Combining Heart Rate Variability with Disease Severity Score Variables for Mortality Risk Stratification in Septic Patients Presenting at the Emergency Department
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population and Eligibility
2.3. Data Collection
2.4. Disease Severity Score Calculation
2.5. Statistical Analysis
3. Results
3.1. Patient Recruitment and Outcomes
3.2. Baseline Characteristics, Clinical and Heart Rate Variability Parameters
3.3. Components of Logistic Regression Models
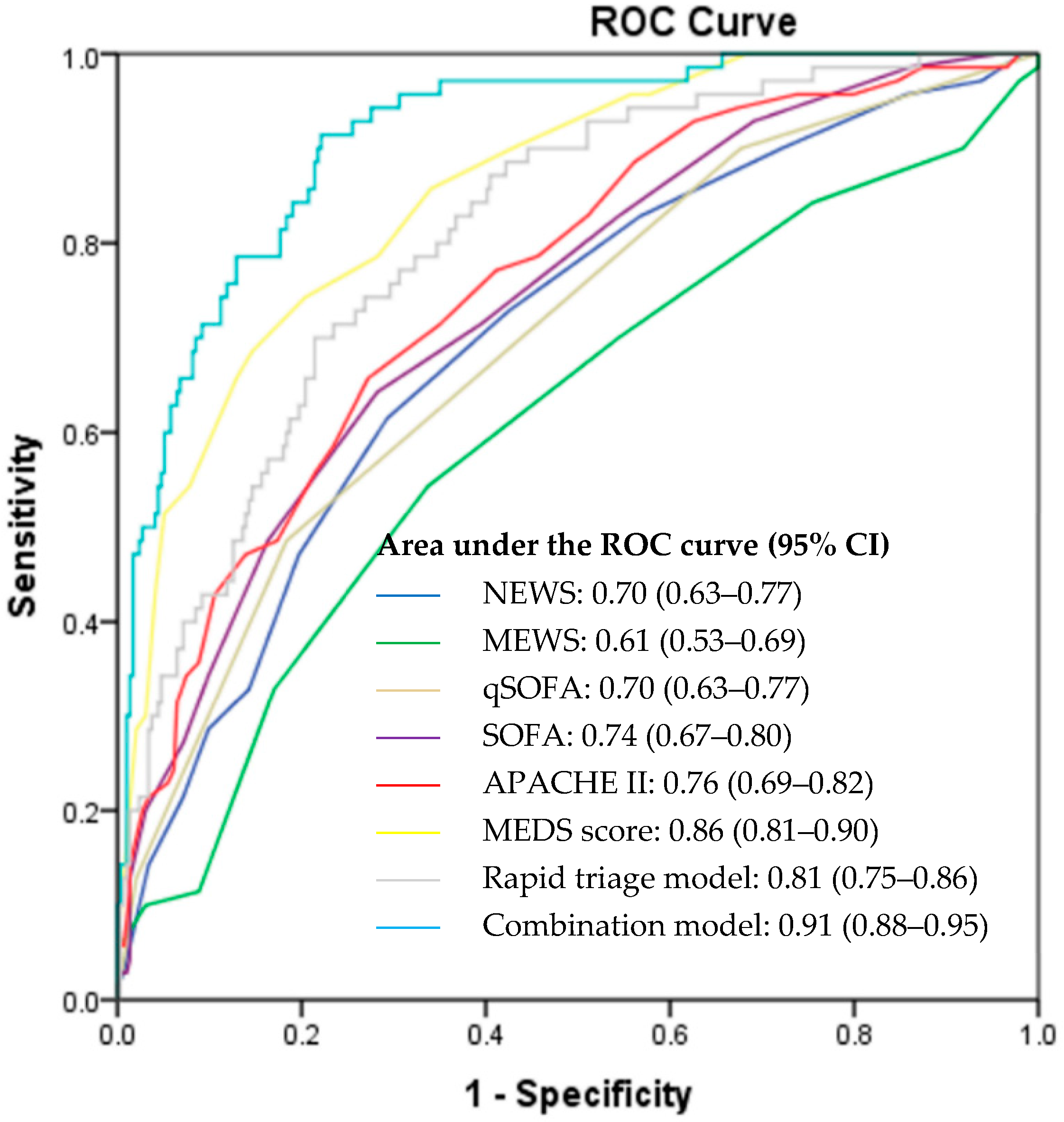
3.4. Prediction of Primary Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Variables | NEWS | MEWS | qSOFA | SOFA | APACHE II | MEDS Score |
|---|---|---|---|---|---|---|
| Age | ✓ | ✓ | ||||
| Nursing home resident | ✓ | |||||
| Chronic health problems | ✓ | |||||
| Terminal illness | ✓ | |||||
| Heart rate | ✓ | ✓ | ✓ | |||
| Blood pressure | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Respiratory rate/supplementary oxygen | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Temperature | ✓ | ✓ | ✓ | |||
| PaO2 | ✓ | ✓ | ||||
| SpO2 | ✓ | ✓ | ||||
| FiO2 | ✓ | ✓ | ||||
| AVPU | ✓ | ✓ | ||||
| GCS | ✓ | ✓ | ✓ | |||
| AMS | ✓ | |||||
| Hematocrit | ✓ | |||||
| White blood cell count | ✓ | |||||
| Percentage bands | ✓ | |||||
| Platelet count | ✓ | ✓ | ||||
| Serum sodium | ✓ | |||||
| Serum potassium | ✓ | |||||
| Serum creatinine | ✓ | ✓ | ||||
| Arterial pH or serum bicarbonate | ✓ | |||||
| Serum bilirubin | ✓ | |||||
| Suspicion of LRTI | ✓ | |||||
| Septic shock | ✓ | |||||
| Acute renal failure | ✓ | |||||
| Use of vasopressors | ✓ |
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K.; the International Forum of Acute Care Trialists. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010, 304, 1787–1794. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Knaus, W.A.; Wagner, D.P.; Lynn, J. Short-term mortality predictions for critically ill hospitalized adults: Science and ethics. Science 1991, 254, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Naylor, C.D.; Baigrie, R.S.; Goldman, B.S.; Cairns, J.A.; Beanlands, D.S.; Berman, N.; Borts, D.; Fitchett, D.H.; Haq, A.; Hess, A. Assigning priority to patients requiring coronary revascularization: Consensus principles from a panel of cardiologists and cardiac surgeons. Can. J. Cardiol. 1991, 7, 207–213. [Google Scholar]
