Cystic Fibrosis Mortality in Childhood. Data from European Cystic Fibrosis Society Patient Registry
Abstract
1. Introduction
2. Patients and Methods
Statistical Analysis
- (1)
- Diagnosis: Genotype, Age at diagnosis, Neonatal screening, Meconium ileus
- (2)
- Microbiology: Chronic Pseudomonas aeruginosa, Chronic Staphylococcus aureus, Chronic Burkholderia cepacia, Nontuberculous mycobacteria, Stenotrophomonas maltophilia
- (3)
- Therapies: Inhaled hypertonic NaCl, Inhaled antibiotic, Inhaled bronchodilators, Use of oxygen, Use of rhDNase, Use of macrolide, Use of ursodeoxycholic acid
- (4)
- Complications: Allergic bronchopulmonary Aspergillosis (ABPA), CFRD, Pneumothorax requiring chest drain, Liver disease, Hemoptysis, Occurrence of malignancy
- (5)
- Growth and lung function: BMI-SDS, FEV1%
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cystic Fibrosis in Australia 2014 17th Annual Report from the Australian Cystic Fibrosis Data Registry. Available online: https://www.cysticfibrosis.org.au/media/wysiwyg/CFAustralia/medical-documents/CFA_DataRegistryReport_2014_Final.pdf (accessed on 25 July 2018).
- Cystic Fibrosis Foundation Patient Registry 2014 Annual Data Report Bethesda, Cystic Fibrosis Foundation. Available online: https://www.cff.org/2014_CFF_Annual_Data_Report_to_the_Center_Directors.pdf (accessed on 25 July 2018).
- ECFSPR Annual Report 2014. Available online: https://www.ecfs.eu/sites/default/files/general-content-files/working-groups/ecfs-patientregistry/ECFSPR_Annual%20Report%202014_Nov2016.pdf (accessed on 25 July 2018).
- Kerem, E.; Viviani, L.; Zolin, A.; MacNeill, S.; Hatziagorou, E.; Ellemunter, H.; Drevinek, P.; Gulmans, V.; Krivec, U.; Olesen, H.; et al. Factors associated with FEV1 decline in cystic fibrosis: Analysis of the ECFS patient registry. Eur. Respir. J. 2014, 43, 125–133. [Google Scholar] [CrossRef] [PubMed]
- McColley, S.A.; Schechter, M.S.; Moran, W.J.; Pasta, D.J.; Craib, M.L.; Konstan, M.W. Risk factors for mortality before age 18 years in cystic fibrosis. Pediatr. Pulmonol. 2017, 52, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.; Macek, M.; Mehta, A. European Registry Working Group. Cystic fibrosis across Europe: EuroCareCF analysis of demographic data from 35 countries. J. Cyst. Fibros. 2010, 9, S5–S21. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.; Mehta, G.; Olsen, H.V.; Viviani, L.; Macek, M. Jr.; Mehta, A.; European Registry Working Group. Comparative demographics of the European cystic fibrosis population: A cross-sectional database analysis. Lancet 2010, 375, 1007–1013. [Google Scholar] [CrossRef]
- Guidelines for the ECFSPR. Available online: https://www.ecfs.eu/projects/efcs-patient-registry/guidelines (accessed on 25 July 2018).
- Viviani, L.; Zolin, A.; Mehta, A.; Olesen, H.V. The European Cystic Fibrosis Society Patient Registry: Valuable lessons learned on how to sustain a disease registry. Orphanet. J. Rare. Dis. 2014, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Annual Reports. Available online: https://www.ecfs.eu/projects/ecfs-patient-registry/annual-reports (accessed on 25 July 2018).
- Gross National Income Per Capita 2017, Atlas Method and PPP. Available online: http://databank.worldbank.org/data/download/GNIPC.pdf (accessed on 25 July 2018).
- Registry Variables and Definitions. Available online: https://www.ecfs.eu/projects/ecfs-patient-registry/Variables-Definitions (accessed on 25 July 2018).
