Building-Related Environmental Intolerance and Associated Health in the General Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Populations and Samples
2.2. Demographics and Health Issues
2.3. Identification of Building-Related Intolerance
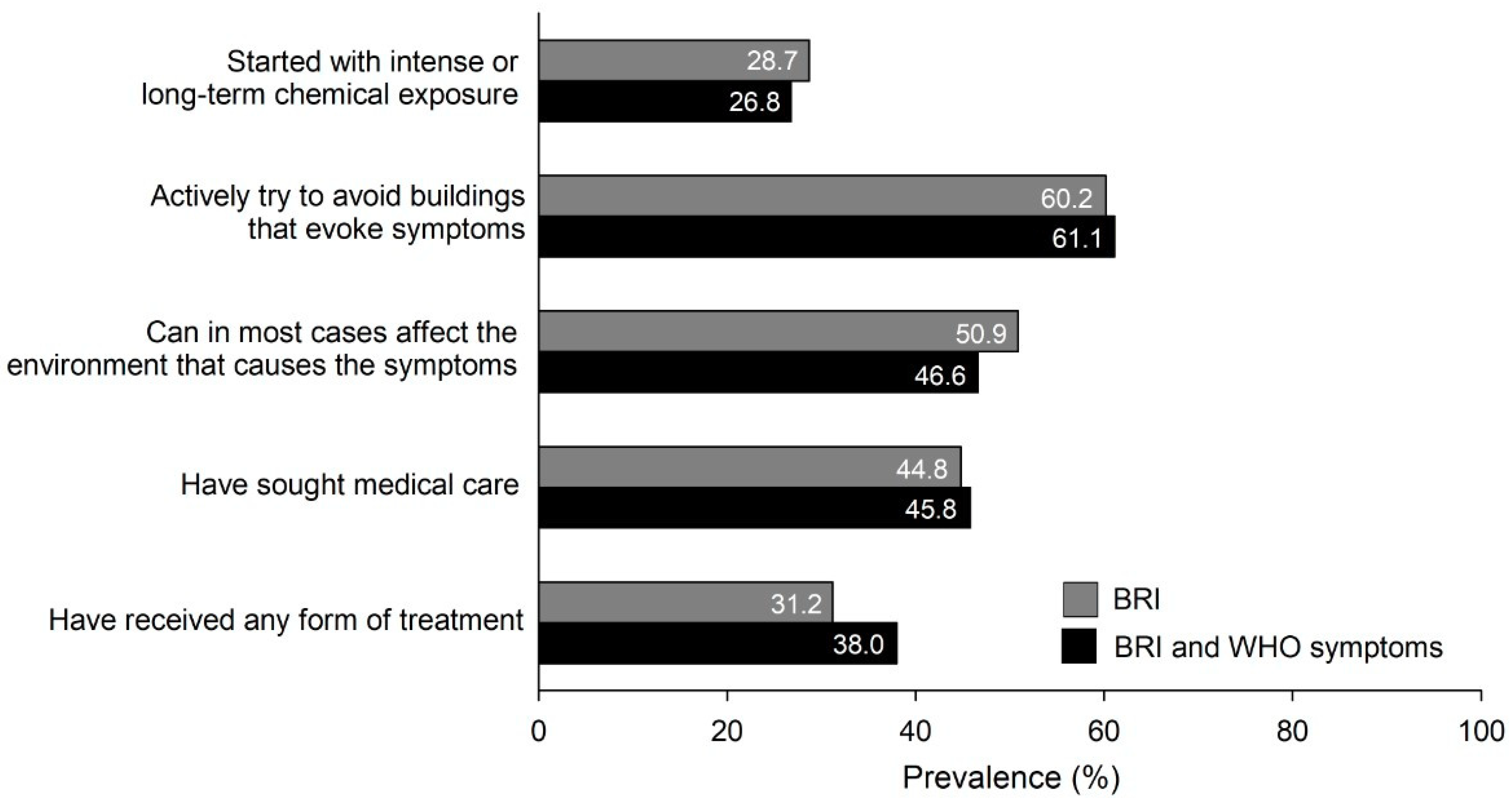
2.4. Duration, Symptom Frequency, and Emotional and Behavioral Impact of BRI
2.5. Coping Strategies
2.6. Statistical Analyses
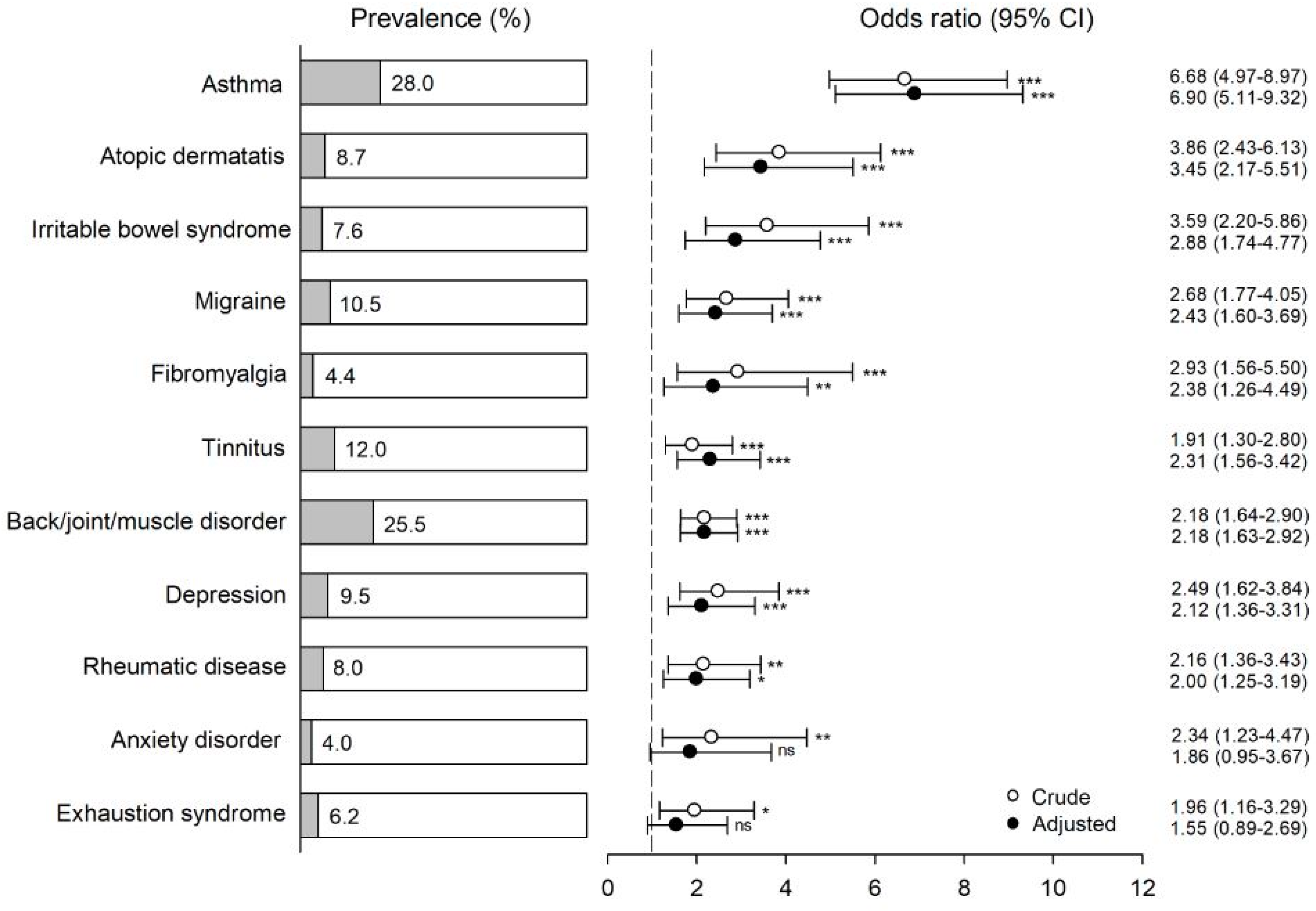
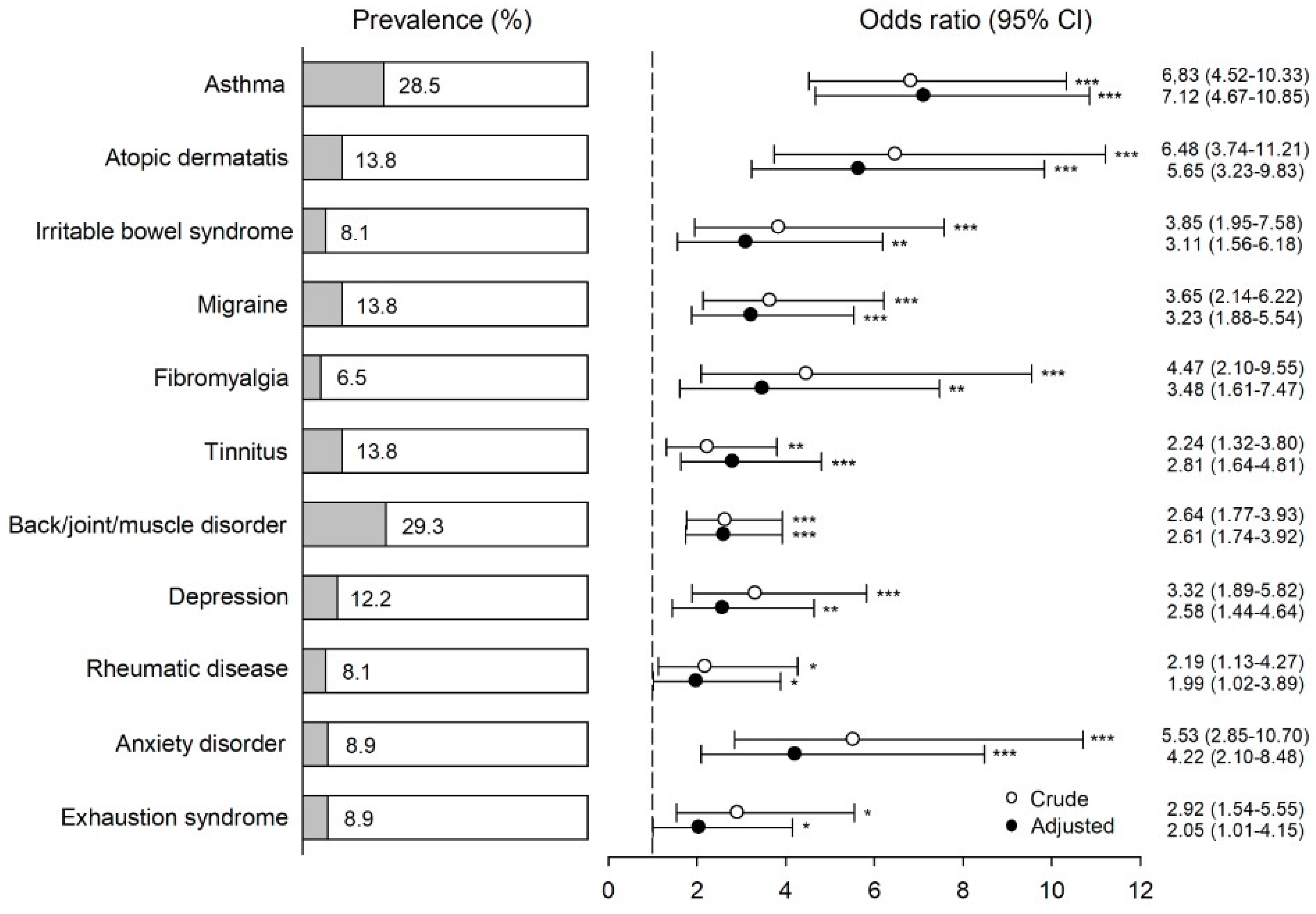
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Indoor Air Pollutants: Exposure and Health Effects. Euro Reports and Studies, 78; WHO Regional Office for Europe: Copenhagen, Denmark, 1983. [Google Scholar]
- Norbäck, D. An update on sick building syndrome. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Edvardsson, B.; Stenberg, B.; Bergdahl, J.; Eriksson, N.; Linden, G.; Widman, L. Medical and social prognoses of non-specific building-related symptoms (Sick Building Syndrome): A follow-up study of patients previously referred to hospital. Int. Arch. Occup. Environ. Health 2008, 81, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Söderholm, A.; Öhman, A.; Stenberg, B.; Nordin, S. Experience of living with nonspecific building-related symptoms. Scand. J. Psychol. 2016, 57, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Tietjen, G.E.; Khubchandani, J.; Ghosh, S.; Bhattacharjee, S.; Kleinfelder, J. Headache symptoms and indoor environmental parameters: Results from the EPA BASE study. Ann. Indian Acad. Neurol. 2012, 15, S95–S99. [Google Scholar] [PubMed]
- Lu, C.Y.; Tsai, M.C.; Muo, C.H.; Kuo, Y.H.; Sung, F.C.; Wu, C.C. Personal, Psychosocial and Environmental Factors Related to Sick Building Syndrome in Official Employees of Taiwan. Int. J. Environ. Res. Public Health 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- IPCS/WHO. Conclusions and recommendations of a workshop on Multiple Chemical Sensitivities (MCS). International Program on Chemical Safety/World Health Organization. Regul. Toxicol. Pharmacol. 1996, 24, 188–189. [Google Scholar] [CrossRef]
