Co-Amendment of S and Si Alleviates Cu Toxicity in Rice (Oryza Sativa L.) Grown on Cu-Contaminated Paddy Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Preparation and Analyses
2.3. Statistical Analyses
3. Results
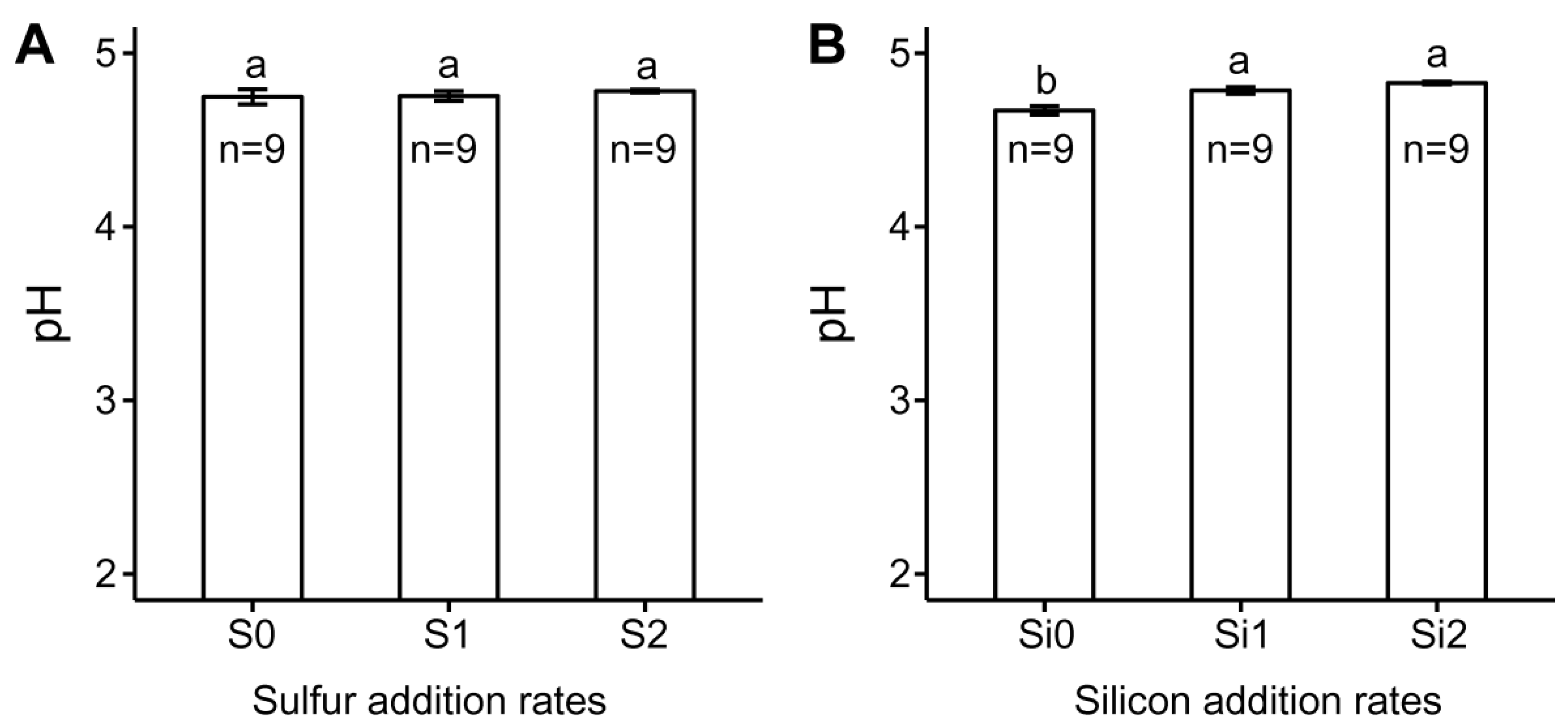
3.1. Variations of Soil Cu Speciation in Response to S and Si Addition
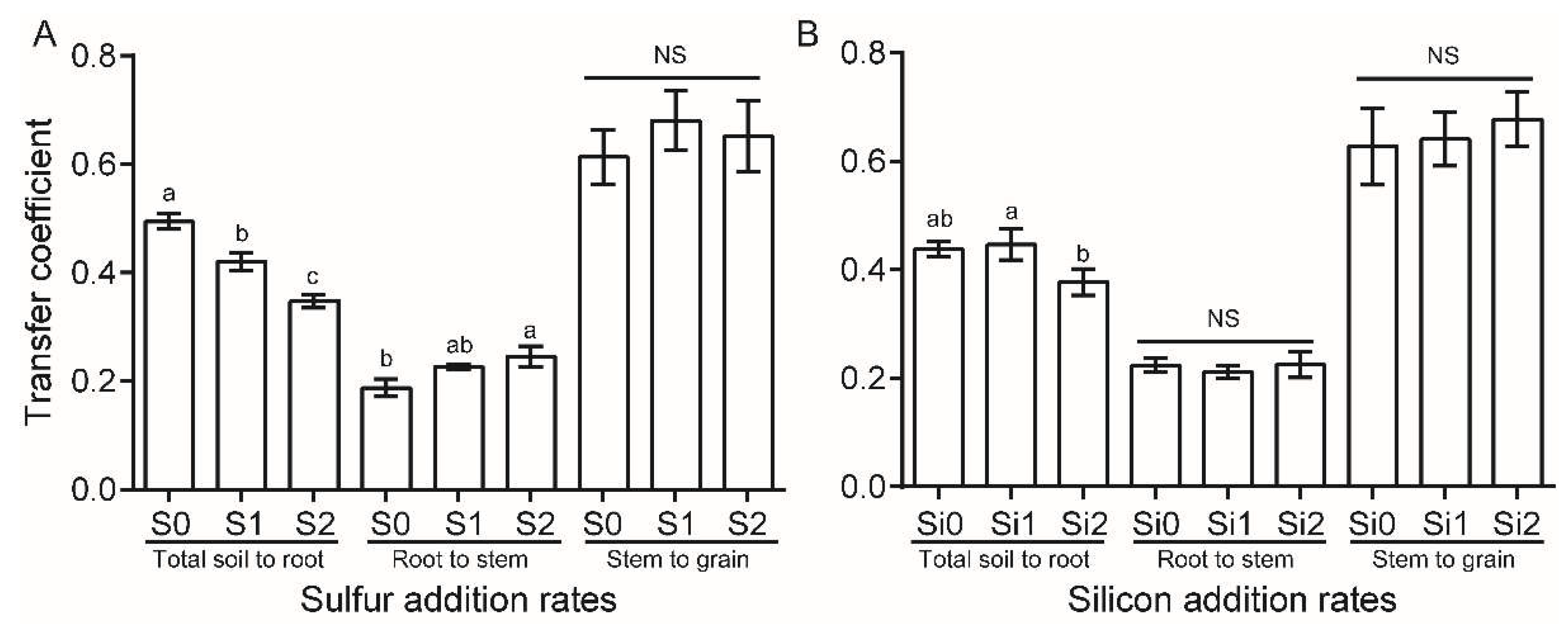
3.2. The Uptake and Transfer of Cu in Rice Plant as Affected by S and Si Additions
3.3. Relationships between Cu in Soil and Its Uptake by Rice Plant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, Z.; Hu, Z. Copper contamination in paddy soils irrigated with wastewater. Chemosphere 2000, 41, 3–6. [Google Scholar] [CrossRef]
- Xu, J.; Yang, L.; Wang, Z.; Dong, G.; Huang, J.; Wang, Y. Toxicity of copper on rice growth and accumulation of copper in rice grain in copper contaminated soil. Chemosphere 2006, 62, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martín, M.; García-Delgado, M.; Lorenzo, L.; Rodríguez-Cruz, M.; Arienzo, M. Heavy metals in sewage sludge amended soils determined by sequential extractions as a function of incubation time of soils. Geoderma 2007, 142, 262–273. [Google Scholar] [CrossRef]
- Das, M.; Maiti, S. Comparison between availability of heavy metals in dry and wetland tailing of an abandoned copper tailing pond. Environ. Monit. Assess. 2008, 137, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef]
- Hodson, M.E.; Donner, E. Managing adverse soil chemical environments. In Soil Conditions and Plant Growth; Gregoryand, P.J., Nortcliff, S., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 195–237. [Google Scholar]