- Prytherch, D.R.; Smith, G.B.; Schmidt, P.E.; Featherstone, P.I. ViEWS—Towards a national early warning score for detecting adult inpatient deterioration. Resuscitation 2010, 81, 932–937. [Google Scholar] [CrossRef]
- Subbe, C.P.; Kruger, M.; Rutherford, P.; Gemmel, L. Validation of a modified Early Warning Score in medical admissions. QJM 2001, 94, 521–526. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Shapiro, N.I.; Wolfe, R.E.; Moore, R.B.; Smith, E.; Burdick, E.; Bates, D.W. Mortality in Emergency Department Sepsis (MEDS) score: A prospectively derived and validated clinical prediction rule. Crit. Care Med. 2003, 31, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Samsudin, M.I.; Liu, N.; Prabhakar, S.M.; Chong, S.-L.; Lye, W.K.; Koh, Z.X.; Guo, D.; Rajesh, R.; Ho, A.F.W.; Ong, M.E.H. A novel heart rate variability based risk prediction model for septic patients presenting to the emergency department. Medicine 2018, 97, e10866. [Google Scholar] [CrossRef]
- Macdonald, S.P.; Arendts, G.; Fatovich, D.M.; Brown, S.G. Comparison of PIRO, SOFA, and MEDS scores for predicting mortality in emergency department patients with severe sepsis and septic shock. Acad. Emerg. Med. 2014, 21, 1257–1263. [Google Scholar] [CrossRef]
- Williams, J.M.; Greenslade, J.H.; Chu, K.; Brown, A.F.; Lipman, J. Severity Scores in Emergency Department Patients with Presumed Infection: A Prospective Validation Study. Crit. Care Med. 2016, 44, 539–547. [Google Scholar] [CrossRef]
- Hilderink, M.J.; Roest, A.A.; Hermans, M.; Keulemans, Y.C.; Stehouwer, C.D.; Stassen, P.M. Predictive accuracy and feasibility of risk stratification scores for 28-day mortality of patients with sepsis in an emergency department. Eur. J. Emerg. Med. 2015, 22, 331–337. [Google Scholar] [CrossRef]
- Malik, M.; Camm, A.J.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Scheff, J.D.; Griffel, B.; Corbett, S.A.; Calvano, S.E.; Androulakis, I.P. On heart rate variability and autonomic activity in homeostasis and in systemic inflammation. Math. Biosci. 