3. Statistical Analysis
3. Results
3.1. Description of Study Population and Tested Samples
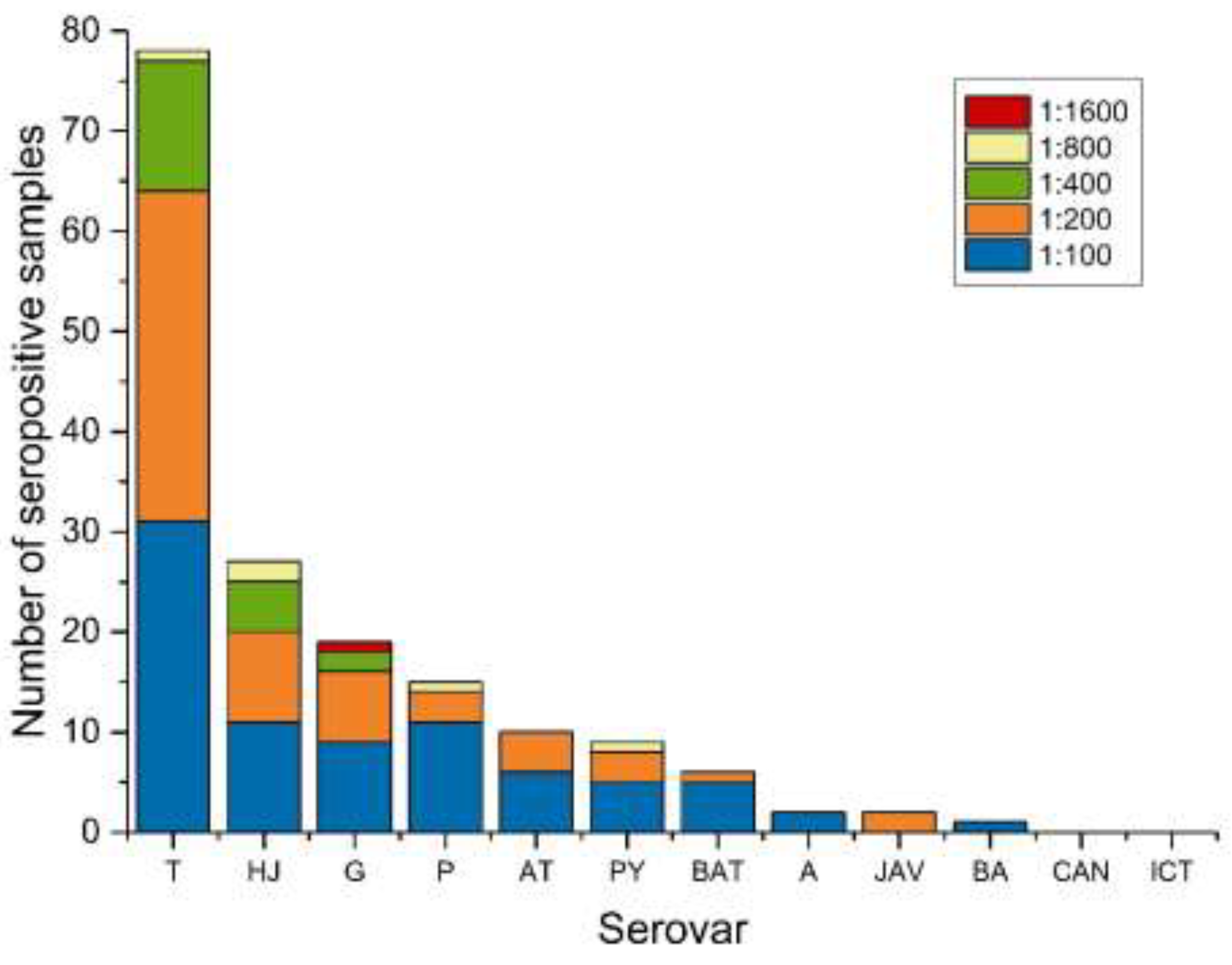
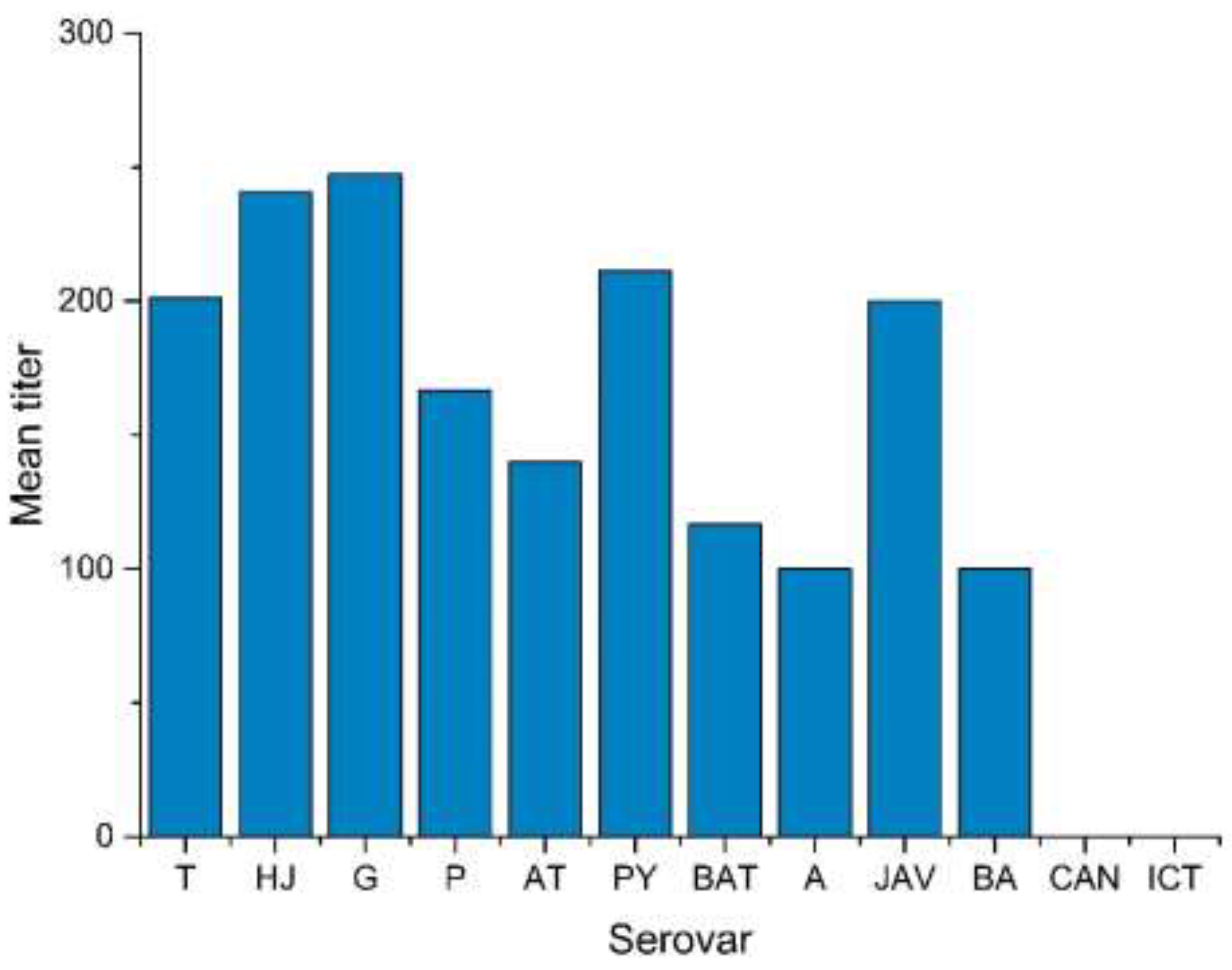
3.2. Serological Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haake, D.A.; Levett, P.N. Leptospirosis in humans. In Leptospira and Leptospirosis. Current Topics in Microbiology and Immunology, 1st ed.; Adler, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 387, pp. 65–97. [Google Scholar]
- Ellis, W.A. Animal leptospirosis. In Leptospira and Leptospirosis. Current Topics in Microbiology and Immunology, 1st ed.; Adler, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 387, pp. 99–137. [Google Scholar]
- Cerqueira, G.M.; Picardeau, M. A century of Leptospira strain typing. Infect. Genet. Evol. 2009, 9, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Hartskeerl, R.A.; Collares-Pereira, M.; Ellis, W.A. Emergence, control and re-emerging leptospirosis: Dynamics of infection in the changing world. Clin. Microbiol. Infect. 2011, 17, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global morbidity and mortality of leptospirosis: A systematic review. PLoS Negl. Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef] [PubMed]
- Kolochine-Erber, B.; Brygoo, E.R. Research on leptospirosis in Madagascar. Bull. Soc. Pathol. Exot. Fil. 1956, 49, 686–698. [Google Scholar]
- Silverie, R.; Monnier, M.; Lataste-Dorolle, C. Recent survey of leptospirosis on Madagascar. Contribution to the study of human, bovine and porcine leptospirosis in the southern region. Bull. Soc. Pathol. Exot. Fil. 1968, 61, 346–359. [Google Scholar]
- Lhuillier, M. Leptospiroses in Madagascar. (Bacteriological and serological study). Arch. Inst. Pasteur Madag. 1978, 46, 429–439. [Google Scholar]
- Ralaiarijaona, R.L.; Bellenger, E.; Chanteau, S.; Roger, F.; Pérolat, P.; Rasolofo Razanamparany, V. Detection of leptospirosis reservoirs in Madagascar using the polymerase chain reaction technique. Arch. Inst. Pasteur Madag. 2001, 67, 34–36. [Google Scholar]
- Pagès, F.; Kuli, B.; Moiton, M.P.; Goarant, C.; Jaffar-Bandjee, M.C. Leptospirosis after a stay in Madagascar. J. Travel Med. 2015, 22, 136–139. [Google Scholar] [CrossRef][Green Version]
