A Case of Listeria monocytogenes ST-219 Meningo-Encephalitis
Abstract
:1. Introduction
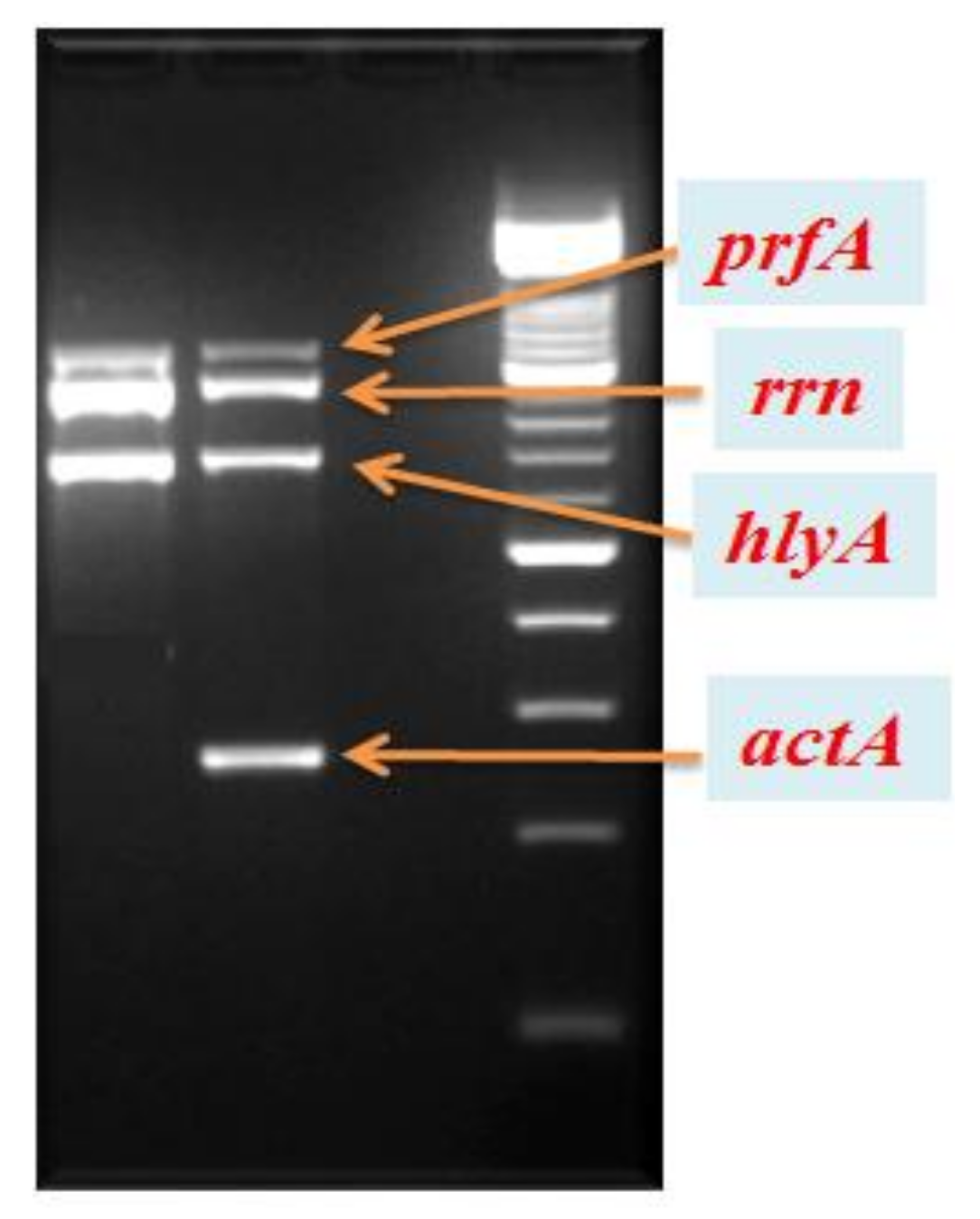
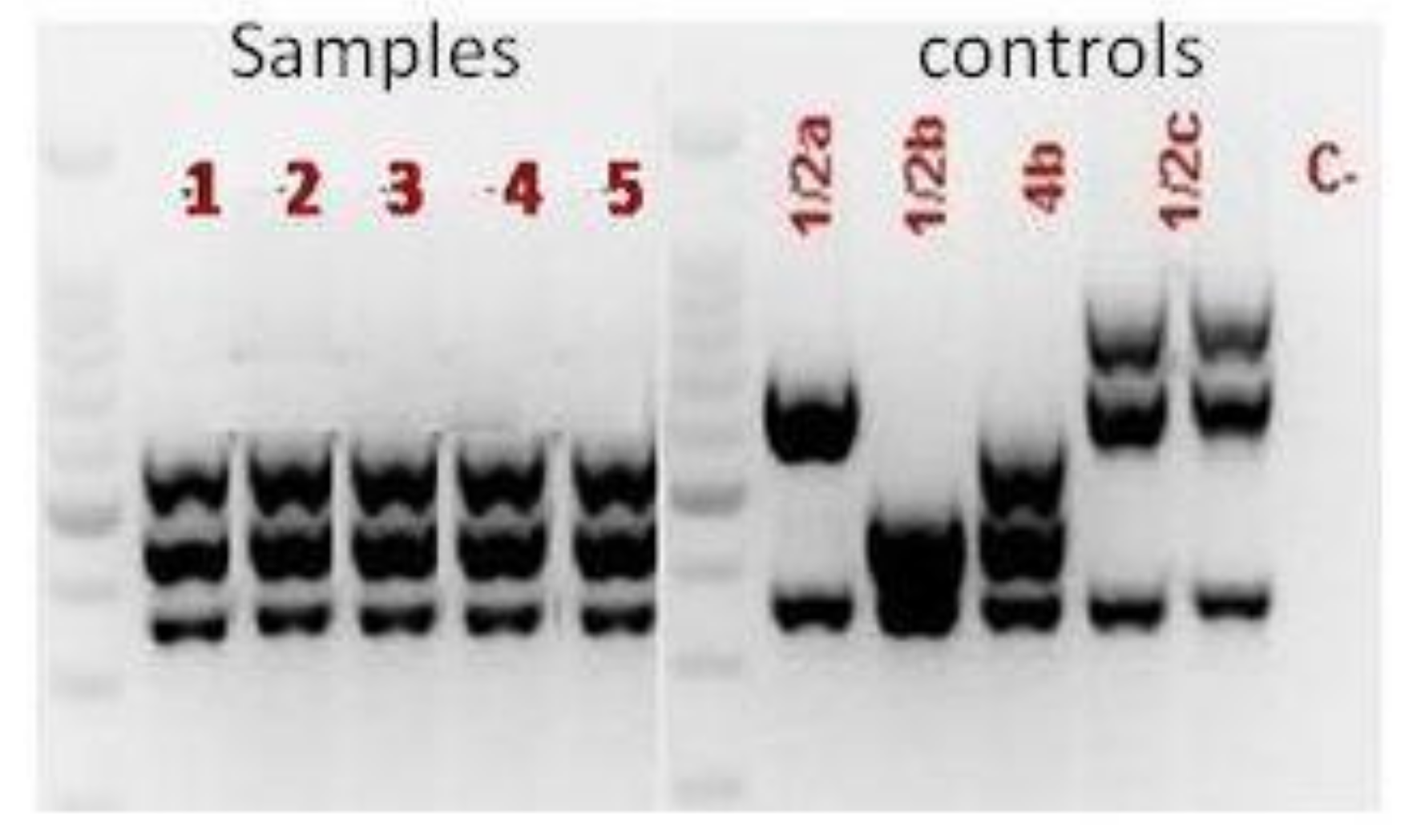
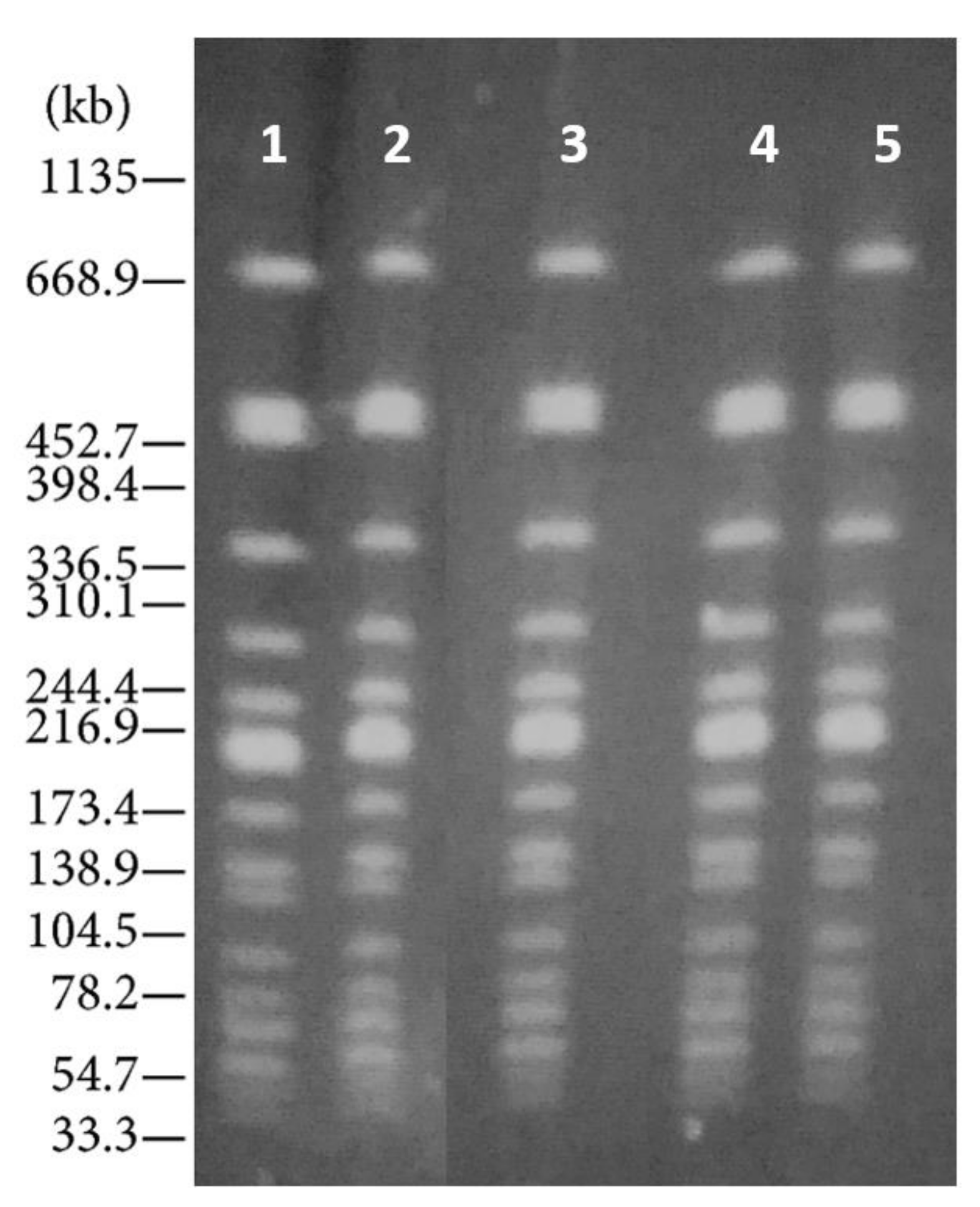
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
Ethics Approval
References
- Scallan, E.; Hoekstra, R.M.; Mahon, B.E.; Jones, T.F.; Griffin, P.M. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol. Infect. 2015, 143, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Vital Signs: Listeria Illnesses, Deaths, and Outbreaks—United States, 2009–2011. Morb. Mortal. Wkly. Rep. 2013, 62, 448–452. [Google Scholar]
