Associated Factors of Drinking Prior to Recognising Pregnancy and Risky Drinking among New Zealand Women Aged 18 to 35 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
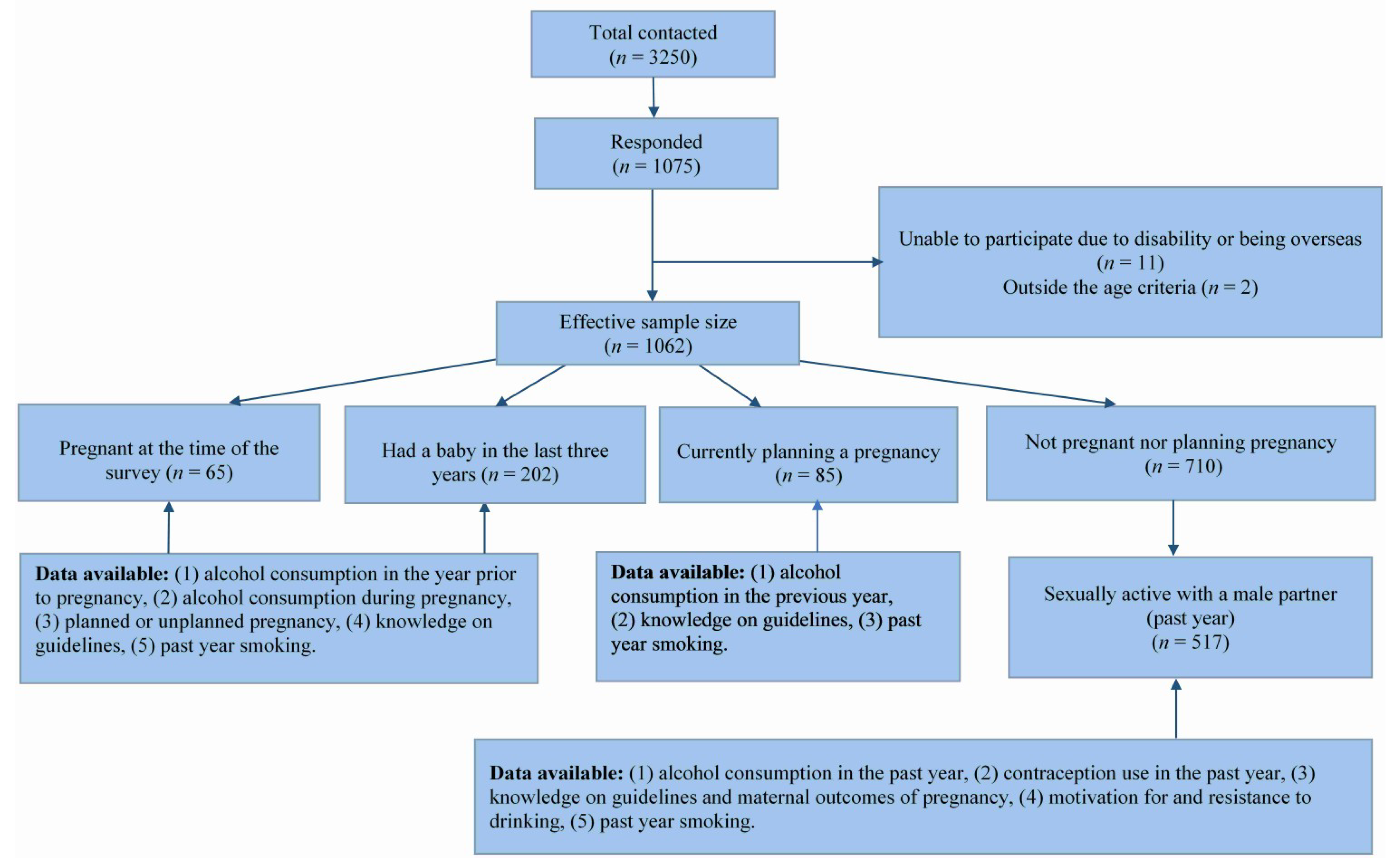
2.2. Sample Size Calculation and Participant Selection
2.3. Data Collection
2.4. Maternal Status
2.5. Demographic Measures
2.6. Knowledge Measures
2.7. Contraception Measures
2.8. Consumption Measures
“A 330 mL bottle/stubby or can of normal strength beer or a 30 mL measure of spirits mixed or straight, or 1 can of ready to drink (RTD) contains around one standard drink. 100 mL of wine is one standard drink, so a small 150 mL glass of wine contains one and a half standard drinks, a medium 200 mL wine glass contains two standard drinks and a typical 750 mL bottle of wine contains around eight standard drinks.”