- Van den Bergh, O.; Brown, R.; Petersen, S.; Witthöft, M. Idiopathic Environmental Intolerance: A Comprehensive Model. Clin. Psychol. Sci. 2017, 5, 551–567. [Google Scholar] [CrossRef]
- Hetherington, L.; Battershill, J. Review of evidence for a toxicological mechanism of idiopathic environmental intolerance. Hum. Exp. Toxicol. 2013, 32, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Karvala, K.; Sainio, M.; Palmquist, E.; Nyback, M.H.; Nordin, S. Prevalence of various environmental intolerances in a Swedish and Finnish general population. Environ. Res. 2018, 161, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, E.; Claeson, A.S.; Neely, G.; Stenberg, B.; Nordin, S. Overlap in prevalence between various types of environmental intolerance. Int. J. Hyg. Environ. Health 2014, 217, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Claeson, A.S.; Andersson, H.; Wikdahl, F.; Nyback, M.H.; Nordin, S. Comorbidity of airway inflammatory diseases in chemical and building-related intolerance. J. Occup. Environ. Med. 2018, 60, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Lind, N.; Söderholm, A.; Palmquist, E.; Andersson, L.; Millqvist, E.; Nordin, S. Comorbidity and Multimorbidity of Asthma and Allergy and Intolerance to Chemicals and Certain Buildings. J. Occup. Environ. Med. 2017, 59, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E.; Janson, C.; Norbäck, D.; Boman, G. Symptoms related to the sick building syndrome in a general population sample: Associations with atopy, bronchial hyper-responsiveness and anxiety. Int. J. Tuberc. Lung Dis. 1998, 2, 1023–1028. [Google Scholar] [PubMed]