- Allerberger, F.; Wagner, M. Listeriosis: A resurgent foodborne infection. Clin. Microbiol. Infect. 2010, 16, 16–23. [Google Scholar] [CrossRef]
- Van Walle, I.; Björkman, J.T.; Cormican, M.; Dallman, T.; Mossong, J.; Moura, A.; Pietzka, A.; Ruppitsch, W.; Takkinen, J. European Listeria WGS Typing Group. Retrospective validation of whole genome sequencing-enhanced surveillance of listeriosis in Europe, 2010 to 2015. Euro Surveill. 2018, 23, 1700798. [Google Scholar] [CrossRef] [PubMed]
- Camejo, A.; Carvalho, F.; Reis, O.; Leitão, E.; Sousa, S.; Cabanes, D. The arsenal of virulence factors deployed by Listeria monocytogenes to promote its cell infection cycle. Virulence 2011, 2, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, D.A.; Chakraborty, T.; Goebel, W.; Cossart, P. Molecular Determinants of Listeria monocytogenes Pathogenesis. Infect. Immun. 1992, 60, 1263–1267. [Google Scholar] [PubMed]
- Doumith, M.; Buchrieser, C.; Glaser, P.; Jacquet, C.; Martin, P. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J. Clin. Microbiol. 2004, 42, 3819–3822. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2015; ECDC: Stockholm, Sweden, 2018; Available online: https://ecdc.europa.eu/sites/portal/files/documents/AER_for_2016-listeriosis.pdf (accessed on 19 November 2018).