2.9. Factors Associated with Drinking Prior to Recognising Pregnancy among Pregnant Women
2.10. Factors Associated with Risky Drinking among Sexually Active Nonpregnant Women Who Consumed Alcohol
2.11. Analysis
3. Results
3.1. Demographic Characteristics, Alcohol and Tobacco Consumption and Knowledge of Alcohol in Pregnancy Guidelines According to Maternal Status
3.2. Pregnant Women
3.2.1. Types of Drinkers in Pregnancy
3.2.2. Factors Associated with Drinking Prior to Pregnancy Recognition among Pregnant Women
3.3. Nonpregnant Women
3.3.1. Drinking Motives and Drinking Refusal Self-Efficacy Scores of Nonpregnant Sexually Active Women
3.3.2. Participants’ Level of Agreement on Outcomes of Maternal Drinking
3.3.3. Factors Associated with Risky Drinking among Nonpregnant Sexually Active Women Who Consumed Alcohol
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- May, P.A.; Chambers, C.D.; Kalberg, W.O.; Zellner, J.; Feldman, H.; Buckley, D.; Taras, H. Prevalence of fetal alcohol spectrum disorders in 4 U.S. communities. JAMA 2018, 379, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Probst, C.; Gmel, G.; Rehm, J.; Burd, L.; Papova, S. Global prevalence of fetal alcohol spectrum disorder among children and youth. A systematic review and meta-analysis. JAMA Pediatr. 2017, 171, 948–956. [Google Scholar] [CrossRef]
- Parackal, S.M.; Parackal, M.K.; Harraway, J.A. Prevalence and correlates of drinking in early pregnancy among women who stopped drinking on pregnancy recognition. Matern. Child Health J. 2013, 17, 520–529. [Google Scholar] [PubMed]
- Floyd, R.L.; Decouflé, P.; Hungerford, D.W. Alcohol use prior to pregnancy recognition. Am. J. Prev. Med. 1999, 17, 101–107. [Google Scholar] [CrossRef]
- Coles, C. Critical periods for prenatal alcohol exposure: Evidence from animal and human studies. Alcohol Res. Health 1994, 18, 22. [Google Scholar]
- Ministry of Health. Food and Nutrition Guidelines for Healthy Pregnant and Breastfeeding Women: A background Paper; Ministry of Health: Wellington, New Zealand, 2006.
- National Health and Medical Research Council. Australian Guidelines to Reduce Health Risks from Drinking Alcohol: Guideline 4: Pregnancy and Breastfeeding; NHMRC: Canberra, Australia, 2009. [Google Scholar]
- Butt, P.; Beirness, D.; Gliksman, L.; Paradis, C.; Stockwell, T. Alcohol and Health in Canada: A Summary of Evidence and Guidelines for Low-Risk Drinking; Canadian Centre on Substance Abuse: Ottawa, ON, USA, 2011. [Google Scholar]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 15 March 2017).
- UK Chief Medical Officers’ Low Risk Drinking Guidelines. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/545937/UK_CMOs__report.pdf (accessed on 15 November 2018).
