Environmental Silica Dust Exposure and Pulmonary Tuberculosis in Johannesburg, South Africa
Abstract
1. Introduction
2. Materials and Methods
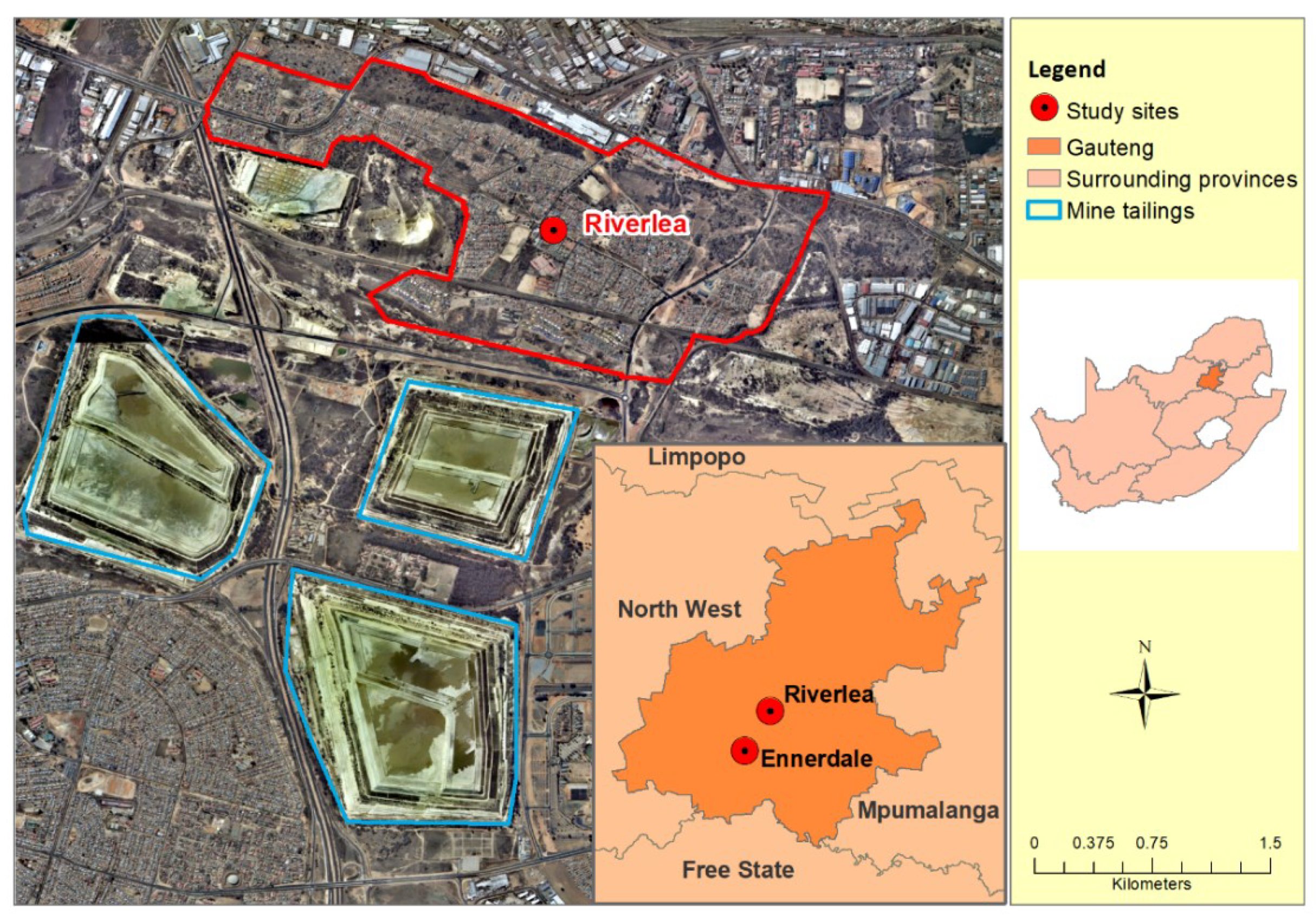
2.1. Study Population and Setting
2.2. Measures
2.3. Radiological Assessment of Tuberculosis
2.4. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hobbs, P.; Oelofse, S.H.; Rascher, J.; Cobbing, J. Pollution reality of gold mining waste on the Witwatersrand. ReSource 2010, 12, 51–55. [Google Scholar]
- Coetzee, H.; Winde, F.; Wade, P. An Assessment of Sources, Pathways, Mechanisms and Risks of Current and Potential Future Pollution of Water and Sediments in Gold-mining Areas of the Wonderfonteinspruit Catchment: Report to the Water Research Commission; Water Research Commission: Pretoria, South Africa, 2004; p. 266. [Google Scholar]
- Rösner, T.; Boer, R.; Reyneke, R.; Aucamp, P.; Vermaak, J. A Preliminary Assessment of Pollution Contained in the Unsaturated and Saturated Zone beneath Reclaimed Gold-Mine Residue Deposits; Water Research Commission: Pretoria, South Africa, 2001. [Google Scholar]
- Andraos, C.; Utembe, W.; Gulumian, M. Exceedance of environmental exposure limits to crystalline silica in communities surrounding gold mine tailings storage facilities in South Africa. Sci. Total Environ. 2018, 619–620, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, V.; Wichmann, J.; Voyi, K. Mine dumps, wheeze, asthma, and rhinoconjunctivitis among adolescents in South Africa: Any association? Int. J. Environ. Health Res. 2015, 25, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, V. How Mine Dumps in South Africa Affect the Health of Communities Living Nearby, 2018. The Conversation. Available online: https://theconversation.com/how-mine-dumps-in-south-africa-affect-the-health-of-communities-living-nearby-77113 (accessed on 20 January 2019).