2014, 252, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Barnaby, D.P.; Fernando, S.M.; Ferrick, K.J.; Herry, C.L.; Seely, A.J.E.; Bijur, P.E.; Gallagher, J. Use of the low-frequency/high-frequency ratio of heart rate variability to predict short-term deterioration in emergency department patients with sepsis. Emerg. Med. J. 2018, 35, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Barnaby, D.P.; Fernando, S.M.; Herry, C.L.; Scales, N.B.; Gallagher, E.J.; Seely, A.J.E. Heart Rate Variability, Clinical and Laboratory Measures to Predict Future Deterioration in Patients Presenting with Sepsis. Shock 2019, 51, 416–422. [Google Scholar] [CrossRef]
- Barnaby, D.; Ferrick, K.; Kaplan, D.T.; Shah, S.; Bijur, P.; Gallagher, E.J. Heart rate variability in emergency department patients with sepsis. Acad. Emerg. Med. 2002, 9, 661–670. [Google Scholar] [CrossRef]
- De Castilho, F.M.; Ribeiro, A.L.P.; da Silva, J.L.P.; Nobre, V.; de Sousa, M.R. Heart rate variability as predictor of mortality in sepsis: A prospective cohort study. PLoS ONE 2017, 12, e0180060. [Google Scholar] [CrossRef]
- Chen, W.L.; Kuo, C.D. Characteristics of heart rate variability can predict impending septic shock in emergency department patients with sepsis. Acad. Emerg. Med. 2007, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Chen, J.H.; Huang, C.C.; Kuo, C.D.; Huang, C.I.; Lee, L.S. Heart rate variability measures as predictors of in-hospital mortality in ED patients with sepsis. Am. J. Emerg. Med. 2008, 26, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.; Green, G.; Bravi, A.; Hollenberg, S.; Seely, A. Impaired heart rate variability predicts clinical deterioration and progressive organ failure in emergency department sepsis patients. Crit. Care 2012, 16, P37. [Google Scholar] [CrossRef][Green Version]
- Prabhakar, S.M.; Tagami, T.; Liu, N.; Samsudin, M.I.; Ng, J.C.J.; Koh, Z.X.; Ong, M.E.H. Combining quick sequential organ failure assessment score with heart rate variability may improve predictive ability for mortality in septic patients at the emergency department. PLoS ONE 2019, 14, e0213445. [Google Scholar] [CrossRef]
- Simpson, S.Q. New Sepsis Criteria: A Change We Should Not Make. Chest 2016, 149, 1117–1118. [Google Scholar] [CrossRef]
- Fernando, S.M.; Tran, A.; Taljaard, M.; Cheng, W.; Rochwerg, B.; Seely, A.J.E.; Perry, J.J. Prognostic Accuracy of the Quick Sequential Organ Failure Assessment for Mortality in Patients with Suspected Infection: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2018, 168, 266–275. [Google Scholar] [CrossRef]