- Ratsitorahina, M.; Rahelinirina, S.; Michault, A.; Rajerison, M.; Rajatonirina, S.; Richard, V. Has Madagascar lost its exceptional leptospirosis free-like status? PLoS ONE 2015, 10, e0122683. [Google Scholar] [CrossRef]
- Kolochine-Erber, B.; Buck, G.; Quesnel, J. Bovine leptospirosis should be suspected in Madagascar. Bull. Soc. Pathol. Exot. Fil. 1956, 49, 681–686. [Google Scholar]
- Keller, C.; Kruger, A.; Schwarz, N.G.; Rakotozandrindrainy, R.; Rakotondrainiarivelo, J.P.; Razafindrabe, T.; Derschum, H.; Silaghi, C.; Pothmann, D.; Veit, A.; et al. High detection rate of Rickettsia africae in Amblyomma variegatum but low prevalence of anti-rickettsial antibodies in healthy pregnant women in Madagascar. Ticks Tick Borne Dis. 2016, 7, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Boone, I.; Henning, K.; Hilbert, A.; Neubauer, H.; von Kalckreuth, V.; Dekker, D.M.; Schwarz, N.G.; Pak, G.D.; Krüger, A.; Hagen, R.M.; et al. Are brucellosis, Q fever and melioidosis potential causes of febrile illness in Madagascar? Acta Trop. 2017, 172, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hagen, R.M.; Frickmann, H.; Ehlers, J.; Krüger, A.; Margos, G.; Hizo-Teufel, C.; Fingerle, V.; Rakotozandrindrainy, R.; Kalckreuth, V.V.; Im, J.; et al. Presence of Borrelia spp. DNA in ticks, but absence of Borrelia spp. and of Leptospira spp. DNA in blood of fever patients in Madagascar. Acta Trop. 2018, 177, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Goris, M.G.; Hartskeerl, R.A. Leptospirosis serodiagnosis by the microscopic agglutination test. Curr. Protoc. Microbiol. 2014, 32, 12E.5.1–12E.5.18. [Google Scholar] [CrossRef]
- Fleiss, J.L.; Levin, B.; Paik, M.C. Statistical Methods for Rates and Proportions, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Desvars, A.; Michault, A.; Bourhy, P. Leptospirosis in the western Indian Ocean islands: What is known so far? Vet. Res. 2013, 44, 80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- FAO. Livestock Sector Brief Madagascar; FAO: Rome, Italy, 2005. [Google Scholar]
- Mannewald, A. Prevalence of Atypical Leptospira Serovars in New Zealand’s Pastoral Livestock. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2016. [Google Scholar]
