Finasteride-Induced Inhibition of 5α-Reductase Type 2 Could Lead to Kidney Damage—Animal, Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Hormone Assays
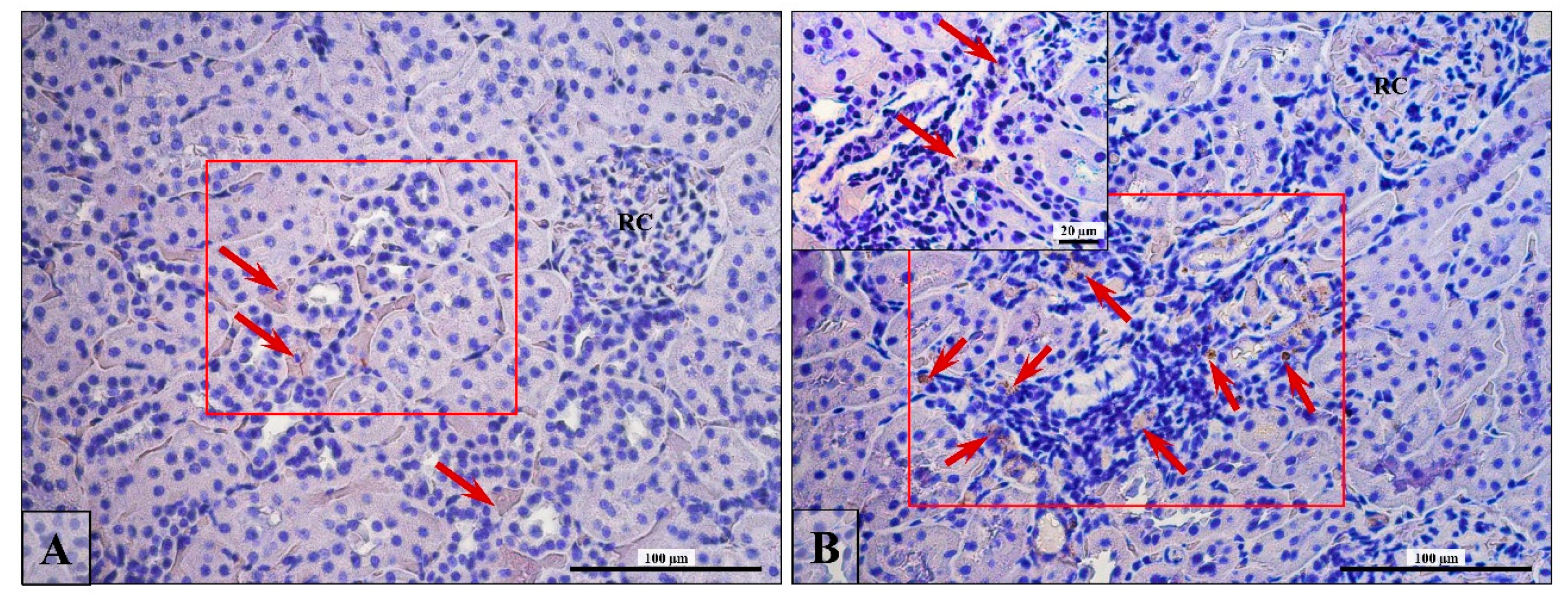
2.3. Immunohistochemistry (IHC)
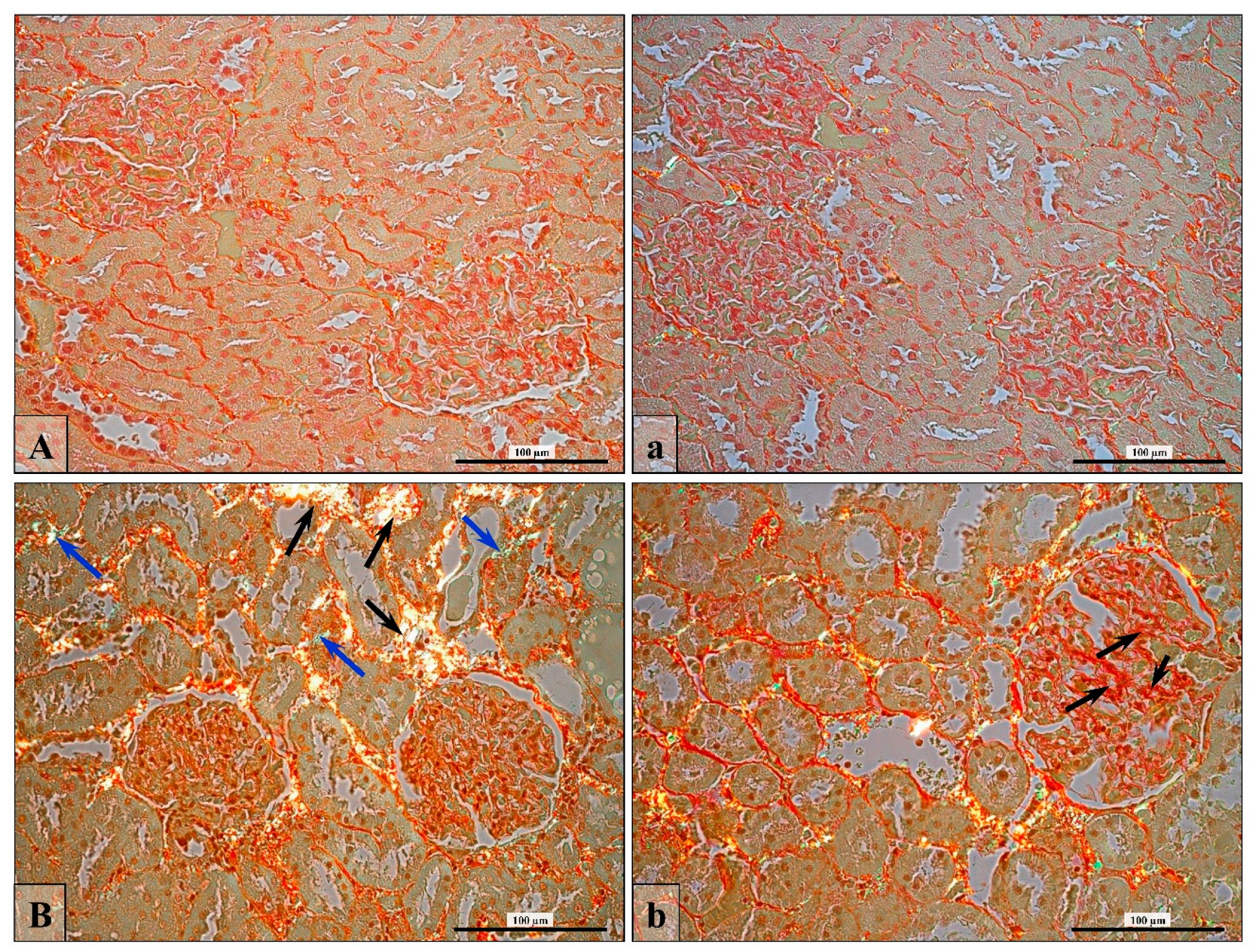
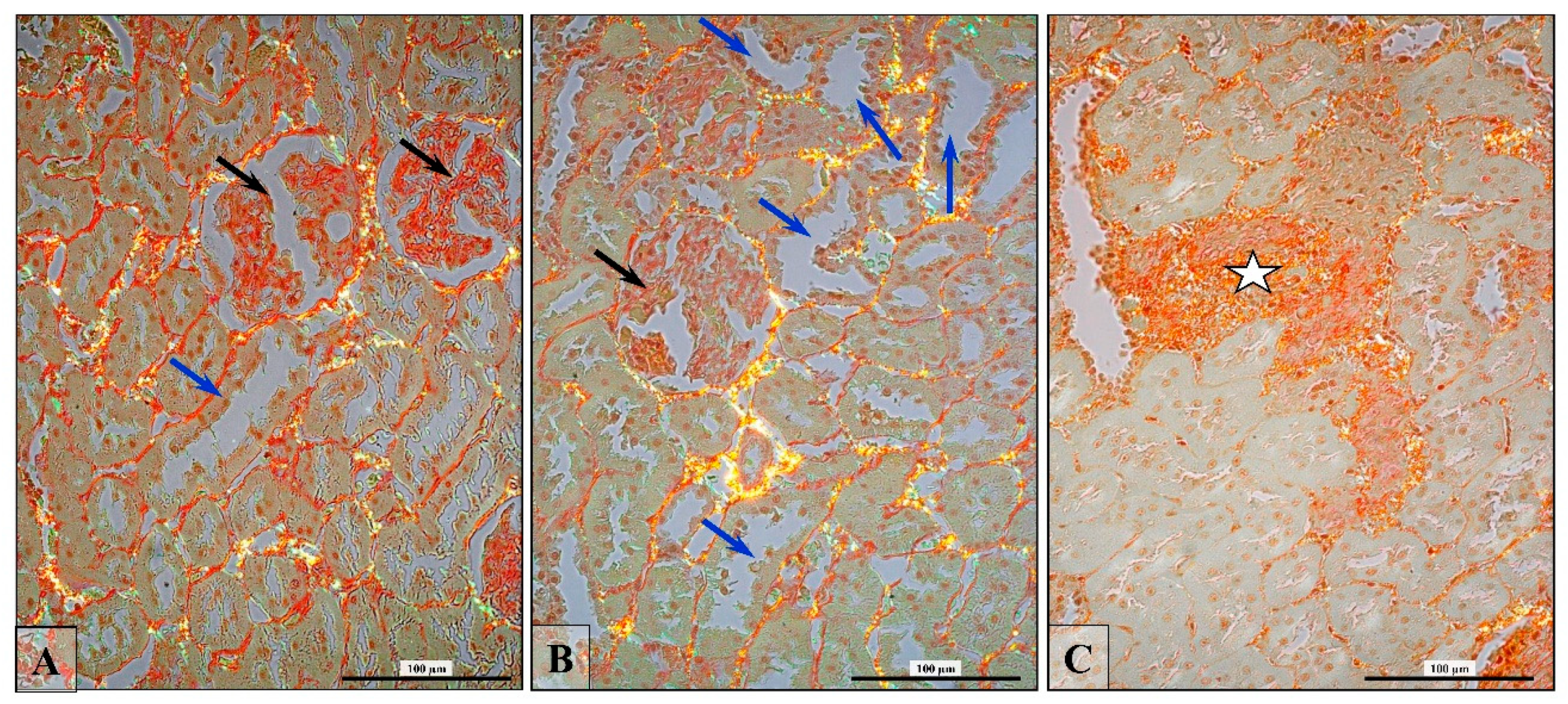
2.4. Collagen Fiber Visualization and Validation