- Van der Woude, S.W.; van Doormaal, F.F.; Hutten, B.A.; Nellen, F.J.; Holleman, F. Classifying sepsis patients in the emergency department using SIRS, qSOFA or MEWS. Neth. J. Med. 2018, 76, 158–166. [Google Scholar]
- Usman, O.A.; Usman, A.A.; Ward, M.A. Comparison of SIRS, qSOFA, and NEWS for the early identification of sepsis in the Emergency Department. Am. J. Emerg. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Pandharipande, P.P.; Shintani, A.K.; Hagerman, H.E.; St Jacques, P.J.; Rice, T.W.; Sanders, N.W.; Ware, L.B.; Bernard, G.R.; Ely, E.W. Derivation and validation of Spo2/Fio2 ratio to impute for Pao2/Fio2 ratio in the respiratory component of the Sequential Organ Failure Assessment score. Crit. Care Med. 2009, 37, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Willson, K.; Francis, D.P.; Wensel, R.; Coats, A.J.; Parker, K.H. Relationship between detrended fluctuation analysis and spectral analysis of heart-rate variability. Physiol. Meas. 2002, 23, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Huikuri, H.V.; Makikallio, T.H.; Peng, C.K.; Goldberger, A.L.; Hintze, U.; Moller, M. Fractal correlation properties of R-R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation 2000, 101, 47–53. [Google Scholar] [CrossRef]
- Ho, K.K.; Moody, G.B.; Peng, C.K.; Mietus, J.E.; Larson, M.G.; Levy, D.; Goldberger, A.L. Predicting survival in heart failure case and control subjects by use of fully automated methods for deriving nonlinear and conventional indices of heart rate dynamics. Circulation 1997, 96, 842–848. [Google Scholar] [CrossRef]
- Liu, N.; Guo, D.; Koh, Z.X.; Ho, A.F.W.; Ong, M.E.H. Heart Rate n-Variability (HRnV): A Novel Representation of Beat-to-Beat Variation in Electrocardiogram. bioRxiv 2019, 449504. [Google Scholar] [CrossRef]
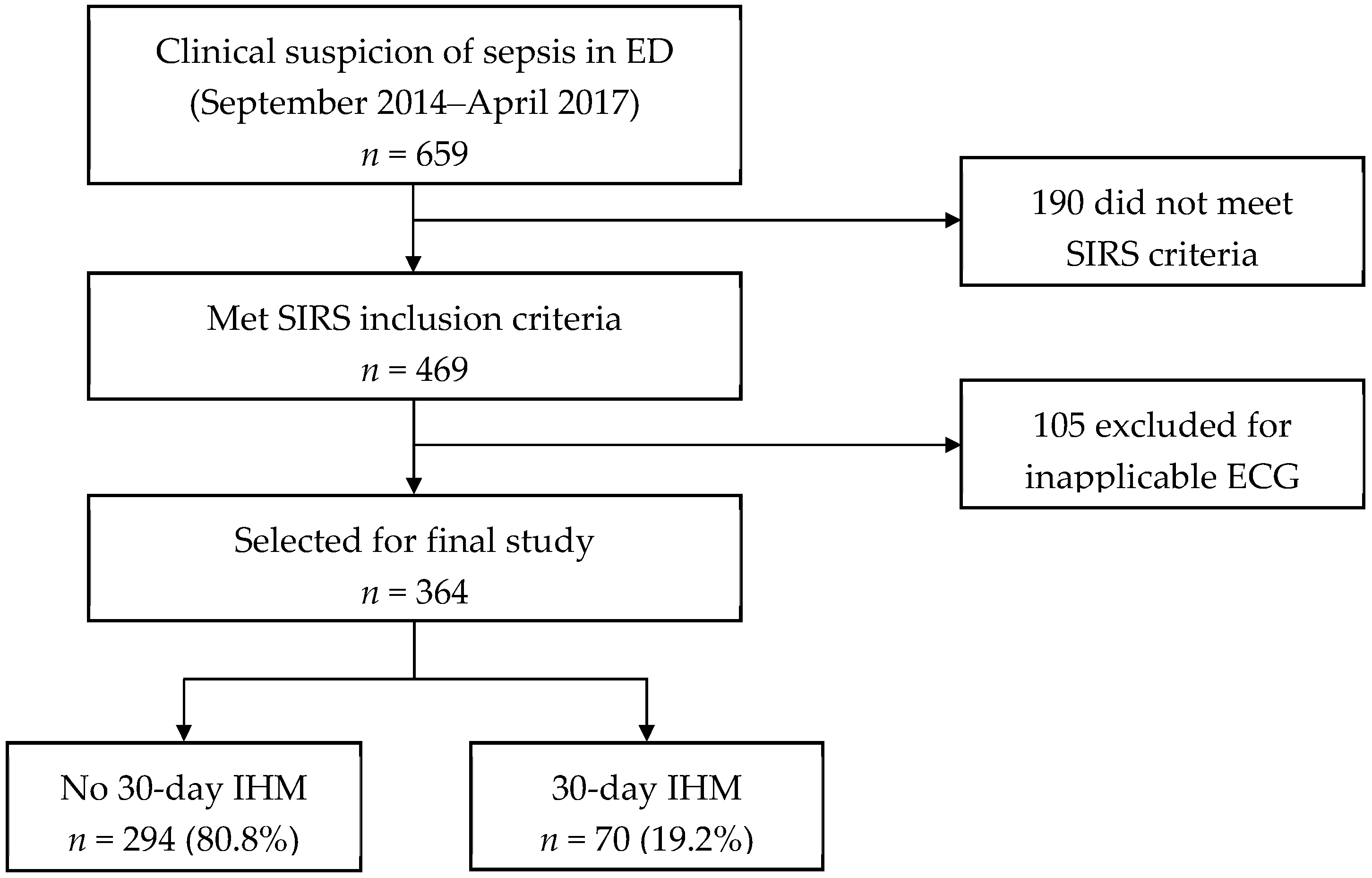
| Variables | No 30-Day IHM (n = 294) | 30-Day IHM (n = 70) | p-Value |
|---|---|---|---|
| Age in years, mean (SD) | 65.7 (16.4) | 72.8 (15.0) | 0.001 |
| Gender, n (%) | 0.595 | ||
| Male | 147 (50.0) | 32 (45.7) | |
| Female | 147 (50.0) | 38 (54.3) | |
| Race, n (%) | 0.659 | ||
| Chinese | 213 (72.4) | 53 (75.7) | |
| Malay | 44 (15.0) | 7 (10.0) | |
| Indian | 22 (7.5) | 7 (10.0) | |
| Other | 15 (5.1) | 3 (4.3) | |
| Medical history, n (%) | |||
| Ischemic heart disease | 76 (25.9) | 23 (32.9) | 0.236 |