- Heuer, C. Human health and leptospirosis. In Proceedings of the Society of Sheep & Beef Cattle Veterinarians of the NZVA, Annual Seminar, Auckland, New Zealand, 25–27 May 2006. [Google Scholar]
- Yatbantoong, N.; Chaiyarat, R. Factors Associated with Leptospirosis in Domestic Cattle in Salakphra Wildlife Sanctuary, Thailand. Int. J. Environ. Res. Public Health 2019, 16, 1042. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.; Gomard, Y.; Lagadec, E.; Ramasindrazana, B.; Le Minter, G.; Guernier, V.; Benlali, A.; Rocamora, G.; Markotter, W.; Goodman, S.M.; et al. Biogeography of Leptospira in wild animal communities inhabiting the insular ecosystem of the western Indian Ocean islands and neighboring Africa. Emerg. Microbes Infect. 2018, 7, 57. [Google Scholar] [CrossRef]
- Rahelinirina, S.; Bourhy, P.; Andriamiaramanana, F.; Garin, B.; Rajerison, M. High Prevalence of Leptospira spp. in Rodents in an Urban Setting in Madagascar. Am. J. Trop. Med. Hyg. 2019, 100, 1079–1081. [Google Scholar] [CrossRef]
- Blanco, R.M.; dos Santos, L.F.; Galloway, R.L.; Romero, E.C. Is the microagglutination test (MAT) good for predicting the infecting serogroup for leptospirosis in Brazil? Comp. Immunol. Microbiol. Infect. Dis. 2016, 44, 34–36. [Google Scholar] [CrossRef]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef]
- Dreyfus, A.; Wilson, P.; Benschop, J.; Collins-Emerson, J.; Verdugo, C.; Heuer, C. Seroprevalence and herd-level risk factors for seroprevalence of Leptospira spp. in sheep, beef cattle and deer in New Zealand. N. Z. Vet. J. 2018, 66, 302–311. [Google Scholar] [CrossRef] [PubMed]
- OIE. Chapter 2.1.12. Leptospirosis. In OIE Terrestrial Manual; OIE: Paris, France, 2014; pp. 1–15. [Google Scholar]
| Genospecies | Serogroup | Serovar | Strain |
|---|---|---|---|
| L. interrogans | Australis | Australis | Ballico |
| Autumnalis | Autumnalis | Akiyami A | |
| Bataviae | Bataviae | Swart | |
| Canicola | Canicola | Hond Utrecht IV | |
| Icterohaemorrhagiae | Icterohaemorrhagiae | Ictero I | |
| Pomona | Pomona | Pomona | |
| Pyrogenes | Pyrogenes | Salinem | |
| Sejroe | Hardjo | Hardjoprajitno | |
| L. borgpetersenii | Ballum | Ballum | Mus 127 |