2.5. Apoptosis In Situ Detection
Validation of TUNEL-Positive (Apoptotic Cell) and PCNA-Positive (Proliferating Cell)
2.6. Statistical Analysis
3. Results
3.1. Sex Hormone Levels
3.2. Androgen Receptor Expression
3.3. Junctional Protein Expression
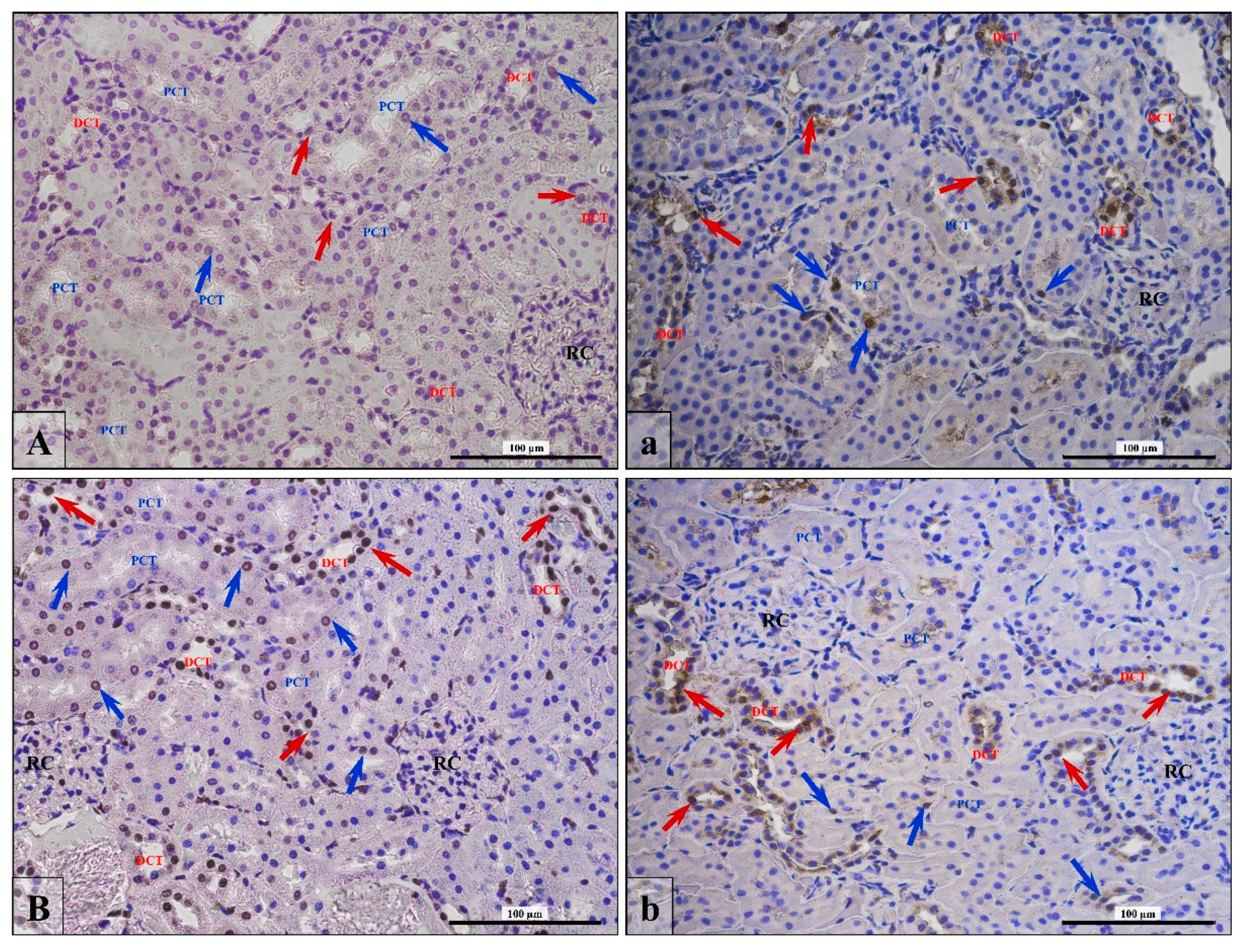
3.4. Apoptosis/Proliferation Ratio
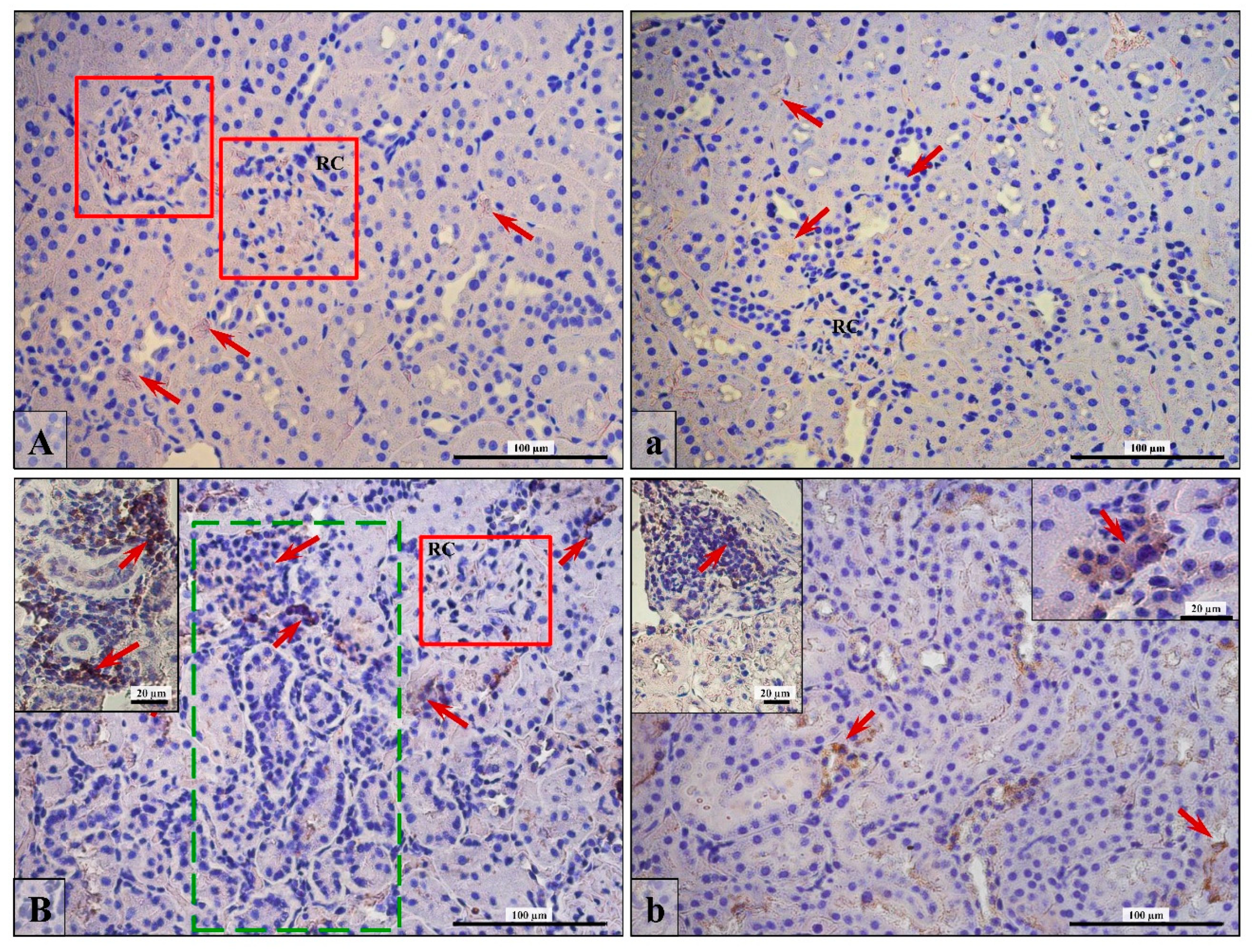