- Tough, S.; Tofflemire, K.; Clarke, M.; Newburn-Cook, C. Do women change their drinking behaviours while trying to conceive? An opportunity for preconception counselling. J. Clin. Med. Res. 2006, 4, 97–105. [Google Scholar] [CrossRef]
- McCormack, C.; Hutchinson, D.; Burns, L.; Wilson, J.; Elliot, E.; Allsop, S.; Najman, J.; Jacons, S.; Rossem, L.; Olsson, C.; et al. Prenatal alcohol consumption between conception and recognition of pregnancy. Alcohol Clin. Exp. Res. 2017, 41, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Mullally, A.; Cleary, B.J.; Barry, J.; Fahey, T.P.; Murphy, D.J. Prevalence, predictors and perinatal outcomes of peri-conceptional alcohol exposure-retrospective cohort study in an urban obstetric population in Ireland. BMC Pregnancy Childbirth 2011, 11, 27. [Google Scholar]
- Muggli, E.; O’Leary, C.; Donath, S.; Orsini, F.; Forster, D.; Anderson, P.J.; Lewis, S.; Nagle, C.; Craig, J.M.; Elliott, E.; et al. “Did you ever drink more?” A detailed description of pregnant women’s drinking patterns. BMC Public Health 2016, 16, 683. [Google Scholar] [CrossRef]
- O’Callaghan, F.V.; O’Callaghan, M.O.; Najman, J.M.; Williams, G.M.; Bor, W. Maternal alcohol consumption during pregnancy and physical outcomes up to 5 years: A longitudinal study. Early Hum. Dev. 2003, 71, 137–148. [Google Scholar] [CrossRef]
- Strandberg-Larsen, K.; Nielsen, N.R.; Andersen, A.M.N.; Olsen, J.; Grønbæk, M. Characteristics of women who binge drink before and after they become aware of their pregnancy. Eur. J. Epidemiol. 2008, 23, 565–572. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.W.; Hicks, M.; Rasmussen, C.; Nagulesapillai, T.; Cook, J.; Tough, S.C. Characteristics of women who consume alcohol before and after pregnancy recognition in a Canadian sample: A prospective cohort study. Alcohol Clin. Exp. Res. 2014, 38, 3008–3016. [Google Scholar] [CrossRef] [PubMed]
- Dott, M.; Rasmussen, S.A.; Hogue, C.J.; Reefhuis, J. Association between pregnancy intention and reproductive health related behaviours before and after pregnancy recognition. Nation birth defects prevention study, 1997–2002. Matern. Child Health J. 2010, 14, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Finer, L.B.; Henshaw, S.K. Disparities of unintended pregnancy in the United States, 1994 and 2001. Perspect. Sex Reprod. Health 2006, 38, 90–96. [Google Scholar] [CrossRef]
- Mosher, W.; Jones, J.; Abma, J.C. Intended and Unintended Births in the United States, 1982–2010. National Health Statistics Report, No 55, 2012, Hyattsville, MD: National Centre for Health Statistics. Available online: https://www.ncbi.nlm.nih.gov/pubmed/23115878 (accessed on 1 July 2017).