- Eriksson, N.M.; Stenberg, B.G. Baseline prevalence of symptoms related to indoor environment. Scand. J. Public Health 2006, 34, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Gomzi, M.; Bobic, J.; Radosevic-Vidacek, B.; Macan, J.; Varnai, V.M.; Milkovic-Kraus, S.; Kanceljak-Macan, B. Sick building syndrome: Psychological, somatic, and environmental determinants. Arch. Environ. Occup. Health 2007, 62, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Brasche, S.; Bullinger, M.; Morfeld, M.; Gebhardt, H.J.; Bischof, W. Why do women suffer from sick building syndrome more often than men?—Subjective higher sensitivity versus objective causes. Indoor Air 2001, 11, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Nordin, S.; Palmquist, E.; Claeson, A.S.; Stenberg, B. The environmental hypersensitivity symptom inventory: Metric properties and normative data from a population-based study. Arch. Public Health 2013, 71, 18. [Google Scholar] [CrossRef] [PubMed]
- Nordin, S.; Liden, E.; Gidlöf-Gunnarsson, A. Cognition and neurosciences development and evaluation of a category ratio scale with semantic descriptors: The Environmental Annoyance Scale. Scand. J. Psychol. 2009, 50, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Bell, I.R.; Miller, C.S.; Schwartz, G.E.; Peterson, J.M.; Amend, D. Neuropsychiatric and somatic characteristics of young adults with and without self-reported chemical odor intolerance and chemical sensitivity. Arch. Environ. Health 1996, 51, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Eis, D.; Helm, D.; Muhlinghaus, T.; Birkner, N.; Dietel, A.; Eikmann, T.; Gieler, U.; Herr, C.; Lacour, M.; Nowak, D.; et al. The German Multicentre Study on Multiple Chemical Sensitivity (MCS). Int. J. Hyg. Environ. Health 2008, 211, 658–681. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Nordin, S.; Heiden, M.; Sandström, M. Symptoms, personality traits, and stress in people with mobile phone-related symptoms and electromagnetic hypersensitivity. J. Psychosom. Res. 2010, 68, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Paulin, J.; Andersson, L.; Nordin, S. Characteristics of