3.5. Lymphocytes T and B Specific Markers and IL-6 Expression
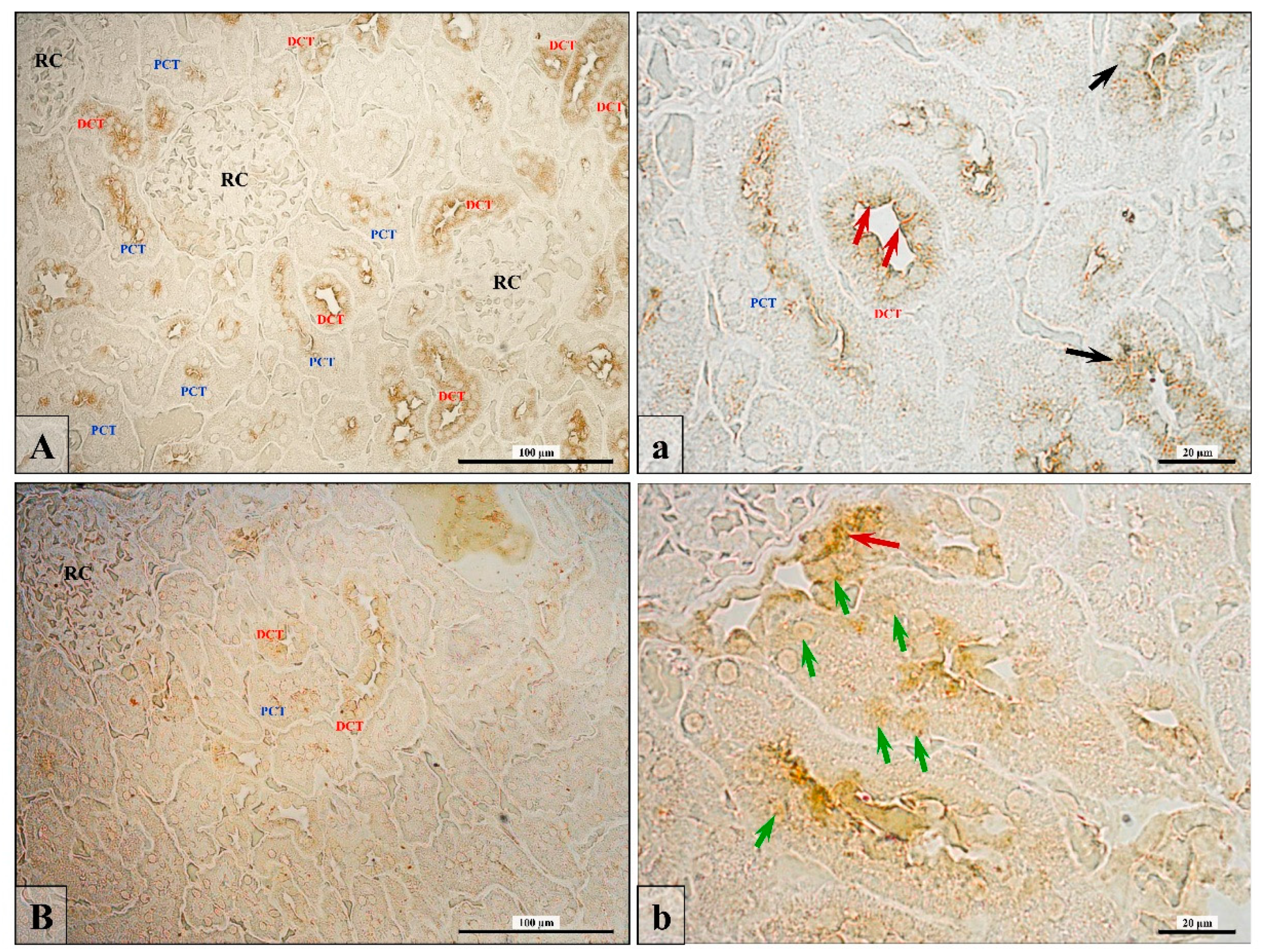
3.6. Renal Fibrosis (Collagen Fiber Thickening)
4. Discussion
5. Conclusions
- The finasteride treatment of adult male rats led to a decrease in androgen receptor expression and its cellular translocation within the kidney cortex.