- Steenland, K. One agent, many diseases: Exposure-response data and comparative risks of different outcomes following silica exposure. Am. J. Ind. Med. 2005, 48, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Statistics South Africa. Mortality and Causes of Death in South Africa, 2015: Findings from Death Notification Form (P0309.3); Statistics South Africa: Pretoria, South Africa, 2018. [Google Scholar]
- Hnizdo, E.; Murray, J. Risk of pulmonary tuberculosis relative to silicosis and exposure to silica dust in South African gold miners. Occup. Environ. Med. 1998, 55, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, R.I.; Churchyard, G.J.; Pemba, L.; Dekker, K.; Vermeis, M. Tuberculosis and silica exposure in South African gold miners. Occup. Environ. Med. 2006, 63, 187–192. [Google Scholar]
- Cowie, R.L. The epidemiology of tuberculosis in gold miners with silicosis. Am. J. Respir. Crit. Care Med. 1994, 150, 1460–1462. [Google Scholar] [CrossRef]
- Bhagia, L. Non-occupational exposure to silica dust in vicinity of slate pencil industry, India. Environ. Monit. Assess. 2009, 151, 477. [Google Scholar] [CrossRef] [PubMed]
- HSRC. The City of Johannesburg (COJ) Economic Overview: 2013 A Review of the State of the Economy and Other Key Indicators; Human Sciences Research Council: City of Johannesburg, South Africa, 2013; Available online: http://bit.ly/13KRrj9 (accessed on 20 January 2019).