hyperacusis in the general population. Noise Health 2016, 18, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Caress, S.M.; Steinemann, A.C. Asthma and chemical hypersensitivity: Prevalence, etiology, and age of onset. Toxicol. Ind. Health 2009, 25, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Janssens, T.; Ritz, T. Perceived triggers of asthma: Key to symptom perception and management. Clin. Exp. Allergy 2013, 43, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Jaen, C.; Dalton, P. Asthma and odors: The role of risk perception in asthma exacerbation. J. Psychosom. Res. 2014, 77, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Miller, G.E. Stress and inflammation in exacerbations of asthma. Brain Behav. Immun. 2007, 21, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Gilmour, H. Medically unexplained physical symptoms (MUPS) among adults in Canada: Comorbidity, health care use and employment. Health Rep. 2017, 28, 3–8. [Google Scholar] [PubMed]
- Yunus, M.B. Editorial review: An update on central sensitivity syndromes and the issues of nosology and psychobiology. Curr. Rheumatol. Rev. 2015, 11, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Dantoft, T.M.; Skovbjerg, S.; Andersson, L.; Claeson, A.S.; Lind, N.; Nordin, S.; Brix, S. Inflammatory Mediator Profiling of n-butanol Exposed Upper Airways in Individuals with Multiple Chemical Sensitivity. PLoS ONE 2015, 10, e0143534. [Google Scholar] [CrossRef] [PubMed]
- Kanchongkittiphon, W.; Mendell, M.J.; Gaffin, J.M.; Wang, G.; Phipatanakul, W. Indoor environmental exposures and exacerbation of asthma: An update to the 2000 review by the Institute of Medicine. Environ. Health Perspect. 