- The pathomorphological changes (glomerulosclerosis, tubulosclerosis, dysplastic glomeruli, and tubules with lumen dilatation) in rats’ kidneys with disturbed steroid hormone imbalance were associated with the diminished expression of intracellular junctional proteins.
- The changed apoptotic/proliferating ratio of nephron cells and the increase in the numberof lymphocytes in the area of pathologically altered convoluted tubules were accompaniedby impaired androgen/estrogen homeostasis.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and ist disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Metcalf, B.W.; Levy, M.A.; Holt, D.A. Inhibitor of steroid 5-reductase in benign prostatic hyperplasia, male pattern baldness and acne. Trends Pharmacol. Sci. 1989, 10, 491–495. [Google Scholar] [CrossRef]
- Peters, D.H.; Sorkin, E.M. Finasteride: A review of its potential in the treatment of benign prostatic hyperplasia. Drugs 1993, 46, 77–208. [Google Scholar] [CrossRef] [PubMed]
- McClellan, K.J.; Markham, A. Finasteride: A review of its use in male pattern hair loss. Drugs 1999, 51, 111–126. [Google Scholar] [CrossRef] [PubMed]
- The Finasteride Male Pattern Hair Loss Group. Long-term (5-year) multinational experience with finasteride 1 mg in the treatment of men with androgenic alopecia. Eur. J. Dermatol. 2002, 12, 38–49. [Google Scholar]
- Lapi, F.; Azoulay, L.; Niazi, M.T.; Yin, H.; Benayoun, S.; Suissa, S. Androgen deprivation therapy and risk of acute kidney injury in patients with prostate cancer. JAMA 2013, 310, 289–296. [Google Scholar] [CrossRef]
- Molinari, C.; Battaglia, A.; Grossini, E.; Mary, D.A.; Vassanelli, C.; Vacca, G. The effect of testosterone on regional blood flow in prepubertal anaesthetized pigs. J. Physiol. 2002, 543, 365–372. [Google Scholar] [CrossRef]
- Hutchens, M.P.; Fujiyoshi, T.; Komers, R.; Herson, P.S.; Anderson, S. Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am. J. Physiol. Ren. Physiol. 2012, 303, F377–F385. [Google Scholar] [CrossRef]
- Sadler, T.W. Urogenital System. In Langman’s Medical Embryology; Sadler, T.W., Ed.; Lippincott Williams & Wilkins/Wolters Kluwer Company: Philadelphia, PA, USA, 2004; pp. 321–362. [Google Scholar]
- Reyes, J.L.; Meléndez, E.; Alegría, A.; Jaramillo-Juárez, F. Influence of sex differences on the renal secretion of organic anions. Endocrinology 1998, 139, 1581–1587. [Google Scholar] [CrossRef]
- Schmidt, A.; Luger, A.; Hörl, W.H. Sexual hormone abnormalities in male patients with renal failure. Nephrol. Dial. Transpl. 2002, 17, 368–371. [Google Scholar] [CrossRef]
- Sharma, K.; Ziyadeh, F.N. The emerging role of the transforming growth factor-β in the kidney disease. Am. J. Physiol. 1994, 266, F829–F842. [Google Scholar] [CrossRef]
- Liang, L.; Li, L.; Tian, J.; Lee, S.O.; Dang, Q.; Huang, C.K.; Yeh, S.; Erturk, E.; Bushinsky, D.; Chang, L.S.; He, D.; Chang, C. Androgen receptor enhances kidney stone-CaOx crystal formation via modulation of oxalate biosynthesis & oxidative stress. Mol. Endocrinol. 2014, 28, 1291–1303. [Google Scholar]
- Khalil, R.; Kim, N.R.; Jardi, F.; Vanderschueren, D.; Claessens, F.; Decallonne, B. Sex steroids and the kidney: Role in renal calcium and phosphate handling. Mol. Cell. Endocrionl. 2018, 465, 61–72. [Google Scholar] [CrossRef]
- Antus, B.; Yao, Y.; Liu, S.; Song, E.; Lutz, J.; Heemann, U. Contribution of androgens to chronic allograft nephropathy is mediated by dihydrotestosterone. Kidney Int. 2001, 60, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Antus, B.; Yao, Y.; Song, E.; Liu, S.; Lutz, J.; Heemann, U. Opposite effects of testosterone and estrogens on chronic allograft nephropathy. Transpl. Int. 2002, 15, 494–501. [Google Scholar] [CrossRef]
- Doublier, S.; Lupia, E.; Catanuto, P.; Periera-Simon, S.; Xia, X.; Korach, K.; Berho, M.; Elliot, S.J.; Karl, M. Testosterone and 17β-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int. 2011, 79, 404–413. [Google Scholar] [CrossRef]
- Sakemi, T.; Baba, N. Castration attenuates proteinuria and glomerular injury in unilaterally nephrectomized male Sprague-Dawley rats. Lab. Investig. 1993, 69, 51–57. [Google Scholar]
- Sakemi, T.; Toyoshima, H.; Morito, F. Testosterone eliminates attenuating effect of castration on the progressive glomerular injury in hypercholesterolemic male Imai rats. Nephron 1994, 67, 469–476. [Google Scholar] [CrossRef]
- Baylis, C. Age-dependent glomerular damage in rat: Dissociation between glomerular injury and both glomerular hypertension and hypertrophy: Male gender as a primary risk factor. J. Clin. Investig. 1994, 94, 1823–1829. [Google Scholar] [CrossRef]
- Reckelhoff, J.F.; Granger, J.P. Role of androgens in mediating hypertension and renal injury. Clin. Exp. Pharmacol. Physiol. 1999, 26, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Neugarten, J.; Acharya, A.; Silbiger, S.R. Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J. Am. Soc. Nephrol. 2000, 11, 319–329. [Google Scholar]
- Iliescu, R.; Reckelhoff, J.F. Sex and the kidney. Hypertension 2008, 51, 1000–1001. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, K. Mechanisms underlying sex differences in progressive renal disease. Gend. Med. 2008, 5, 10–23. [Google Scholar] [CrossRef]
- Goldberg, I.; Krause, I. The role of gender in chronic kidney diseases. EMJ 2016, 1, 58–64. [Google Scholar]