- Roberts, S.C.M.; Wilsnack, S.C.; Foster, D.G.; Delucchi, K.L. Alcohol use before and during pregnancy. Alcohol Clin. Exp. Res. 2014, 38, 2844–2852. [Google Scholar] [CrossRef] [PubMed]
- Terplan, M.; Cheng, D.; Chisolm, M.S. The relationship between pregnancy intention and alcohol use behavior: An analysis of PRAMS data. J. Subst. Abuse Treat. 2014, 46, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Tyden, T.; Stern, J.; Nydahl, M.; Berglund, A.; Larsson, M.; Rosenblad, A.; Aarts, C. Pregnancy planning in Sweden—A pilot study among 270 women attending antenatal clinics. Acta. Obstet. Gyn. Scan. 2011, 90, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Backhausen, M.G.; Ekstrand, M.; Tyden, T.; Magnussen, B.K.; Shawe, J.; Stern, J.; Hegaard, H.K. Pregnancy planning and lifestyle prior to conception and during early pregnancy among Danish women. Eur. J. Contracept Reprod. Health Care 2014, 19, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.; Joelsson, L.S.; Tyden, T.; Berglund, A.; Ekstrand, M.; Heggard, H.; Aarts, C.; Rosebland, A.; Larsson, M.; Kristiansson, P. Is pregnancy planning associated with background characteristics and pregnancy-planning behaviour? Acta. Obstet. Gyn. Scan. 2015, 95, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Pryor, J.; Patrick, S.W.; Sundermann, A.C.; Wu, P.; Hartmann, K.E. Pregnancy intention and maternal alcohol consumption. Obstet. Gynecol. 2017, 129, 727–733. [Google Scholar] [CrossRef]
- Ethen, M.K.; Ramadhani, T.A.; Scheuerle, A.E.; Canfield, M.A.; Wyszynski, D.F.; Druschel, C.M.; Romitti, P.A. Alcohol Consumption by Women Before and During Pregnancy. Matern. Child Health J. 2009, 13, 274–285. [Google Scholar] [CrossRef]
- Kaplowitz, M.D.; Hadlock, T.D.; Levine, R. A comparison of web and mail survey response rates. Public Opin. Q. 2004, 68, 94–101. [Google Scholar] [CrossRef]
- Statistics New Zealand. Births and Deaths: Year ended December 2013. Available online: http://www.stats.govt.nz/browse_for_stats/population/births/BirthsAndDeaths_HOTPYeDec13/Commentary.aspx#woman (accessed on 5 April 2015).
- Maclennan, B.; Kypri, K.; Langley, J.; Room, R. Non-response bias in a community survey of drinking, alcohol-related experiences and public opinion on alcohol policy. Drug Alcohol Depend. 2012, 126, 189–194. [Google Scholar] [CrossRef]
- Statistics NZ. Census Form and Guide Notes. Available online: http://www.stats.govt.nz/Census/2013-census/info-about-the-census/forms-guidenotes.aspx (accessed on 1 June 2015).
- Annual Update of Key Results 2015/16: New Zealand Health Survey. Available online: https://minhealthnz.shinyapps.io/nz-health-survey-2015-16-annual-update/ (accessed on 13 March 2019).
- McLeod, D.; Pullon, S.; Cookson, T.; Cornford, E. Factors influencing alcohol consumption during pregnancy and after giving birth. N. Z. Med. J. 2002, 115, 1157. [Google Scholar]
- Saunders, J.B.; Aasland, O.G.; Babor, T.F.; de la Fuente, J.R.; Grant, M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 1993, 88, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.; Gray, R.; Smith, L.A. Brief screening questionnaires to identify problem drinking during pregnancy: A systematic review. Addiction 2010, 105, 601–614. [Google Scholar] [CrossRef]
- Cooper, M.L. Motivations for alcohol use among adolescents: Development and validation of a four-factor model. Psychol. Assess. 1994, 6, 117–128. [Google Scholar] [CrossRef]
- Oei, T.P.; Hasking, P.A.; Young, R.M. Drinking refusal self-efficacy questionnaire-revised (DRSEQ-R): A new factor structure with confirmatory factor analysis. Drug Alcohol Depend. 2005, 78, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Bradley, K.A.; Bush, K.R.; Epler, A.J.; Dobie, D.J.; Davis, T.M.; Sporleder, J.L.; Kivlahan, D.R. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): Validation in a female Veterans Affairs patient population. Arch. Intern. Med. 2003, 163, 821–829. [Google Scholar] [CrossRef]
- Crawford-Williams, F.; Fielder, A.; Mikocka-Walus, A.; Esterman, A.; Steen, M. A public health intervention to change knowledge, attitudes and behaviour regarding alcohol consumption in pregnancy. Evid. Based Midwifery 2016, 14, 4–10. [Google Scholar]
- O’Connor, M.J.; Tomlinson, M.; LeRoux, I.M.; Stewart, J.; Greco, E.; Rotheram-Borus, M.J. Predictors of alcohol use prior to pregnancy recognition among township women in Cape Town, South Africa. Soc. Sci. Med. 2011, 72, 83–90. [Google Scholar] [CrossRef]
- Guo, Y.; Kopec, J.A.; Cibere, J.; Li, L.C.; Goldsmith, C.H. Population survey features and response rates: A randomized experiment. Am. J. Public Health 2016, 106, 1422–1426. [Google Scholar] [CrossRef]
- Lin, I.F.; Schaeffer, N.C. Using survey participants to estimate the impact of nonparticipation. Public Opin. Q. 1995, 59, 236–258. [Google Scholar] [CrossRef]
- Ministry of Health, New Zealand. Report on Maternity 2015. Available online: https://www.health.govt.nz/publication/report-maternity-2015 (accessed on 13 March 2019).