- Dobermann, A.; Cassman, K.; Mamaril, C.; Sheehy, J. Management of phosphorus, potassium, and sulfur in intensive, irrigated lowland rice. Field Crop. Res. 1998, 56, 113–138. [Google Scholar] [CrossRef]
- Zhou, W.; Wan, M.; He, P.; Li, S.; Lin, B. Oxidation of elemental sulfur in paddy soils as influenced by flooded condition and plant growth in pot experiment. Biol. Fert. Soils 2002, 36, 384–389. [Google Scholar] [CrossRef]
- Wang, G.; Koopmans, G.F.; Song, J.; Temminghoff, E.J.; Luo, Y.; Zhao, Q.; Japenga, J. Mobilization of heavy metals from contaminated paddy soil by EDDS, EDTA, and elemental sulfur. Environ. Geochem. Health 2007, 29, 221–235. [Google Scholar] [CrossRef]
- Shi, J.Y.; Lin, H.R.; Yuan, X.F.; Chen, X.C.; Shen, C.F.; Chen, Y.X. Enhancement of copper availability and microbial community changes in rice rhizospheres affected by sulfur. Molecules 2011, 16, 1409–1417. [Google Scholar] [CrossRef]
- Sun, L.; Zheng, C.; Yang, J.; Peng, C.; Xu, C.; Wang, Y.; Feng, J.; Shi, J. Impact of sulfur (S) fertilization in paddy soils on copper (Cu) accumulation in rice (Oryza sativa L.) plants under flooding conditions. Biol. Fert. Soils 2016, 52, 31–39. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Zhu, Y.G.; Li, M.; Zhang, L.G.; Cao, Z.H.; Smith, F.A. Sulfur (S)-induced enhancement of iron plaque formation in the rhizosphere reduces arsenic accumulation in rice (Oryza sativa L.) seedlings. Environ. Pollut. 2007, 147, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. USA 1994, 91, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Sun, W.; Zhu, Y.-G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.; Liang, Y.; Liu, Z.; Xiao, J.; Duan, A. Impacts of steel-slag-based silicate fertilizer on soil acidity and silicon availability and metals-immobilization in a paddy soil. PLoS ONE 2016, 11, e0168163. [Google Scholar] [CrossRef]