- Sullivan, J.C.; Gillis, E.E. Sex and gender differences in hypertensive kidney injury. Am. J. Physiol. Ren. Physiol. 2017, 313, F1009–F1017. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Roeher, O.; Hickstein, H.; Korht, S. Blood pressure guided profiling of ultrafiltration during hemodialysis. Saudi J. Kidney Dis. Transpl. 2001, 12, 337–344. [Google Scholar]
- Neugarten, J.; Golestaneh, L. Gender and the prevalence and progression of renal disease. Adv. Chronic Kidney Dis. 2013, 20, 390–395. [Google Scholar] [CrossRef]
- Silbiger, S.R.; Neugarten, J. The impact of gender on the progression of chronic renal disease. Am. J. Kidney Dis. 1995, 25, 515–533. [Google Scholar] [CrossRef]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Cell Physiol 2004, 286, C1213–C1228. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, C.T.; Macara, I.G. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell 2007, 18, 189–200. [Google Scholar] [CrossRef]
- Theard, D.; Steiner, M.; Kalicharan, D.; Hoekstra, D.; van Ijzendoorn, S.C. Cell polarity development and protein trafficking in hepatocytes lacking E-cadherin/beta-catenin-based adherens junctions. Mol. Biol. Cell 2007, 18, 2313–2321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Firestone, G.L.; Kapadia, B.J. Minireview: Regulation of gap junction dynamics by nuclear hormone receptors and their ligands. Mol. Endocrinol. 2012, 26, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Gary, L.; Firestone, G.L.; Kapadia, B.J. Minireview: Steroid/nuclear receptor-regulated dynamics of occluding and anchoring junctions. Mol. Endocrinol. 2014, 28, 1769–1784. [Google Scholar]
- Gonzalez-Mariscal, L.; Namorado, M.C.; Martin, D.; Luna, J.; Alarcon, L.; Islas, S.; Valencia, L.; Muriel, P.; Ponce, L.; Reyes, J.L. Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules. Kidney Int. 2000, 57, 2386–2402. [Google Scholar] [CrossRef]
- Balkovetz, D.F. Claudins at the gate: Determinants of renal epithelial tight junction paracellular permeability. Am. J. Physiol. Ren. Physiol. 2006, 290, F572–F579. [Google Scholar] [CrossRef]
- Balkovetz, D.F. Tight junction claudins and the kidney in sickness and in health. Biochim. Biophys. Acta 2009, 1788, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Lamar, P.C.; Appelt, D.M. Differential expression of E-cadherin, N-cadherin and beta-catenin in proximal and distal segments of the rat nephron. BMC Physiol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Z.; Niu, B.; Zhang, J.; Tan, T.K.; Lee, S.R.; Zhao, Y.; Harris, D.C.; Zheng, G. E-cadherin/β-catenin complex and the epithelial barrier. J. Biomed. Biotechnol. 2011, 2011, 567305. [Google Scholar] [CrossRef] [PubMed]
- Hanner, F.; Sorensen, C.M.; Holstein-Rathlou, N.H.; Peti-Peterdi, J. Connexins and the kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1143–R1155. [Google Scholar] [CrossRef]
- Mese, G.; Richard, G.; White, T.W. Gap Junctions: Basic structure and function. J. Investig. Dermatol. 2007, 127, 2516–2524. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.T.; Alpert, L.; Laird, D.W.; Batias, G.; Chalifour, L.; Alaoui-Jamali, M.A. Regulation of the gap junction connexin 43 gene by androgens in the prostate. J. Mol. Endocrinol. 2001, 26, 1–10. [Google Scholar] [CrossRef]
- Kolasa, A.; Marchlewicz, M.; Wenda-Różewicka, L.; Wiszniewska, B. DHT deficiency perturbs the integrity of the rat seminiferous epithelium by disrupting tight and adherens junctions. Folia Histochem. Cytobiol. 2011, 49, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Kolasa-Wołosiuk, A.; Misiakiewicz-Has, K.; Baranowska-Bosiacka, I.; Gutowska, I.; Wiszniewska, B. Androgen levels and apoptosis in the testis during postnatal development of finasteride-treated male rat offspring. Folia Histiochem. Cytobiol. 2015, 53, 236–248. [Google Scholar] [CrossRef]
- Kolasa-Wołosiuk, A.; Misiakiewicz-Has, K.; Baranowska-Bosiacka, I.; Gutowska, I.; Tarnowski, M.; Tkacz, M.; Wiszniewska, B. Connexin 43 expression in the testes during postnatal development of finasteride-treated male rat offspring. Arch. Med. Sci. 2018, 14, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Ashby, J.; Tinwell, H.; Odum, J.; Lefevre, P. Natural variability and the influence of concurrent control values on the detection and interpretation of low-dose or weak endocrine toxicities. Environ. Health Perspect. 2004, 112, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Kearbey, J.D.; Nair, V.A.; Chung, K.; Parlow, A.F.; Miller, D.D.; Dalton, J.T. Comparison of the pharmacological effects of a novel selective androgen receptor modulator, the 5alpha-reductase inhibitor finasteride, and the anti-androgen hydroxyl flutamide in intact rats: New approach for benign prostate hyperplasia. Endocrinology 2004, 145, 5420–5428. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Kędzierska, K.; Sporniak-Tutak, K.; Kolasa, A.; Domański, L.; Domański, M.; Sindrewicz, K.; Smektała, T.; Bober, J.; Safranow, K.; Osekowska, B.; et al. The effect of immunosuppressive therapy on renal cell apoptosis in native rat kidneys. Histol. Histopathol. 2015, 30, 105–116. [Google Scholar] [CrossRef]
- PROPECIA—Finasteride Tablet, Film Coated. Available online: http://archive.is/xdDiK#selection-131.0-131.621 (accessed on 24 March 2019).