2015, 123, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Wolkoff, P. Indoor air pollutants in office environments: Assessment of comfort, health, and performance. Int. J. Hyg. Environ. Health 2013, 216, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Leeuw, M.; Goossens, M.E.; Linton, S.J.; Crombez, G.; Boersma, K.; Vlaeyen, J.W. The fear-avoidance model of musculoskeletal pain: Current state of scientific evidence. J. Behav. Med. 2007, 30, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Beesdo-Baum, K.; Jenjahn, E.; Hofler, M.; Lueken, U.; Becker, E.S.; Hoyer, J. Avoidance, safety behavior, and reassurance seeking in generalized anxiety disorder. Depress. Anxiety 2012, 29, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Dantoft, T.M.; Andersson, L.; Nordin, S.; Skovbjerg, S. Chemical intolerance. Curr. Rheumatol. Rev. 2015, 11, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Tomenson, B.; Essau, C.; Jacobi, F.; Ladwig, K.H.; Leiknes, K.A.; Lieb, R.; Meinlschmidt, G.; McBeth, J.; Rosmalen, J.; Rief, W.; et al. Total somatic symptom score as a predictor of health outcome in somatic symptom disorders. Br. J. Psychiatry 2013, 203, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Eliasen, M.; Kreiner, S.; Ebstrup, J.F.; Poulsen, C.H.; Lau, C.J.; Skovbjerg, S.; Fink, P.K.; Jørgensen, T. Somatic Symptoms: Prevalence, Co-Occurrence and Associations with Self-Perceived Health and Limitations Due to Physical Health—A Danish Population-Based Study. PLoS ONE 2016, 11, e0150664. [Google Scholar] [CrossRef] [PubMed]
- Baliatsas, C.; van Kamp, I.; Hooiveld, M.; Yzermans, J.; Lebret, E. Comparing non-specific physical symptoms in environmentally sensitive patients: Prevalence, duration, functional status and illness behavior. J. Psychosom. Res. 2014, 76, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Black, D.W.; Okiishi, C.; Schlosser, S. The Iowa follow-up of chemically sensitive persons. Ann. N. Y. Acad. Sci. 2001, 933, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, E. Environmental Intolerance: Psychological Risk and Health Factors. Ph.D. Thesis, Umeå Universitet, Umeå, Sweden, 2017. Available online: http://umu.diva-portal.org/ (accessed on 18 August 2018).