- Wang, X.; Dockery, D.W.; Wypij, D.; Gold, D.R.; Speizer, F.E.; Ware, J.H.; Ferris, B.G. Pulmonary function between 6 and 18 years of age. Pediatr. Pulmonol. 1993, 15, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, J.L.; Odencrantz, J.R.; Fedan, K.B. Spirometric reference values from a sample of the general US population. Am. J. Respir. Crit. Care Med. 1999, 159, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Kuczmarski, R.J.; Ogden, C.L.; Guo, S.S.; Grummer-Strawn, L.M.; Flegal, K.M.; Mei, Z.; Wei, R.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. 2000 CDC Growth Charts for the United States: Methods and development. Vital Health Stat. 2002, 11, 1–190. [Google Scholar]
- Morgan, W.J.; Butler, S.M.; Johnson, C.A.; Colin, A.A.; FitzSimmons, S.C.; Geller, D.E.; Konstan, M.W.; Light, M.J.; Rabin, H.R.; Regelmann, W.E.; et al. Epidemiologic study of cystic fibrosis: Design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the U.S. and Canada. Pediatr. Pulmonol. 1999, 28, 231–241. [Google Scholar] [CrossRef]
- Tridello, G.; Castellani, C.; Meneghelli, I.; Tamanini, A.; Assael, B.M. Early diagnosis from newborn screening maximises survival in severe cystic fibrosis. ERJ Open Res. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Alicandro, G.; Frova, L.; Di Fraia, G.; Colombo, C. Cystic fibrosis mortality trend in Italy from 1970 to 2011. J. Cyst. Fibros. 2015, 14, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.C.; Kosorok, M.R.; Laxova, A.; Makholm, L.M.; Farrell, P.M. Delayed diagnosis of US females with cystic fibrosis. Am. J. Epidemiol. 2002, 156, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Corey, M.; Farewell, V. Determinants of mortality from cystic fibrosis in Canada, 1970–1989. Am. J. Epidemiol. 1996, 143, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.E.; Calloway, E.H.; Kiser, M.; Yeatts, J.; Davis, S.D.; Drumm, M.L.; Schechter, M.S.; Leigh, M.W.; Emond, M.; van Rie, A.; et al. Age of Pseudomonas aeruginosa acquisition and subsequent severity of cystic fibrosis lung disease. Pediatr. Pulmonol. 2011, 46, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Sweezey, N.B.; Ratjen, F. The cystic fibrosis gender gap: Potential role of estrogen. Pediatr. Pulmonol. 2014, 49, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Emerson, J.; Rosenfeld, M.; McNamara, S.; Ramsey, B.; Gibson, R.L. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 2002, 34, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Stick, S.M.; Brennan, S.; Murray, C.; Douglas, T.; von Ungern-Sternberg, B.S.; Garratt, L.W.; Gangell, C.L.; De Klerk, N.; Linnane, B.; Ranganathan, S.; et al. Bronchiectasis in infants and preschool children diagnosed with CF after NBS. J. Pediatr. 2009, 155, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.B.; Emerson, J.; Ren, C.L.; Schechter, M.S.; Gibson, R.L.; Morgan, W.; Rosenfeld, M.; EPIC Study Group. Early childhood risk factors for decreased FEV1 at age six to seven years in young children with CF. Ann. ATS 2015, 12, 1170–1176. [Google Scholar] [CrossRef]
- Moran, A.; Brunzell, C.; Cohen, R.C.; Katz, M.; Marshall, B.C.; Onady, G.; Robinson, K.A.; Sabadosa, K.A.; Stecenko, A.; Slovis, B.; et al. Clinical Care Guidelines for Cystic Fibrosis-Related Diabetes: A position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010, 33, 2697–2708. [Google Scholar] [CrossRef] [PubMed]