- Amory, J.K.; Watts, N.B.; Easley, K.A.; Sutton, P.R.; Anawalt, B.D.; Matsumoto, A.M.; Bremner, W.J.; Tenover, J.L. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J. Clin. Endocrinol. Metab. 2004, 89, 503–510. [Google Scholar] [CrossRef]
- Vaughan, C.; Goldstein, F.C.; Tenover, J.L. Exogenous testosterone alone or with finasteride does not improve measurements of cognition in healthy older men with low serum testosterone. J. Androl. 2007, 28, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Chodak, G.; Mutchnik, S.; Nakamoto, T.; Chang, C. Immunohistochemical localization of androgen receptors with mono- and polyclonal antibodies to androgen receptor. J. Endocrinol. 1999, 126, 17–25. [Google Scholar] [CrossRef]
- Kimura, N.; Mizokami, A.; Oonuma, T.; Sasano, H.; Nagura, H. Immunocytochemical localization of androgen receptor with polyclonal antibody in paraffin-embedded human tissues. J. Histochem. Cytochem. 1993, 41, 671–678. [Google Scholar] [CrossRef]
- Langner, C.; Ratschek, M.; Rehak, P.; Schips, L.; Zigeuner, R. Steroid hormone receptor expression in renal cell carcinoma: An immunohistochemical analysis of 182 tumors. J. Urol. 2004, 171, 611–614. [Google Scholar] [CrossRef]
- Li, J.Y.; Zhou, T.; Gao, X.; Xu, C.; Sun, Y.; Peng, Y.; Chang, Z.; Zhang, Y.; Jiang, J.; Wang, L.; et al. Testosterone and androgen receptor in human nephrolithiasis. J. Urol. 2010, 184, 2360–2363. [Google Scholar] [CrossRef] [PubMed]
- Yakirevich, E.; Matoso, A.; Morris, D.J.; Resnick, M.B. Steroid Receptors in Renal Cell Carcinoma. In Emerging Research and Treatments in Renal Cell Carcinoma; Robert, A., Ed.; In Tech: Ramsey, MN, USA, 2012; pp. 99–126. [Google Scholar]
- Turner, K.J.; Morley, M.; MacPherson, S.; Millar, M.R.; Wilson, J.A.; Sharpe, R.M.; Saunders, P.T. Modulation of gene expression by androgen and oestrogens in the testis and prostate of the adult rat following androgen withdrawal. Mol. Cell. Endocrinol. 2001, 178, 73–87. [Google Scholar] [CrossRef]
- Liu, T.; Wu, Z.; Fulton, M.D.; Johnson, J.M.; Berkman, E.C. Prolonged androgen deprivation leads to downregulation of androgen receptor and prostate-specific membrane antigen in prostate cancer cells. Int. J. Oncol. 2012, 41, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Deslypere, J.-P.; Young, M.; Wilson, J.D.; McPhaul, M.J. Testosterone and 5α-dihydrotestosterone interact differently with the androgen receptor to enhance transcription of the MMTV-CAT reporter gene. Mol. Cell. Endocrinol. 1992, 88, 15–22. [Google Scholar] [CrossRef]
- Chang, C.; Lee, S.O.; Yeh, S.; Chang, T.M. Androgen receptor (AR) differential roles in hormone-related tumors including prostate, bladder, kidney, lung, breast and liver. Oncogene 2014, 33, 3225–3234. [Google Scholar] [CrossRef] [PubMed]
- Bass, R.; Perry, B.; Langenstroer, P.; Thrasher, J.B.; Dennis, K.L.; Tawfik, O.; Holzbeierlein, J. Effects of short-term finasteride on apoptotic factors and androgen receptors in prostate cancer cells. J. Urol. 2009, 181, 615–619. [Google Scholar] [CrossRef]
- Iguchi, M.; Takamura, C.; Umekawa, T.; Kurita, T.; Kohri, K. Inhibitory effects of female sex hormones on urinary stone formation in rats. Kidney Int. 1999, 56, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Panet-Raymond, V.; Gottlieb, B.; Umekawa, T.; Kurita, T.; Kohri, K. Interactions between androgen and estrogen receptors and the effects on their transactional properties. Mol. Cell. Endocrinol. 2000, 167, 139–150. [Google Scholar] [CrossRef]
- Lee, S.H. Coexistence of cytoplasmic and nuclear estrogen receptors. A histochemical study on human mammary cancer and rabbit uterus. Cancer 1989, 64, 1461–1466. [Google Scholar] [CrossRef]
- Benten, W.P.M.; Stephan, C.; Lieberherr, M.; Wunderlich, F. Estradiol signaling via sequestrable surface receptors. Endocrinology 2001, 142, 1669–1677. [Google Scholar] [CrossRef]
- Gruber, C.J.; Tschugguel, W.; Schneeberger, C.; Huber, J.C. Production and actions of estrogens. N. Engl. J. Med. 2002, 346, 340–352. [Google Scholar] [CrossRef]
- Migliaccio, A.; Castoria, D.; Di Domenico, M.; de Falco, A.; Bilancio, A.; Lombardi, M.; Bottero, D.; Varricchio, L.; Nanayakkara, M.; Rotondi, A.; et al. Sex steroid hormones act as growth factor. J. Steroid Biochem. Mol. Biol. 2002, 83, 31–35. [Google Scholar] [CrossRef]
- Shihan, M.; Bulldan, A.; Scheiner-Bobis, G. Non-classical testosterone signaling is mediated by a G-protein-coupled receptor interacting with Gnα11. Biochim. Biophys. Acta 2014, 1843, 1172–1181. [Google Scholar] [CrossRef]
- Thomas, P.; Converse, A.; Berg, H.A. ZIP9, a novel membrane androgen receptor and zinc transporter protein. Gen. Comp. Endocriol. 2018, 257, 130–136. [Google Scholar] [CrossRef]
- Zhu, G.; Liang, L.; Li, L.; Dang, Q.; Song, W.; Yeh, S.; He, D.; Chang, C. The expression and evaluation of androgen receptor in human renal cell carcinoma. Urology 2014, 83, e19–e24. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y. Nuclear receptors link gender dimorphism of renal disease progression. Kidney Int. 2006, 70, 1889–1890. [Google Scholar] [CrossRef]