- Limmer, M.A.; Mann, J.; Amaral, D.C.; Vargas, R.; Seyfferth, A.L. Silicon-rich amendments in rice paddies: Effects on arsenic uptake and biogeochemistry. Sci. Total Environ. 2018, 624, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Collin, B.; Doelsch, E.; Keller, C.; Cazevieille, P.; Tella, M.; Chaurand, P.; Panfili, F.; Hazemann, J.-L.; Meunier, J.-D. Evidence of sulfur-bound reduced copper in bamboo exposed to high silicon and copper concentrations. Environ. Pollut. 2014, 187, 22–30. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006; First Update 2007; FAO: Rome, Italy, 2007. [Google Scholar]
- Ministry of Ecology and Environment of PR China. Soil Environmental Quality, Risk Control Standard for Soil Contamination of Agricultural Land; Chinese National Standard Agency: Beijing, China, 2018.
- Elliott, C.L.; Snyder, G.H. Autoclave-induced digestion for the colorimetric determination of silicon in rice straw. J. Agric. Food Chem. 1991, 39, 1118–1119. [Google Scholar] [CrossRef]
- Davidson, C.M.; Thomas, R.P.; McVey, S.E.; Perala, R.; Littlejohn, D.; Ure, A.M. Evaluation of a sequential extraction procedure for the speciation of heavy metals in sediments. Anal. Chim. Acta 1994, 291, 277–286. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research, R Package Version 1.1; Available online: http://CRAN.R-project.org/package=agricolae (accessed on 25 December 2018).