- Ministry of Health, New Zealand. Fetal and Infant Deaths 2015. Available online: https://www.health.govt.nz/publication/fetal-and-infant-deaths-2015 (accessed on 13 March 2019).
- Papova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e290–e299. [Google Scholar] [CrossRef]
| Maternal Status % [95% CI] | ||||
|---|---|---|---|---|
| Nonpregnant N = 710 | Planning Pregnancy N = 85 | Previously Pregnant N = 202 | Currently Pregnant N = 65 | |
| Total | 67.6 [64.5–70.6] | 8.7 [6.9–10.9] | 18.4 [16.0–21.1] | 5.2 [4.0–6.8] |
| Age Category (years) | ||||
| 18–24 | 57.0 [53.1–60.9] | 21.6 [13.0–33.7] | 12.1 [7.6–18.9] | 9.2 [3.8–20.7] |
| 25–29 | 24.9 [21.7–28.3] | 37.2 [26.2–49.6] | 30.1 [23.4–37.8] | 45.6 [33.1–58.8] |
| 30–34 | 18.1 [15.4–21.1] | 41.2 [30.5–52.9] | 57.8 [50.0–65.2] | 45.2 [32.7–58.3] |
| Chi 2 = 206.1413; p < 0.001 | ||||
| Prioritised Ethnicity 2 | ||||
| Māori | 17.9 [14.9–21.3] | 22.7 [14.5–33.7] | 14.9 [10.3–20.9] | 10.1 [4.2–22.2] |
| Pacific | 5.3 [3.1–8.7] | 20.6 [10.7–35.8] | 11.4 [6.2–20.2] | 3.0 [0.4–18.3] |
| Asian | 17.0 [13.6–21.1] | 16.2 [8.9–27.6] | 18.9 [13.1–26.4] | 19.9 [10.0–35.5] |
| New Zealand European/other | 59.9 [55.5–64.0] | 40.5 [30.2–51.8] | 54.8 [47.0–62.5] | 67.1 [52.2–79.2] |
| Chi 2 = 41.4504; p = 0.002 | ||||
| Marital Status 3 | ||||
| Single/widowed/divorced | 68.2 [64.4–71.7] | 24.5 [15.1–37.1] | 14.2 [9.6–20.5] | 18.2 [9.8–31.2] |
| Permanent relationship | 31.8 [28.3–35.6] | 75.5 [62.9–84.9] | 85.8 [79.5–90.4] | 81.8 [68.8–90.2] |
| Chi 2 = 235.0809; p < 0.001 | ||||
| Highest level of Education 4 | ||||
| No secondary education | 1.0 [0.4–2.3] | 2.0 [0.5–7.8] | 3.8 [1.8–7.9] | 2.0 [0.3–12.7] |
| Some/Completed secondary education | 25.0 [21.4–28.8] | 20.3 [12.0–32.5] | 17.7 [12.5–24.5] | 17.8 [9.9–29.9] |
| Some University | 29.5 [25.9–33.3] | 22.0 [13.4–33.9] | 23.5 [17.7–30.5] | 17.0 [8.7–30.5] |
| Completed University | 44.6 [40.6–48.6] | 55.7 [43.4–67.3] | 55.0 [47.4–62.4] | 63.2 [49.5–75.1] |
| Chi 2 = 24.0947; p = 0.015 | ||||
| Current Employment 5 | ||||
| Employed | 77.0 [73.3–80.3] | 75.8 [64.1–84.6] | 60.3 [52.8–67.4] | 83.4 [70.1–91.5] |
| Unemployed | 23.0 [19.7–26.7] | 24.2 [15.4–35.9] | 39.7 [32.6–47.2] | 16.6 [8.5–29.9] |
| Chi 2 = 24.7187; p < 0.001 | ||||
| Use of Community Service Card 6 | ||||
| Yes | 19.7 [16.9–22.8] | 16.5 [8.6–24.4] | 20.8 [15.2–26.4] | 10.8 [3.2–18.3] |
| Chi 2 = 3.716; p = 0.294 | ||||
| Consumption Measures | ||||