- van Dessel, N.; den Boeft, M.; van der Wouden, J.C.; Kleinstäuber, M.; Leone, S.S.; Terluin, B.; Numans, M.E.; van der Horst, H.E.; van Marwijk, H. Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst. Rev. 2014, CD011142. [Google Scholar] [CrossRef]
| Age (Years) | Västerbotten | Österbotten | ||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| 18–29 | 307 (32.1) | 179 (17.3) | 128 (28.6) | 70 (14.2) |
| 30–39 | 266 (40.3) | 177 (24.7) | 121 (36.0) | 80 (21.3) |
| 40–49 | 288 (40.5) | 230 (31.0) | 140 (37.4) | 80 (19.7) |
| 50–59 | 367 (50.9) | 295 (39.5) | 192 (46.0) | 123 (29.5) |
| 60–69 | 405 (58.4) | 356 (50.7) | 186 (44.2) | 169 (39.5) |
| 70–79 | 265 (53.8) | 271 (63.9) | 131 (44.9) | 115 (43.5) |
| Total sample | 1898 (45.2) | 1508 (34.9) | 898 (39.7) | 637 (27.2) |
| Variable | Self-Reported BRI 1 | Self-Reported BRI and World Health Organization (WHO) Symptoms 2 | Referents |
|---|---|---|---|
| (n = 275) | (n = 123) | (n = 4180) | |
| Age (years), mean (SD) | 51.8 (14.5) ns | 51.3 (14.4) | 51.4 (16.9) |
| Women, n (%) | 201 (73.1) *** | 96 (78.0) *** | 2259 (54.0) |
| Married/cohabitant, n (%) | 185 (67.3) * | 78 (63.4) * | 3098 (74.1) |
| No response | 4 (1.5) | 2 (1.6) | 34 (0.8) |
| University education, n (%) | 121 (44.0) ns | 50 (40.7) ns | 1614 (38.6) |
| No response | 4 (1.5) | 2 (1.6) | 71 (1.7) |
| Smoking, n (%) | 28 (10.2) ns | 15 (12.2) ns | 376 (9.0) |
| No response | 0 (0) | 0 (0) | 29 (0.7) |
| Physical exercise, n (%) | |||
| Once a month or less | 37 (13.5) ns | 15 (12.2) ns | 601 (14.4) |
| 2–4 times/month | 53 (19.3) | 25 (20.3) | 913 (21.8) |
| 2–3 times/week | 101 (36.7) | 47 (38.2) | 1564 (37.4) |
| More than 3 times/week | 79 (28.7) | 33 (26.8) | 1035 (24.8) |
| No response | 5 (1.8) | 3 (2.4) | 67 (1.6) |
| Perceived general health, n (%) | |||
| Excellent/very good | 75 (27.3) *** | 27 (22.0) *** | 1665 (39.8) |
| Good | 81 (29.5) | 31 (25.2) | 1449 (34.7) |
| Fairly good/poor | 118 (42.9) | 65 (52.8) | 1019 (24.4) |
| No response | 1 (0.4) | 0 (0) | 47 (1.1) |
| Duration of BRI (years), mean (SD) | 12.4 (11.6) | 12.8 (12.3) | |
| Frequency of BRI-related symptoms | |||
| Daily | 55 (20.0) | 32 (26.0) | |
| Weekly | 64 (23.3) | 33 (26.8) | |
| Monthly | 129 (46.9) | 51 (41.5) | |
| No response | 27 (9.8) | 7 (5.7) | |
| Degree of negative impact of visiting buildings that evoke symptoms, mean (SD) | |||
| Emotionally | 2.43 (1.68) | 2.85 (1.66) | |
| Behaviorally | 2.13 (1.71) | 2.51 (1.73) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karvala, K.; Sainio, M.; Palmquist, E.; Claeson, A.-S.; Nyback, M.-H.; Nordin, S. Building-Related Environmental Intolerance and Associated Health in the General Population. Int. J. Environ. Res. Public Health 2018, 15, 2047. https://doi.org/10.3390/ijerph15092047
Karvala K, Sainio M, Palmquist E, Claeson A-S, Nyback M-H, Nordin S. Building-Related Environmental Intolerance and Associated Health in the General Population. International Journal of Environmental Research and Public Health. 2018; 15(9):2047. https://doi.org/10.3390/ijerph15092047
Chicago/Turabian StyleKarvala, Kirsi, Markku Sainio, Eva Palmquist, Anna-Sara Claeson, Maj-Helen Nyback, and Steven Nordin. 2018. "Building-Related Environmental Intolerance and Associated Health in the General Population" International Journal of Environmental Research and Public Health 15, no. 9: 2047. https://doi.org/10.3390/ijerph15092047
APA StyleKarvala, K., Sainio, M., Palmquist, E., Claeson, A.-S., Nyback, M.-H., & Nordin, S. (2018). Building-Related Environmental Intolerance and Associated Health in the General Population. International Journal of Environmental Research and Public Health, 15(9), 2047. https://doi.org/10.3390/ijerph15092047