- Charpentier, E.; Courvalin, P. Antibiotic Resistance in Listeria spp. Antimicrob. Agents Chemother. 1999, 43, 2103–2108. [Google Scholar] [CrossRef]
- Hadorn, K.; Hachler, H.; Schaffner, A.; Kayser, F.H. Genetic characterization of plasmid-encoded multiple antibiotic resistance in a strain of Listeria monocytogenes causing endocarditis. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12, 928–937. [Google Scholar] [CrossRef]
- Quentin, C.; Thibaut, M.C.; Horovitz, J.; Bebear, C. Multiresistant strain of Listeria monocytogenes in septic abortion. Lancet 1990, 336, 375. [Google Scholar] [CrossRef]
- Strategie di Sorveglianza attiva e networking per il controllo delle infezioni da L. monocytogenes. Available online: www.listeriaweb.uniss.it/index.php/progetto (accessed on 19 November 2018).
- Van de Beek, D.; Cabellos, C.; Dzupova, O.; Esposito, S.; Klein, M.; Kloek, A.T.; Leib, S.L.; Mourvillier, B.; Ostergaard, C.; Pagliano, P.; et al. ESCMID guideline: Diagnosis and treatment of acute bacterial meningitis. Clin. Microbiol. Infect. 2016, 22, S37–S62. [Google Scholar] [CrossRef]
- Piana, A.; Are, R.; Orrù, M.; Saba, F.; Dettori, M.; Maida, I.; Sotgiu, G.; Rais, C.; Mura, M.S. Listeria monocytogenes meningoencephalitis: Molecular methods for diagnosis and for monitoring the response to chemotherapy. Ital. J. Public Health 2005, 2, 29–34. [Google Scholar]
- ISO 11290-1:1996/Amd 1:2004. Microbiology of Food and Animal Feeding Stuffs Horizontal Method for the Detection and Enumeration of Listeria monocytogenes. Part 1; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Mureddu, A.; Mazza, R.; Fois, F.; Meloni, D.; Bacciu, R.; Piras, F.; Mazzette, R. Listeria monocytogenes persistence in ready-to-eat sausages and in processing plants. Ital. J. Food Saf. 2014, 3, 1697. [Google Scholar] [CrossRef] [PubMed]
- Centers of Disease Control and Prevention. Standard Operating Procedure for PulseNet PFGE of Listeria monocytogenes; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013. Available online: http://www.cdc.gov/pulsenet/PDF/listeria-pfge-protocol-508c.pdf (accessed on 19 November 2018).
- Tenover, F.C.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 1995, 33, 2233–2339. [Google Scholar]
- Primers Used for Amplification and Sequencing (MLST). Available online: http://bigsdb.pasteur.fr/listeria/listeria.html (accessed on 19 November 2018).
- Surveillance Atlas of Infectious Diseases (ECDC). Available online: www.ecdc.europa.eu/en/surveillance-atlas-infectious-diseases- (accessed on 19 November 2018).