| Diabetes | 118 (40.1) | 27 (38.6) | 0.892 |
| Hypertension | 162 (55.1) | 35 (50.0) | 0.505 |
| Cancer | 88 (29.9) | 25 (35.7) | 0.389 |
| Serious infection | 121 (41.2) | 30 (42.9) | 0.789 |
| Drug history, n (%) | |||
| Beta-blocker | 96 (32.7) | 16 (22.9) | 0.116 |
| Digoxin | 11 (3.7) | 2 (2.9) | 1.000 |
| Calcium channel blocker | 73 (24.8) | 17 (24.3) | 1.000 |
| Amiodarone | 4 (1.4) | 0 (0.0) | 1.000 |
| Vital signs at presentation, mean (SD) | |||
| Heart rate, bpm | 117.5 (21.8) | 116.7 (23.4) | 0.622 |
| Systolic blood pressure, mm Hg | 115.5 (33.1) | 103.3 (31.1) | 0.004 |
| Diastolic blood pressure, mm Hg | 63.4 (17.7) | 60.2 (16.0) | 0.200 |
| Respiratory rate, bpm | 20.2 (3.9) | 23.4 (5.7) | <0.001 |
| Temperature, °C | 38.1 (1.2) | 37.2 (1.4) | <0.001 |
| Glasgow coma scale (3–15) | 13.4 (3.0) | 12.2 (3.9) | 0.002 |
| Vital signs worst recorded, mean (SD) | |||
| Systolic blood pressure, mm Hg | 96.0 (25.3) | 80.5 (21.3) | <0.001 |
| Respiratory rate, bpm | 22.8 (5.3) | 27.9 (6.8) | <0.001 |
| Glasgow coma scale (3–15) | 13.2 (3.3) | 12.1 (3.9) | 0.002 |
| WBC count, mean (SD) | 14.3 (7.2) | 14.0 (10.2) | 0.291 |
| Source of infection, n (%) | 0.041 | ||
| Respiratory | 83 (28.2) | 32 (45.7) | |
| Urinary tract | 71 (24.1) | 6 (8.6) | |
| Gastrointestinal | 16 (5.4) | 5 (7.1) | |
| Musculoskeletal | 11 (3.7) | 2 (2.9) | |
| Hepatobiliary | 21 (7.1) | 3 (4.3) | |
| Peritoneum | 3 (1.0) | 1 (1.4) | |
| Skin | 10 (3.4) | 0 (0.0) | |
| Line | 9 (3.1) | 0 (0.0) | |
| Cardiac | 6 (2.0) | 2 (2.9) | |
| Central nervous system | 1 (0.3) | 0 (0.0) | |
| Unknown | 45 (15.3) | 16 (22.9) | |
| No infection | 18 (6.1) | 3 (4.3) | |
| Disposition from ED, n (%) | <0.001 | ||
| General ward | 251 (85.4) | 52 (74.3) | |
| High dependency | 26 (8.8) | 3 (4.3) | |
| Intensive care unit | 17 (5.8) | 15 (21.4) |
| Variable, Mean (SD) | No 30-Day IHM (n = 294) | 30-Day IHM (n = 70) | p-Value |
|---|---|---|---|
| Time domain | |||
| Mean NN, s | 559.20 (117.90) | 561.47 (130.81) | 0.866 |
| Standard deviation of NN, s | 21.96 (24.85) | 31.98 (34.38) | 0.050 |
| Mean heart rate, bpm | 111.95 (21.44) | 112.79 (23.93) | 0.742 |
| Standard deviation of heart rate, bpm | 4.84 (5.87) | 6.78 (7.37) | 0.011 |
| RMSSD, s | 26.56 (39.08) | 42.66 (50.00) | <0.001 |
| NN50, n | 48.60 (114.22) | 72.80 (121.94) | <0.001 |