| Javanica | Javanica | Veldrat Batavia 46 | |
| Tarassovi | Tarassovi | Perepelitsin | |
| L. kirschneri | Grippotyphosa | Grippotyphosa | Moskva V |
| Serovar | Seropositive Samples n | Seroprevalence % | CI 95% |
|---|---|---|---|
| L. Tarassovi | 78 | 40.2 | 33.3–47.5 |
| L. Hardjo | 27 | 13.9 | 9.5–19.8 |
| L. Grippotyphosa | 19 | 9.8 | 6.2–15.1 |
| L. Pomona | 15 | 7.7 | 4.5–12.7 |
| L. Autumnalis | 10 | 5.2 | 2.6–9.5 |
| L. Pyrogenes | 9 | 4.6 | 2.3–8.9 |
| L. Bataviae | 6 | 3.1 | 1.3–6.9 |
| L. Australis | 2 | 1 | 0.2–4.1 |
| L. Javanica | 2 | 1 | 0.2–4.1 |
| L. Ballum | 1 | 0.5 | 0.0–3.3 |
| L. Canicola | 0 | 0 | - |
| L. Icterohaemorrhagiae | 0 | 0 | - |
| Positive Serovars n | Samples with Multiple Reactions n (%) | Serovars with Frequent Reactions (n) |
|---|---|---|
| 2 | 32 (27.8) | T 1 + HJ 2 (9) |
| 3 | 8 (7.0) | |
| 4 | 2 (1.7) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schafbauer, T.; Dreyfus, A.; Hogan, B.; Rakotozandrindrainy, R.; Poppert, S.; Straubinger, R.K. Seroprevalence of Leptospira spp. Infection in Cattle from Central and Northern Madagascar. Int. J. Environ. Res. Public Health 2019, 16, 2014. https://doi.org/10.3390/ijerph16112014
Schafbauer T, Dreyfus A, Hogan B, Rakotozandrindrainy R, Poppert S, Straubinger RK. Seroprevalence of Leptospira spp. Infection in Cattle from Central and Northern Madagascar. International Journal of Environmental Research and Public Health. 2019; 16(11):2014. https://doi.org/10.3390/ijerph16112014
Chicago/Turabian StyleSchafbauer, Theresa, Anou Dreyfus, Benedikt Hogan, Raphael Rakotozandrindrainy, Sven Poppert, and Reinhard K. Straubinger. 2019. "Seroprevalence of Leptospira spp. Infection in Cattle from Central and Northern Madagascar" International Journal of Environmental Research and Public Health 16, no. 11: 2014. https://doi.org/10.3390/ijerph16112014
APA StyleSchafbauer, T., Dreyfus, A., Hogan, B., Rakotozandrindrainy, R., Poppert, S., & Straubinger, R. K. (2019). Seroprevalence of Leptospira spp. Infection in Cattle from Central and Northern Madagascar. International Journal of Environmental Research and Public Health, 16(11), 2014. https://doi.org/10.3390/ijerph16112014