- Müller, V.; Szabo, A.; Viklicky, O. Sex hormones and gender related differences: Their influence on chronic renal allograft rejection. Kidney Int. 1999, 55, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Robertson, H.; Ali, S.; McDonnell, B.J.; Burt, A.D.; Kirby, J.A. Chronic renal allograft dysfunction: The role of T cell-mediated tubular epithelial to mesenchymal cell transition. J. Am. Sci. Nephrol. 2004, 15, 390–397. [Google Scholar] [CrossRef]
- Araneo, B.A.; Dowell, T.; Diegel, M.; Daynes, R.A. Dihydrotestosterone exerts a depressive influence on the production of interleukin-4 (IL-4), IL-5, and γ-interferon, but not IL-2 by activated murine T cells. Blood 1991, 78, 688–699. [Google Scholar]
- Benten, W.P.M.; Lieberherr, M.; Giese, G.; Wrehlke, C.; Stamm, O.; Sekeris, C.E.; Mossmann, H.; Wunderlich, F. Functional testosterone receptors in plasma membranes of T cells. FASEB J. 1999, 13, 123–133. [Google Scholar] [CrossRef]
- Grossman, C. Possible underlying mechanisms of sexual dimorphism in the immune response, fact and hypothesis. J. Steroid Biochem. 1989, 34, 241–251. [Google Scholar] [CrossRef]
- Huynh, H. Induction of apoptosis in rat ventral prostate by finasteride is associated with alteration in MAP kinase pathways and Bcl-2 related family of proteins. Int. J. Oncol. 2002, 20, 1297–1303. [Google Scholar] [CrossRef]
- Huynh, H.; Seyam, R.M.; Brock, G.B. Reduction of central prostate weight by finasteride is associated with suppression of insulin-like growth factor I (IGF-I), IGF-I receptor and an increase in IGF binding protein 3. Cancer Res. 1998, 58, 215–218. [Google Scholar] [PubMed]
- Huynh, H.; Alpert, L.; Alaoui-Jamali, M.A.; Ng, C.Y. Co-administration of finasteride and pure anti-estrogen ICI 182,780 act synergistically in modulating the IGF system in rat prostate. J. Endocrinol. 2001, 171, 109–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Metcalfe, P.D.; Leslie, J.A.; Campbell, M.T.; Meldrum, D.R.; Hile, K.L.; Meldrum, KK. Testosterone exacerbates obstructive renal injury by stimulating TNF-alpha production and increasing proapoptotic and profibrotic signaling. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E435–443. [Google Scholar] [CrossRef] [PubMed]
- Verzola, D.; Gandolfo, M.T.; Salvatore, F.; Villaggio, B.; Gianiorio, F.; Traverso, P.; Deferrari, G.; Garibotto, G. Testosterone promotes apoptotic damage in human renal tubular cells. Kidney Int. 2004, 65, 1252–1261. [Google Scholar] [CrossRef]
- Verzola, D.; Villaggio, B.; Procopio, V.; Gandolfo, M.T.; Gianiorio, F.; Famà, A.; Tosetti, F.; Traverso, P.; Deferrari, G.; Garibotto, G. Androgen-mediated apoptosis of kidney tubule cells: Role of c-Jun amino terminal kinase. Biochem. Biophys. Res. Commun. 2009, 387, 531–536. [Google Scholar] [CrossRef]
- Rittmaster, R.S.; Norman, R.W.; Thomas, L.N.; Rowden, G. Evidence for atrophy and apoptosis in the prostates of men given finasteride. J. Clin. Endocrinol Metab. 1996, 81, 814–819. [Google Scholar] [PubMed]
- Sirinarumitr, K.; Sirnarumitr, T.; Johnston, S.D.; Sarkar, D.K.; Kustritz, M.V. Finasteride-induced prostatic involution by apoptosis in dogs with benign prostatic hypertrophy. Am. J. Vet. Res. 2002, 63, 495–498. [Google Scholar] [CrossRef]
- Gilleron, J.; Carette, D.; Carpentier, F.; Segretain, D.; Pointis, G. Three dimensional analysis of connexin 43 gap junction in the ex vivo rat seminiferous tubules: Short-term effects of hormonal effectors. Microsc. Res. Tech. 2009, 72, 845–855. [Google Scholar] [CrossRef]
- Hejmej, A.; Bilińska, B. A role of junction-mediated interactions in cells of the male reproductive tract: Impact of prenatal, neonatal, and prepubertal exposure to anti-androgens on adult reproduction. Histol. Histopathol. 2014, 29, 815–830. [Google Scholar]
- Yao, J.; Oite, T.; Kitamura, M. Gap junctional intercellular communication in the juxtaglomerular apparatus. Am. J. Physiol. Ren. Physiol. 2009, 296, F939–F946. [Google Scholar] [CrossRef]
- Pechere-Bertschi, A.; Burnier, M. Gonadal steroids, salt-sensitivity and renal function. Curr. Opin. Nephrol. Hypertens. 2007, 16, 16–21. [Google Scholar] [CrossRef]
- Rastaldi, M.P.; Ferrario, F.; Giardino, L.; Dell’Antonio, G.; Grillo, C.; Grillo, P.; Strutz, F.; Müller, G.A.; Colasanti, G.; D’Amico, G. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 2002, 62, 137–146. [Google Scholar] [CrossRef]
- Zheng, G.; Lyons, J.G.; Tan, T.K.; Wang, Y.; Hsu, T.T.; Min, D.; Succar, L.; Rangan, G.K.; Hu, M.; Henderson, B.R.; Alexander, S.I.; Harris, D.C. Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-β1 in renal tubular epithelial cells. Am. J. Pathol. 2009, 175, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.J.; Oh, S.; Lee, G.T.; Chung, J.; Min, K.; Yoon, J.; Kim, W.; Ryu, D.S.; Kim, I.Y.; Kang, D.I. Clinical Significance of Wnt/β-Catenin Signaling and Androgen Receptor Expression in Prostate Cancer. World J. Men’s Health 2013, 31, 36–46. [Google Scholar] [CrossRef]