| pNN50, % | 7.47 (17.43) | 11.98 (20.12) | <0.001 |
| NN triangular index | 3.87 (3.45) | 4.55 (5.39) | 0.918 |
| TINN | 137.60 (147.02) | 194.86 (180.16) | 0.004 |
| Frequency domain | |||
| Total power, ms2 | 514.81 (1737.84) | 1276.95 (3254.90) | 0.176 |
| VLF power, ms2 | 117.57 (307.02) | 254.08 (833.86) | 0.939 |
| LF power, ms2 | 120.47 (476.99) | 305.57 (826.58) | 0.485 |
| HF power, ms2 | 274.43 (1005.67) | 713.44 (1763.77) | 0.011 |
| LF power, nu | 47.32 (28.82) | 35.46 (25.76) | 0.002 |
| HF power, nu | 51.93 (28.33) | 63.73 (25.44) | 0.002 |
| LF/HF | 2.59 (4.47) | 1.65 (4.88) | 0.002 |
| Non-linear domain | |||
| Poincaré plot standard deviation 1, ms | 18.80 (27.66) | 30.19 (35.39) | <0.001 |
| Poincaré plot standard deviation 2, ms | 23.05 (23.39) | 32.42 (34.39) | 0.147 |
| Approximate entropy | 0.98 (0.34) | 1.02 (0.35) | 0.304 |
| Sample entropy | 1.07 (0.55) | 1.13 (0.59) | 0.454 |
| DFA, alpha 1 | 0.67 (0.38) | 0.54 (0.27) | 0.016 |
| DFA, alpha 2 | 0.95 (0.42) | 0.71 (0.41) | <0.001 |
| Variables | Combination Model | Rapid Triage Model |
|---|---|---|
| Adjusted Odds Ratio (95% CI) | ||
| Clinical parameters | ||
| Systolic blood pressure (presenting) | 0.99 (0.98–1.00) | |
| Respiratory rate (presenting) | 1.13 (1.06–1.20) | |
| Temperature (presenting) | 0.61 (0.45–0.81) | 0.60 (0.47–0.77) |
| Glasgow coma scale (presenting) | 0.91 (0.84–0.99) | |
| Respiratory rate (worst) | 1.09 (1.02–1.16) | |
| HRV parameters | ||
| Poincaré plot standard deviation 2 | 1.03 (1.01–1.04) | |
| DFA, alpha 2 | 0.28 (0.14–0.57) | |
| MEDS score variables | ||
| Terminal illness | 5.93 (2.85–12.35) | |
| LRTI suspicion | 3.09 (1.45–6.59) | |
| Respiratory distress | 7.17 (1.88–27.40) | |
| Septic shock | 3.11 (1.51–6.37) | |
| SOFA variables | ||
| Coagulation (platelet count) | ||
| ≥150,000 | Reference | |
| 100,00–149,999 | 0.54 (0.11–2.58) | |
| 50,000–99,999 | 1.76 (0.48–6.52) | |
| 20,000–49,999 | 9.43 (1.89–47.07) | |
| <20,000 | 1.19 (0.10–15.00) | |
| Clinical Scores | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|
| Quick scoring systems | ||||
| Rapid triage model ≥ 0.201 | 68.6 (56.2–78.9) | 78.6 (73.3–83.0) | 43.2 (34.0–53.0) | 91.3 (87.0–94.3) |
| NEWS ≥ 8 | 61.4 (49.0–72.8) | 70.8 (65.2–75.9) | 33.3 (27.9–39.3) | 88.5 (85.0–91.3) |