- Maury, M.M.; Chenal-Francisque, V.; Bracq-Dieye, H.; Han, L.; Leclercq, A.; Vales, G.; Moura, A.; Gouin, E.; Scortti, M.; Disson, O.; et al. Spontaneous loss of virulence in natural populations of Listeria monocytogenes. Infect Immun. 2017, 85, e00541-17. [Google Scholar] [CrossRef] [PubMed]
- Maury, M.M.; Tsai, Y.; Charlier, C.; Touchon, M.; Chenal-Francisque, V.; Leclercq, A.; Criscuolo, A.; Gaultier, C.; Roussel, S.; Brisabois, A.; et al. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Genet. 2016, 48, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Drevets, D.A. Listeria monocytogenes. Virulence factors that stimulate endothelial cells. Infect. Immun. 1998, 66, 232–238. [Google Scholar]
- McLauchlin, J. Distribution of serovars of Listeria monocytogenes isolated from different categories of patients with listeriosis. Eur. J. Clin. Microbiol. Infect. Dis. 1990, 9, 210–213. [Google Scholar] [CrossRef]
- Pontello, M.; Guaita, A.; Sala, G.; Cipolla, M.; Gattuso, A.; Sonnessa, M.; Gianfranceschi, M.V. Listeria monocytogenes serotypes in human infections (Italy, 2000–2010). Ann. Ist. Super. Sanita 2012, 48, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Gambarin, P.; Magnabosco, C.; Losio, M.N.; Pavoni, E.; Gattuso, A.; Arcangeli, G.; Favretti, M. Listeria monocytogenes in Ready-to-Eat Seafood and Potential Hazards for the Consumers. Int. J. Microbiol. 2012, 2012, 497635. [Google Scholar] [CrossRef]
- Maijala, R.; Lyytikäinen, O.; Autio, T.; Aalto, T.; Haavisto, L.; Honkanen-Buzalski, T. Exposure of Listeria monocytogenes within an epidemic caused by butter in Finland. Int. J. Food Microbiol. 2001, 70, 97–109. [Google Scholar] [CrossRef]
- Colagiorgi, A.; Bruini, I.; Di Ciccio, P.A.; Zanardi, E.; Ghidini, S.; Ianieri, A. Listeria monocytogenes Biofilms in the Wonderland of Food Industry. Pathogens 2017, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Di Ciccio, P.; Conter, M.; Zanardi, E.; Ghidini, S.; Vergara, A.; Paludi, D.; Festino, A.R.; Ianieri, A. Listeria monocytogenes: Biofilms in food processing. Ital. J. Food Sci. 2012, 24, 203–213. [Google Scholar]
| Antibiotic | Susceptibility | Disc Content (μg) |
|---|---|---|
| Ampicillin | Susceptible | 10 |
| Chloramphenicol | Susceptible | 30 |
| Ciprofloxacin | Susceptible | 5 |
| Clindamycin | Resistant | 2 |
| Erythromycin | Resistant | 15 |
| Gentamicin | Susceptible | 10 |
| Imipenem | Susceptible | 10 |
| Oxacillin | Resistant | 1 |
| Penicillin | Susceptible | 10 U |
| Tetracycline | Susceptible | 30 |
| Trimethoprim/Sulfamethoxazole | Resistant | 1.25/23.75 |
| Vancomycin | Susceptible | 30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotgiu, G.; Muresu, N.; Dettori, M.; Mura, E.; Cossu, A.; Dolores Masia, M.; Murgia, P.; Cocuzza, C.; De Santis, E.P.L.; Scarano, C.; et al. A Case of Listeria monocytogenes ST-219 Meningo-Encephalitis. Int. J. Environ. Res. Public Health 2019, 16, 8. https://doi.org/10.3390/ijerph16010008
Sotgiu G, Muresu N, Dettori M, Mura E, Cossu A, Dolores Masia M, Murgia P, Cocuzza C, De Santis EPL, Scarano C, et al. A Case of Listeria monocytogenes ST-219 Meningo-Encephalitis. International Journal of Environmental Research and Public Health. 2019; 16(1):8. https://doi.org/10.3390/ijerph16010008
Chicago/Turabian StyleSotgiu, Giovanni, Narcisa Muresu, Marco Dettori, Erica Mura, Andrea Cossu, Maria Dolores Masia, Paola Murgia, Clementina Cocuzza, Enrico Pietro Luigi De Santis, Christian Scarano, and et al. 2019. "A Case of Listeria monocytogenes ST-219 Meningo-Encephalitis" International Journal of Environmental Research and Public Health 16, no. 1: 8. https://doi.org/10.3390/ijerph16010008
APA StyleSotgiu, G., Muresu, N., Dettori, M., Mura, E., Cossu, A., Dolores Masia, M., Murgia, P., Cocuzza, C., De Santis, E. P. L., Scarano, C., Spanu, C., & Piana, A. (2019). A Case of Listeria monocytogenes ST-219 Meningo-Encephalitis. International Journal of Environmental Research and Public Health, 16(1), 8. https://doi.org/10.3390/ijerph16010008