- World Health Organization. Systematic Screening for Active Tuberculosis: Principles and Recommendations; Geneva, World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- ILO. Guidelines for the Use of the ILO International Classification of Radiographs of Pneumoconiosis: Revised Edition 2011; International Labor Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Lönnroth, K.; Jaramillo, E.; Williams, B.G.; Dye, C.; Raviglione, M. Drivers of tuberculosis epidemics: The role of risk factors and social determinants. Soc. Sci. Med. 2009, 68, 2240–2246. [Google Scholar] [CrossRef] [PubMed]
- Duarte, R.; Lönnroth, K.; Carvalho, C.; Lima, F.; Carvalho, A.C.; Munoz-Torrico, M.; Centis, R. Tuberculosis, social determinants and co-morbidities (including HIV). Pulmonology 2018, 24, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-H.; Ezzati, M.; Murray, M. Tobacco smoke, indoor air pollution and tuberculosis: A systematic review and meta-analysis. PLoS Med. 2007, 4, e20. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, V.; Wichmann, J.; Voyi, K. Comorbidity of respiratory and cardiovascular diseases among the elderly residing close to mine dumps in South Africa: A cross-sectional study. SAMJ S. Afr. Med. J. 2016, 106, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Den Boon, S.; van Lill, S.W.P.; Borgdorff, M.W.; Enarson, D.A.; Verver, S.; Bateman, E.D. High Prevalence of Tuberculosis in Previously Treated Patients, Cape Town, South Africa. Emerg. Infect. Dis. 2007, 13, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.; Bhatia, M.; Suraweera, W.; Morris, S.K.; Patra, C.; Gupta, P.C.; Jha, P. Exposure to Second-Hand Smoke and the Risk of Tuberculosis in Children and Adults: A Systematic Review and Meta-Analysis of 18 Observational Studies. PLoS Med. 2015, 12, e1001835. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Aerts, S.L.; Verma, D.; Ordway, D.J.; Chan, E.D. Epidemiologic Evidence of and Potential Mechanisms by Which Second-Hand Smoke Causes Predisposition to Latent and Active Tuberculosis. Imm. Netw. 2018, 18, e22. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Laso, J.M.; González-García, S.; González-Cortés, C.; Diez-Tascón, C.; López-Medrano, R.; Rivero-Lezcano, O.M. Macrophages from elders are more permissive to intracellular multiplication of Mycobacterium tuberculosis. Age 2013, 35, 1235–1250. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.; Glass, B.; t Mannetje, A.; Douwes, J. Exposure to respirable crystalline silica in the construction industry-do we have a problem. NZ Med. J. 2017, 130, 78–82. [Google Scholar]
- Bang, K.M.; Weissman, D.N.; Wood, J.M.; Attfield, M.D. Tuberculosis mortality by industry in the United States, 1990–1999. Int. J. Tuberc. Lung Dis. 2005, 9, 437–442. [Google Scholar] [PubMed]
- Circular Instruction No. 178. Regarding Compensation for Pulmonary TB in Health Care Workers; National Department of Labour: Pretoria, South Africa, 2003.
- Koppaka, R.; Bock, N. How reliable is chest radiography? In Toman’s Tuberculosis; Friedman, T., Ed.; World Health Organisation: Geneva, Switzerland, 2004; pp. 51–60. [Google Scholar]