- Green, I.D.; Tibbett, M. Differential Uptake, Partitioning and Transfer of Cd and Zn in the Soil-Pea Plant-Aphid System. Environ. Sci. Technol. 2007, 42, 450–455. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Z.; Yuan, X.; Browne, P.; Chen, L.; Ji, J. The influences of soil properties on Cu and Zn availability in soil and their transfer to wheat (Triticum aestivum L.) in the Yangtze River delta region, China. Geoderma 2013, 193, 131–139. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Amelung, W.; Cao, Z.; Fiedler, S.; Frenzel, P.; Jahn, R.; Kalbitz, K.; Kölbl, A.; Schloter, M. Biogeochemistry of paddy soils. Geoderma 2010, 157, 1–14. [Google Scholar] [CrossRef]
- Havlin, J.; Beaton, J.D.; Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers: An Introduction to Nutrient Management; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2005; Volume 515. [Google Scholar]
- Lu, R.K. Principle and Apply Fertilizer of Soil-Plant Nutrition; Chemical Industry Press: Beijing, China, 1998; pp. 209–249.
- Li, P.; Wang, X.X.; Zhang, T.L.; Zhou, D.M.; He, Y.Q. Distribution and accumulation of copper and cadmium in soil–rice system as affected by soil amendments. Water Air Soil Pollut. 2009, 196, 29–40. [Google Scholar] [CrossRef]
- National Health Commission of PR China. Tolerance Limit of Copper in Foods; Chinese National Standard Agency: Beijing, China, 1994.


| Treatment | Sulfur Addition Rate (g kg−1 soil) | Silicon Addition Rate (g kg−1 soil) | |
|---|---|---|---|
| S0 | Si0 | 0 | 0 |
| Si1 | 0 | 0.05 | |
| Si2 | 0 | 0.1 | |
| S1 | Si0 | 0.013 | 0 |
| Si1 | 0.013 | 0.05 | |
| Si2 | 0.013 | 0.1 | |
| S2 | Si0 | 0.026 | 0 |
| Si1 | 0.026 | 0.05 | |
| Si2 | 0.026 | 0.1 | |
| Treatment | F1 | F2 | F3 | F4 | |
|---|---|---|---|---|---|
| mg kg−1 | |||||
| S0 | Si0 | 187.48 (0.99) ab | 87.06 (0.60) d | 136.93 (0.51) b | 108.30 (1.87) bc |
| Si1 | 200.25 (2.84) a | 100.89 (0.83) a | 131.71 (2.01) bc | 75.84 (1.35) de | |
| Si2 | 182.40 (4.94) bc | 93.45 (1.05) c | 133.97 (8.96) bc | 119.39 (11.92) ab | |
| S1 | Si0 | 199.01 (2.30) a | 96.99 (0.21) bc | 156.62 (2.68) a | 69.36 (0.90) e |
| Si1 | 183.90 (1.04) b | 88.11 (1.41) d | 115.78 (5.09) c | 139.55 (3.21) a | |
| Si2 | 182.79 (4.91) b | 100.50 (0.29) ab | 131.72 (0.49) bc | 101.94 (3.18) bc | |
| S2 | Si0 | 168.94 (1.34) c | 89.12 (0.60) d | 133.08 (2.84) bc | 139.56 (0.41) a |
| Si1 | 189.04 (0.92) ab | 102.79 (0.29) a | 142.12 (1.69) ab | 93.00 (0.42) cd | |
| Si2 | 187.66 (0.35) ab | 97.08 (0.55) bc | 140.67 (1.53) ab | 111.32 (0.72) bc | |
| ANOVA | |||||
| S | ** | ** | NS | ** | |
| Si | * | ** | ** | NS | |
| S×Si | ** | ** | ** | ** | |
| Treatment | Yield | Biomass (DW) | Root | Stem | Grain | Brown Rice | |
|---|---|---|---|---|---|---|---|
| g pot−1 | g pot−1 | mg kg−1 | |||||