- Wang, K.; Li, N.; Yeung, C.H.; Li, J.Y.; Wang, H.Y.; Cooper, TG. Oncogenic Wnt/β-catenin signaling pathways in the cancer-resistant epididymis have implications for cancer research. Mol. Hum. Reprod. 2013, 19, 57–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kawakami, T.; Ren, S.; Duffield, J.S. Wnt signaling in kidney diseases: Dual roles in renal injury and repair. J. Pathol. 2013, 229, 221–231. [Google Scholar] [CrossRef] [PubMed]
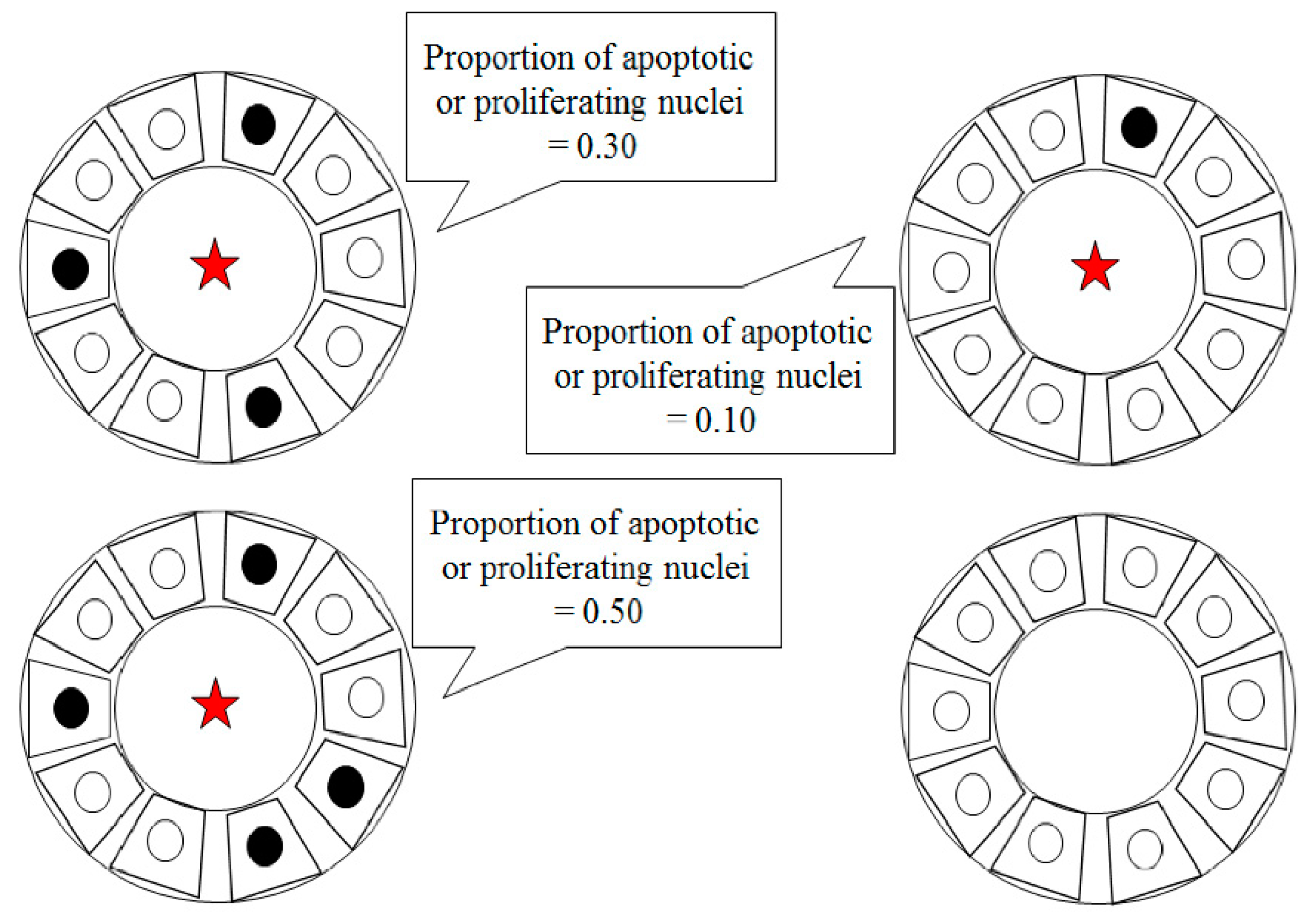
| DHT (ng/mL) | T (ng/mL) | E2 (pg/mL) | |||
|---|---|---|---|---|---|
| Control | Fin | Control | Fin | Control | Fin |
| 0.67 ± 0.03 | 0.25 ± 0.09 ** vs. Control | 1.53 ± 0.19 | 0.57 ± 0.25 ** vs. Control | 56.28 ± 9.47 | 31.33 ± 3.63 |
| Region | AR | |
|---|---|---|
| Control | Fin | |
| RC | 0.14 ± 0.12 | 0.06 ± 0.03 |
| PCT | 0.69 ± 0.32 | 0.16 ± 0.17 * |
| DCT | 0.91 ± 0.12 | 0.5 ± 0.26 * |
| Occludin | Control | Fin |
|---|---|---|
| RC mean ± SD | 100% (1) 1 ± 0 | 100% (1) 1 ± 0 |
| PCT mean ± SD | 18.5% (1); 81.5% (0) 0.185 ± 0.39 | 33.3% (1); 66.6% (0) 0.33 ± 0.48 |
| DCT mean ± SD | 58% (2); 42% (1) 1.58 ± 0.5 | 40% (2); 60 % (1) 1.4 ± 0.54 |
| Connexin 43 | ||
| RC mean ± SD | 100% (1) 1 ± 0 | 66.6% (2); 33.3% (1) 1.67 ± 0.58 *** |
| PCT mean ± SD | 24% (3); 24% (2); 52% (1) 1.71 ± 0.84 | 54.5% (3); 31.8% (2); 9.2% (1); 4.5% (0) 2.36 ± 0.84 * |
| DCT mean ± SD | 33.3% (4); 50% (3); 16.6% (2) 3.62 ± 0.71 | 80% (4); 20% (3) 3.8 ± 0.44 |
| E-cadherin | ||
| RC mean ± SD | 100% (2) 2 ± 0 | 100% (1) 1 ± 0 *** |
| PCT mean ± SD | 48,2% (2); 51.8% (1) 1.48 ± 0.51 | 7.2% (2); 17.8% (1); 75% (0) 0.32 ± 0.61 *** |
| DCT mean ± SD | 42.8% (4); 57.2% (3) 3.43 ± 0.51 | 31.6% (2); 68.4% (1) 1.3 ± 0.47 *** |
| N-cadherin | ||
| RC mean ± SD | 100% (3) 3 ± 0 | 100% (1) 1 ± 0 *** |
| PCT mean ± SD | 28,6 (1); 71.4% (0) 1.83 ± 0.41 | 21% (2); 52.6% (1); 26.4% (0) 0.95 ± 0.7 ** |
| DCT mean ± SD | 53.3% (3); 46.6% (2) 2.57 ± 0.5 | 83.3% (2); 16,6% (1) 0.28 ± 0.46 *** |
| β-catenin | ||
| RC mean ± SD | 100% (1) 1 ± 0 | 100% (1) 1 ± 0 |
| PCT mean ± SD | 45% (2); 54% (1) 1.46 ± 0.5 | 27.8% (1); 72.2% (0) 0.28 ± 0.45 |
| DCT mean ± SD | 62% (2); 28,6 (2); 9.4% (3) 3.33 ± 0.9 | 16.7% (4); 55.5% (3); 27.8% (2) 2.89 ± 0.68 |
| Apoptosis | Control | Fin |
|---|---|---|
| TUNEL+DCT | 0.77 ± 0.006 | 0.90 ± 0.0 6 * vs. Control |
| TUNEL+PCT | 0.73 ± 0.01 | 0.81 ± 0.14 |
| TUNEL+ cells per DCT | 0.18 ± 0.007 | 0.30 ± 0.07 |
| TUNEL+ cells per PCT | 0.15 ± 0.002 | 0.21 ± 0.043 |
| Proliferation | ||
| PCNA+DCT | 0.57 ± 0.14 | 0.86 ± 0.02 * vs. Control |
| PCNA+PCT | 0.35 ± 0.17 | 0.46 ± 0.16 |
| PCNA+ cells per DCT | 0.08 ± 0.002 | 0.17 ± 0.004 *** vs. Control |
| PCNA+ cells per PCT | 0.04 ± 0.01 | 0.07 ± 0.03 |
| Thickness of Collagen Fibers | Collagen Type I Fibers (µm) | Collagen Type III Fibers (µm) | ||
|---|---|---|---|---|
| Control | Fin | Control | Fin | |
| in the Interstitial Cortical Region | ||||
| Mean ± SD | 1.111 ± 0.469 | 3.298 ± 1.760 vs. Control *** | 1.561 ± 0.755 | 2.121 ± 1.154 |
| within the Renal Corpuscle | ||||
| Mean ± SD | 2.542 ± 0.974 | 2.287 ± 0.964 | 0.925 ± 0.532 | 1.426 ± 0.569 vs. Control * |
| Percentage of Area Occupied by the Collagen in Correlation to the Entire Area of the Section | ||||
| Control | Fin | |||
| Mean ± SD | 3.63 ± 1.55 | 8.56 ± 0.89 ** | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baig, M.S.; Kolasa-Wołosiuk, A.; Pilutin, A.; Safranow, K.; Baranowska-Bosiacka, I.; Kabat-Koperska, J.; Wiszniewska, B. Finasteride-Induced Inhibition of 5α-Reductase Type 2 Could Lead to Kidney Damage—Animal, Experimental Study. Int. J. Environ. Res. Public Health 2019, 16, 1726. https://doi.org/10.3390/ijerph16101726
Baig MS, Kolasa-Wołosiuk A, Pilutin A, Safranow K, Baranowska-Bosiacka I, Kabat-Koperska J, Wiszniewska B. Finasteride-Induced Inhibition of 5α-Reductase Type 2 Could Lead to Kidney Damage—Animal, Experimental Study. International Journal of Environmental Research and Public Health. 2019; 16(10):1726. https://doi.org/10.3390/ijerph16101726
Chicago/Turabian StyleBaig, Mirza Saim, Agnieszka Kolasa-Wołosiuk, Anna Pilutin, Krzysztof Safranow, Irena Baranowska-Bosiacka, Joanna Kabat-Koperska, and Barbara Wiszniewska. 2019. "Finasteride-Induced Inhibition of 5α-Reductase Type 2 Could Lead to Kidney Damage—Animal, Experimental Study" International Journal of Environmental Research and Public Health 16, no. 10: 1726. https://doi.org/10.3390/ijerph16101726
APA StyleBaig, M. S., Kolasa-Wołosiuk, A., Pilutin, A., Safranow, K., Baranowska-Bosiacka, I., Kabat-Koperska, J., & Wiszniewska, B. (2019). Finasteride-Induced Inhibition of 5α-Reductase Type 2 Could Lead to Kidney Damage—Animal, Experimental Study. International Journal of Environmental Research and Public Health, 16(10), 1726. https://doi.org/10.3390/ijerph16101726