- World Health Organization. Chest Radiography in Tuberculosis Detection: Summary of Current WHO Recommendations and Guidance on Programmatic Approaches; Report No. 924151150; Geneva, World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization. Tuberculosis Prevalence Surveys: A Handbook; Geneva, World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]

| Characteristics | Riverlea * n (%) | Ennerdale * n (%) | p-Value |
|---|---|---|---|
| N | 125 | 53 | |
| Socio-demographic | |||
| Median age in years (IQR) | 53 (23–79) | 45 (20–66) | 0.006 |
| Female % | 64.8 | 62.3 | 0.346 |
| Average monthly income | |||
| No income | 22 (17.9) | 9 (16.9) | |
| <R 1000 | 15 (12.2) | 3 (5.7) | |
| ≥R 1000 | 86 (69.9) | 41 (77.4) | 0.397 |
| Overcrowding (>3 persons per bedroom) | 18 (14.4) | 1 (1.9) | 0.013 |
| Biomass fuel used for cooking/heating | 11 (8.8) | 13 (24.5) | 0.009 |
| Smoking history | 70 (56.5) | 29 (54.7) | 0.831 |
| Total pack-years smoked | |||
| Non-smoker (<0.5) | 52 (41.6) | 23 (43.4) | |
| 0.5–10 | 32 (25.6) | 19 (35.8) | |
| 11–20 | 21 (16.8) | 7 (13.2) | |
| >21 | 20 (16.0) | 4 (7.6) | 0.299 |
| Exposed to second-hand smoke | 10 (8.0) | 2 (3.8) | 0.304 |
| Environmental dust exposure | |||
| Median years living in the study area (IQR) | 32 (8–60) | 17 (5–39) | <0.001 |
| Perception of outdoor dust during windy weather | 114 (92.7) | 48 (90.6) | 0.635 |
| Dust inside the house | 81 (65.9) | 36 (67.9) | 0.789 |
| Occupational dust exposure | |||
| Worked in a dusty environment > 1 year | 35 (28.5) | 10 (18.9) | 0.181 |
| Median years worked in a dusty environment (IQR) | 6.0 (1–37) | 5.5 (5–10) | 0.365 |
| Types of dust exposure | |||
| No dust exposure | 88 (71.5) | 43 (81.1) | |
| Wood/saw dust | 13 (10.6) | 2 (3.8) | |
| Metal/welding | 6 (4.9) | 1 (1.9) | |
| Sand/construction/mining | 12 (9.8) | 2 (3.8) | |
| Other | 4 (3.2) | 5 (9.4) | 0.102 |
| Medical history | |||
| Median BMI (kg/m2) | 26.3 (16.6–44.7) | 27.2 (18.3–39.0) | 0.609 |
| Previous diagnosis of PTB | 8 (6.6) | 6 (11.5) | 0.276 |
| Self-reported diagnosis of diabetes (n, %) | 12 (9.6) | 4 (7.6) | 0.661 |
| Self-reported HIV status (n, %) | 2 (1.6) | 2 (3.8) | 0.371 |
| Characteristic | n | Crude OR (95% CI) | p-Value |
|---|---|---|---|
| Socio-demographic | |||
| Age in years | 178 | 1.03 (1.00–1.07) | 0.033 |
| Sex | |||
| Female | 64 | Reference | |
| Male | 114 | 1.94 (0.79–4.78) | 0.147 |
| Average monthly income | |||
| ≥R 1000 | 127 | Reference | |
| No income | 31 | 3.19 (1.17–8.66) | 0.051 |
| <R 1000 | 18 | 1.90 (0.48–7.58) | 0.354 |
| Overcrowding (≥3 persons/ bedroom) | |||
| No | 159 | Reference | |
| Yes | 19 | 1.38 (0.37–5.19) | 0.632 |
| Biomass fuel used for cooking/ heating | |||
| No | 154 | Reference | |
| Yes | 24 | 1.51 (0.52–5.97) | 0.493 |
| Smoking history | |||
| Non-smoker | 78 | Reference | |
| Smoker | 99 | 1.78 (0.57–3.63) | 0.216 |
| Total pack-years smoked | |||
| Non-smoker (<0.5) | 75 | Reference | |
| 0.5–10 | 51 | 1.25 (0.42–3.75) | 0.285 |
| 11–19 | 28 | 1.61 (0.43–6.02) | 0.472 |
| ≥20 | 24 | 1.38 (0.33–5.84) | 0.655 |
| Exposed to second-hand smoke | |||
| No | 166 | Reference | |
| Yes | 12 | 4.11 (1.12–15.07) | 0.033 |
| BMI (kg/m2) | 178 | 0.92 (0.85–0.99) | 0.030 |
| Environmental dust exposure | |||
| Exposure to gold mine tailing dust | |||
| No (Ennerdale) | 53 | Reference | |
| Yes (Riverlea) | 125 | 2.06 (0.66–6.41) | 0.212 |
| Years living in the study area | 178 | 0.69 (0.25–1.91) | 0.480 |