| S0 | Si0 | 10.31 (1.42) e | 51.93 (4.93) e | 241.83 (5.29) bc | 59.86 (3.16) a | 29.99 (0.78) c | 7.59 (0.04) ab |
| Si1 | 29.40 (1.53) cd | 60.91 (5.44) e | 278.42 (3.16) a | 46.87 (0.97) c | 24.67 (0.59) e | 7.72 (0.56) ab | |
| Si2 | 35.32 (0.63) bc | 91.19 (4.06) abcd | 248.86 (3.06) b | 36.51 (0.46) d | 29.63 (0.17) c | 8.23 (0.63) ab | |
| S1 | Si0 | 22.38 (1.78) cde | 72.72 (4.98) cde | 242.22 (4.23) bc | 58.83 (0.83) a | 27.62 (0.22) cd | 7.31 (0.02) b |
| Si1 | 46.52 (0.67) ab | 96.62 (2.04) abc | 233.21 (1.95) c | 50.52 (0.38) b | 42.20 (0.27) a | 7.39 (0.04) b | |
| Si2 | 52.85 (2.51) a | 117.83 (1.70) a | 184.63 (1.39) e | 40.47 (1.10) cd | 29.76 (0.15) c | 7.05 (0.09) b | |
| S2 | Si0 | 16.74 (0.96) de | 64.76 (4.79) de | 204.96 (3.70) d | 36.65 (0.58) d | 33.33 (0.30) b | 7.90 (0.46) ab |
| Si1 | 49.10 (7.06) ab | 89.25 (3.42) bcd | 184.61 (1.86) e | 46.20 (0.20) c | 25.92 (0.80) de | 8.06 (0.44) ab | |
| Si2 | 49.25 (6.32) ab | 106.26 (11.22) ab | 163.64 (0.59) f | 50.42 (2.31) b | 24.34 (0.68) e | 9.24 (0.32) a | |
| ANOVA | |||||||
| S | ** | ** | ** | ** | ** | ||
| Si | ** | ** | ** | ** | NS | ||
| S×Si | NS | NS | ** | ** | NS | ||
| Variable | F1 | F2 | F3 | F4 | Root | Stem | Leaf | Chaff | Brown Rice |
|---|---|---|---|---|---|---|---|---|---|
| F1 | 1 | ||||||||
| F2 | 0.48 * | 1 | |||||||
| F3 | 0.38 * | 0.4 * | 1 | ||||||
| F4 | −0.81 ** | −0.7 ** | −0.67 ** | 1 | |||||
| Root | 0.38 * | −0.23 | −0.08 | −0.25 | 1 | ||||
| Stem | 0.56 ** | −0.13 | 0.32 | −0.41 * | 0.17 | 1 | |||
| Leaf | 0.22 | −0.09 | 0.01 | −0.1 | −0.25 | 0.68 ** | 1 | ||
| Chaff | −0.17 | −0.41 * | −0.31 | 0.28 | 0.01 | −0.24 | 0.44 * | 1 | |
| Brown rice | 0.05 | 0.06 | 0.11 | 0.11 | −0.31 | −0.11 | −0.02 | −0.32 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Yan, X.; Wei, Z.; Wu, J. Co-Amendment of S and Si Alleviates Cu Toxicity in Rice (Oryza Sativa L.) Grown on Cu-Contaminated Paddy Soil. Int. J. Environ. Res. Public Health 2019, 16, 57. https://doi.org/10.3390/ijerph16010057
Lu Z, Yan X, Wei Z, Wu J. Co-Amendment of S and Si Alleviates Cu Toxicity in Rice (Oryza Sativa L.) Grown on Cu-Contaminated Paddy Soil. International Journal of Environmental Research and Public Health. 2019; 16(1):57. https://doi.org/10.3390/ijerph16010057
Chicago/Turabian StyleLu, Zhihong, Xiao Yan, Zongqiang Wei, and Jianfu Wu. 2019. "Co-Amendment of S and Si Alleviates Cu Toxicity in Rice (Oryza Sativa L.) Grown on Cu-Contaminated Paddy Soil" International Journal of Environmental Research and Public Health 16, no. 1: 57. https://doi.org/10.3390/ijerph16010057
APA StyleLu, Z., Yan, X., Wei, Z., & Wu, J. (2019). Co-Amendment of S and Si Alleviates Cu Toxicity in Rice (Oryza Sativa L.) Grown on Cu-Contaminated Paddy Soil. International Journal of Environmental Research and Public Health, 16(1), 57. https://doi.org/10.3390/ijerph16010057