| MEWS ≥ 6 | 54.3 (41.9–66.3) | 66.3 (60.6–71.7) | 27.7 (22.7–33.4) | 85.9 (82.3–88.9) |
| qSOFA ≥ 2 | 48.6 (36.4–60.8) | 81.6 (76.7–85.9) | 38.6 (30.9–47.0) | 87.0 (84.1–89.4) |
| Comprehensive scoring systems | ||||
| Combination model ≥ 0.156 | 91.4 (81.6–96.5) | 77.9 (72.6–82.4) | 49.6 (40.7–58.5) | 97.4 (94.3–99.0) |
| APACHE II ≥ 23 | 65.7 (53.3–76.4) | 72.8 (67.3–77.7) | 36.5 (28.3–45.6) | 89.9 (85.2–93.3) |
| SOFA ≥ 6 | 64.3 (51.9–75.4) | 71.8 (66.3–76.8) | 35.2 (29.6–41.1) | 89.4 (85.9–92.1) |
| MEDS ≥ 12 | 74.3 (62.4–84.0) | 79.6 (74.5–84.1) | 46.4 (40.0–53.0) | 92.9 (89.7–95.1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pong, J.Z.; Fook-Chong, S.; Koh, Z.X.; Samsudin, M.I.; Tagami, T.; Chiew, C.J.; Wong, T.H.; Ho, A.F.W.; Ong, M.E.H.; Liu, N. Combining Heart Rate Variability with Disease Severity Score Variables for Mortality Risk Stratification in Septic Patients Presenting at the Emergency Department. Int. J. Environ. Res. Public Health 2019, 16, 1725. https://doi.org/10.3390/ijerph16101725
Pong JZ, Fook-Chong S, Koh ZX, Samsudin MI, Tagami T, Chiew CJ, Wong TH, Ho AFW, Ong MEH, Liu N. Combining Heart Rate Variability with Disease Severity Score Variables for Mortality Risk Stratification in Septic Patients Presenting at the Emergency Department. International Journal of Environmental Research and Public Health. 2019; 16(10):1725. https://doi.org/10.3390/ijerph16101725
Chicago/Turabian StylePong, Jeremy Zhenwen, Stephanie Fook-Chong, Zhi Xiong Koh, Mas’uud Ibnu Samsudin, Takashi Tagami, Calvin J. Chiew, Ting Hway Wong, Andrew Fu Wah Ho, Marcus Eng Hock Ong, and Nan Liu. 2019. "Combining Heart Rate Variability with Disease Severity Score Variables for Mortality Risk Stratification in Septic Patients Presenting at the Emergency Department" International Journal of Environmental Research and Public Health 16, no. 10: 1725. https://doi.org/10.3390/ijerph16101725
APA StylePong, J. Z., Fook-Chong, S., Koh, Z. X., Samsudin, M. I., Tagami, T., Chiew, C. J., Wong, T. H., Ho, A. F. W., Ong, M. E. H., & Liu, N. (2019). Combining Heart Rate Variability with Disease Severity Score Variables for Mortality Risk Stratification in Septic Patients Presenting at the Emergency Department. International Journal of Environmental Research and Public Health, 16(10), 1725. https://doi.org/10.3390/ijerph16101725