| Dust inside the home | |||
| No | 59 | Reference | |
| Yes | 117 | 1.09 (0.42–2.85) | 0.859 |
| Occupational dust exposure | |||
| Worked in a dusty environment >1 year | |||
| No | 133 | Reference | |
| Yes | 45 | 2.88 (1.14–7.22) | 0.024 |
| Types of dust exposure | |||
| No dust exposure | 131 | Reference | |
| Wood/saw dust | 15 | 1.52 (0.30–7.57) | 0.605 |
| Metal/welding | 7 | 1.65 (0.18–14.89) | 0.654 |
| Sand/construction/mining | 14 | 5.51 (1.58–19.11) | 0.007 |
| Other | 9 | 2.83 (0.52–15.20) | 0.224 |
| Years worked in a dusty environment | 178 | 1.02 (0.98–1.07) | 0.307 |
| Medical history | |||
| Self-reported HIV status | |||
| Negative | 174 | Reference | |
| Positive | 4 | 7.38 (0.98–55.20) | 0.052 |
| Self-reported diagnosis of diabetes | |||
| No | 161 | Reference | |
| Yes | 16 | 0.41 (0.05–3.28) | 0.404 |
| Previous diagnosis of TB | |||
| No | 163 | Reference | |
| Yes | 14 | 7.76 (2.36–25.59) | 0.001 |
| Characteristic | n | Adjusted OR * (95% CI) | p-Value |
|---|---|---|---|
| Socio-demographic | |||
| Exposure to gold mine tailing dust | |||
| No (Ennerdale) | 53 | Reference | |
| Yes (Riverlea) | 125 | 2.02 (0.35–11.48) | 0.423 |
| Mean age in years (SD) | 178 | 1.04 (0.99–1.09) | 0.106 |
| Average monthly income | |||
| ≥R 1000 | 127 | Reference | |
| No income | 31 | 3.19 (0.85–11.97) | 0.085 |
| <R 1000 | 18 | 0.81 (0.48–7.58) | 0.826 |
| Exposed to second-hand smoke | |||
| No | 166 | Reference | |
| Yes | 12 | 8.13 (1.16–57.22) | 0.035 |
| BMI (kg/m2) | 178 | 0.88 (0.80–0.98) | 0.017 |
| Occupational history | |||
| Types of dust exposure | |||
| No dust exposure | 131 | Reference | |
| Wood/saw dust | 15 | 0.78 (0.11–5.56) | 0.808 |
| Metal/welding | 7 | 0.82 (0.05–12.35) | 0.884 |
| Sand/construction/mining | 14 | 10.2 (2.10–50.11) | 0.004 |
| Other | 9 | 7.42 (0.83–65.7) | 0.071 |
| Medical history | |||
| Previous diagnosis of TB | |||
| No | 163 | ||
| Yes | 14 | 8.98 (1.98–40.34) | 0.004 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kootbodien, T.; Iyaloo, S.; Wilson, K.; Naicker, N.; Kgalamono, S.; Haman, T.; Mathee, A.; Rees, D. Environmental Silica Dust Exposure and Pulmonary Tuberculosis in Johannesburg, South Africa. Int. J. Environ. Res. Public Health 2019, 16, 1867. https://doi.org/10.3390/ijerph16101867
Kootbodien T, Iyaloo S, Wilson K, Naicker N, Kgalamono S, Haman T, Mathee A, Rees D. Environmental Silica Dust Exposure and Pulmonary Tuberculosis in Johannesburg, South Africa. International Journal of Environmental Research and Public Health. 2019; 16(10):1867. https://doi.org/10.3390/ijerph16101867
Chicago/Turabian StyleKootbodien, Tahira, Samantha Iyaloo, Kerry Wilson, Nisha Naicker, Spo Kgalamono, Tanya Haman, Angela Mathee, and David Rees. 2019. "Environmental Silica Dust Exposure and Pulmonary Tuberculosis in Johannesburg, South Africa" International Journal of Environmental Research and Public Health 16, no. 10: 1867. https://doi.org/10.3390/ijerph16101867
APA StyleKootbodien, T., Iyaloo, S., Wilson, K., Naicker, N., Kgalamono, S., Haman, T., Mathee, A., & Rees, D. (2019). Environmental Silica Dust Exposure and Pulmonary Tuberculosis in Johannesburg, South Africa. International Journal of Environmental Research and Public Health, 16(10), 1867. https://doi.org/10.3390/ijerph16101867