| Risky Drinking (AUDIT-C ≥ 3) 7 YES | 61.0 [56.9–65.0] | 56.4 [44.2–67.9] | 55.6 8 [47.5–63.4] | 56.0 7 [42.2–68.9] |
| Chi 2 = 4.668; p = 0.198 | ||||
| Smoked YES | 23.2 [20.0–26.8] | 34.5 [24.1–46.7] | 18.1 7 [13.0–24.6] | 15.3 7 [7.9–27.7] |
| Chi 2 = 12.986; p = 0.005 | ||||
| Contraception use among sexually active nonpregnant women (n = 517) | ||||
| Always | 74.5 [69.9–78.6] | Not collected | ||
| Sometimes | 18.3 [14.8–22.4] | |||
| Never | 7.2 [4.8–10.7] | |||
| Knowledge measures 9 | ||||
| Knowledge about the government guideline on drinking in pregnancy: Pregnant women or those planning to get pregnant drink NO alcohol as there is no known safe level of alcohol use at any stage of pregnancy | ||||
| Yes | 88.1 [85.7–90.5] | 82.9 [74.8–90.3] | 92.4 [88.7–96.1] | 96.9 [92.6–99.9] |
| Chi 2 = 10.118; p = 0.018 | ||||
| Response Options | Maternal Status % [95%CI] | ||
|---|---|---|---|
| Currently Pregnant | Previously Pregnant | All Pregnant Women | |
| I had some alcohol but only before I knew I was pregnant and stopped | 46.0 [33.3–59.2] | 51.5 [43.7–59.2] | 50.2 [43.5–56.9] |
| I had some alcohol before I knew I was pregnant and continued | 8.7 [3.9–18.4] | 9.9 [6.6–14.7] | 9.6 [6.7–13.7] |
| I drink/drank being aware of my pregnancy | 1.4 [0.2–9.7] | 2.3 [0.9–5.5] | 2.1 [0.9–4.6] |
| I stopped drinking alcohol before I became pregnant | 28.8 [18.2–42.5] | 16.8 [11.7–23.5] | 19.5 [14.7–25.5] |
| Not sure | 0.0 | 0.9 [0.1–6.2] | 0.7 [0.1–4.8] |
| I don’t drink alcohol anyway | 15.0 [7.3–28.3] | 18.6 [13.1–25.8] | 17.8 [13.0–23.9] |
| Chi2 = 4.6208; p = 0.546 | |||
| Factor | Odds Ratio [95% CI of Odds] | p-Value |
|---|---|---|
| AUDIT-C ≥ 3 prior to pregnancy (Risky drinking) | 5.20 [2.78–9.75] | <0.001 |
| Smoke: Yes | 5.51 [1.51–20.11] | 0.010 |
| Planned pregnancy: Yes | 0.47 [0.22–1.01] | 0.052 |
| Use of community service card: Yes | 0.28 [0.12–0.67] | 0.004 |
| Minimum and Maximum Domain Scores | Mean (SD) | Median | |
|---|---|---|---|
| Drinking motives | |||
| Social 1 | 5–25 | 14.1 (5.2) | 14 |
| Coping 2 | 5–25 | 8.0 (3.3) | 7 |
| Enhancement 3 | 5–25 | 11.0 (4.9) | 10 |
| Conformity 4 | 5–25 | 6.7 (2.4) | 6 |
| Drinking Refusal Self-Efficacy | |||
| Social Pressure 5 | 5–30 | 24.5 (5.5) | 26 |
| Emotional Relief 6 | 7–42 | 38.5 (6.0) | 42 |
| Opportunistic 7 | 7–42 | 40.5 (3.8) | 42 |
| Knowledge Statements | Mean Score (SD) |
|---|---|
| A baby is particularly vulnerable to harm from alcohol during the early stages of pregnancy, i.e., the first 8 to 10 weeks of pregnancy | 6.43 (1.2) |
| If you have drunk some alcohol while you are pregnant stopping at any time is good for your baby 1 | 6.10 (1.7) |
| Drinking 1 or 2 drinks once or twice a week is okay when you are pregnant 2 | 6.41 (1.3) |
| Not drinking any alcohol at all during pregnancy is best for the baby | 6.72 (1.0) |
| When a pregnant women drinks, alcohol can pass through the placenta to the baby 2 | 6.54 (1.0) |
| Drinking alcohol at any time during pregnancy can harm the baby | 6.44 (1.2) |
| Drinking alcohol during pregnancy can lead to life-long disabilities in a child | 6.53 (1.0) |
| Composite Score | 6.54 (0.7) |
| Factor | Odds Ratio [95% CI of Odds] | p-Value |
|---|---|---|
| Ethnicity | ||
| New Zealand European/Other (Referent) | ||
| Māori or Pacific | 1.25 [0.55–2.82] | 0.595 |
| Asian | 0.08 [0.03–0.24] | 0.000 * |
| Motivation for drinking | ||
| Drinking for social reasons | 1.09 [1.01–1.18] | 0.021 * |
| Drinking for enhancement | 1.23 [1.11–1.36] | 0.000 * |
| Drinking for coping reasons | 1.25 [1.08–1.44 | 0.003 * |
| Smoking 3 | ||
| Yes | 2.70 [1.14–6.28] | 0.025 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parackal, S.; Parackal, M.; Harraway, J. Associated Factors of Drinking Prior to Recognising Pregnancy and Risky Drinking among New Zealand Women Aged 18 to 35 Years. Int. J. Environ. Res. Public Health 2019, 16, 1822. https://doi.org/10.3390/ijerph16101822
Parackal S, Parackal M, Harraway J. Associated Factors of Drinking Prior to Recognising Pregnancy and Risky Drinking among New Zealand Women Aged 18 to 35 Years. International Journal of Environmental Research and Public Health. 2019; 16(10):1822. https://doi.org/10.3390/ijerph16101822
Chicago/Turabian StyleParackal, Sherly, Mathew Parackal, and John Harraway. 2019. "Associated Factors of Drinking Prior to Recognising Pregnancy and Risky Drinking among New Zealand Women Aged 18 to 35 Years" International Journal of Environmental Research and Public Health 16, no. 10: 1822. https://doi.org/10.3390/ijerph16101822
APA StyleParackal, S., Parackal, M., & Harraway, J. (2019). Associated Factors of Drinking Prior to Recognising Pregnancy and Risky Drinking among New Zealand Women Aged 18 to 35 Years. International Journal of Environmental Research and Public Health, 16(10), 1822. https://doi.org/10.3390/ijerph16101822