- Sermet-Gaudelus, I.; Mayell, S.J.; Southern, K.W. Guidelines on the early management of infants diagnosed with cystic fibrosis following newborn screening. J. Cyst. Fibros. 2010, 9, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 2016, 35, 557–577. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Pillay, K.; Becker, D.J.; Acerini, C.L. Management of cystic fibrosis-related diabetes in children and adolescents. Pediatr. Diabetes 2014, 15, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, T.; Hempstead, S.E.; Brady, C.; Cannon, C.L.; Clark, K.; Condren, M.E.; Guill, M.F.; Guillerman, R.P.; Leone, C.G.; Maguiness, K.; et al. Clinical practice guidelines from the cystic fibrosis foundation for preschoolers with cystic fibrosis. Pediatrics 2016, 137, e20151784. [Google Scholar] [CrossRef] [PubMed]
- Yen, E.H.; Quinton, H.; Borowitz, D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J. Pediatr. 2013, 162, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Tiddens, H.A. Chest computed tomography scans should be considered as a routine investigation in cystic fibrosis. Paediatr. Respir. Rev. 2006, 7, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Oates, G.R.; Schechter, M.S. Socioeconomic status and health outcomes: Cystic fibrosis as a model. Expert Rev. Respir. Med. 2016, 10, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Dijk, F.N.; McKay, K.; Barzi, F.; Gaskin, K.J.; Fitzgerald, D.A. Improved survival in cystic fibrosis patients diagnosed by newborn screening compared to a historical cohort from the same centre. Arch. Dis. Child. 2011, 96, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Coffey, M.J.; Whitaker, V.; Gentin, N.; Junek, R.; Shalhoub, C.; Nightingale, S.; Hilton, J.; Wiley, V.; Wilcken, B.; Gaskin, K.; et al. Differences in outcomes between early and late diagnosis of cystic fibrosis in the newborn screening era. J. Pediatr. 2017, 181, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Barben, J.; Castellani, C.; Dankert-Roelse, J.; Gartner, S.; Kashirskaya, N.; Linnane, B.; Mayell, S.; Munck, A.; Sands, D.; Sommerburg, O.; et al. The expansion and performance of national newborn screening programmes for cystic fibrosis in Europe. J. Cyst. Fibros. 2017, 16, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.A.; Aurora, P. Monitoring early lung disease in cystic fibrosis: Where are we now? Breathe 2014, 10, 34–47. [Google Scholar] [CrossRef]
| Deceased (N = 186) | Alive (N = 186) | |||
|---|---|---|---|---|
| Gender | Male Female | 64 (34.41) 122 (65.59) | 109 (58.60) 77 (41.40) | |
| Diagnosis | Genotype | F508del homozygote F508del heterozygote No F508del Unknown | 87 (46.77) 66 (35.48) 25 (13.44) 8 (4.30) | 83 (44.62) 73 (39.25) 25 (13.44) 5 (2.69) |
| Age at diagnosis (years), median (N) | 0.30 (175) | 0.31 (174) | ||
| Age at diagnosis | <1 year 1–17 years Unknown | 132 (70.97) 43 (23.12) 11 (5.91) | 114 (61.29) 60 (32.26) 12 (6.45) | |
| Neonatal screening | Not performed Performed Unknown | 87 (46.77) 36 (19.35) 63 (33.87) | 79 (42.47) 53 (28.49) 54 (29.03) | |
| Meconium ileus | No Yes Unknown | 130 (69.89) 37 (19.89) 19 (10.22) | 145 (77.96) 23 (12.37) 18 (9.68) | |
| Transplant | Liver transplant | No Yes Unknown | 178 (95.70) 1 (0.54) 7 (3.76) | 180 (96.77) 0 (0.00) 6 (3.23) |
| Lung transplant | No Yes Unknown | 152 (81.72) 30 (16.13) 4 (2.15) | 182 (97.85) 0 (0.00) 4 (2.15) | |
| Microbiology | Chronic Pseudomonas aeruginosa | No Yes Unknown | 73 (39.25) 77 (41.40) 36 (19.35) | 132 (70.97) 38 (20.43) 16 (8.60) |
| Chronic Staphylococcus aureus | No Yes Unknown | 66 (35.48) 31 (16.67) 89 (47.85) | 71 (38.17) 45 (24.19) 70 (37.63) | |
| Chronic Burkholderia cepacia | No Yes Unknown | 142 (76.34) 12 (6.45) 32 (17.20) | 167 (89.78) 2 (1.08) 17 (9.14) | |
| Nontuberculous mycobacteria | No Yes Unknown | 139 (74.73) 4 (2.15) 43 (23.12) | 151 (81.18) 3 (1.61) 32 (17.20) | |
| Stenotrophomonas maltophilia | No Yes Unknown | 141 (75.81) 9 (4.84) 36 (19.35) | 146 (78.49) 18 (9.68) 22 (11.83) | |
| Therapy | Inhaled hypertonic NaCl | No Yes Unknown | 115 (61.83) 37 (19.89) 34 (18.28) | 129 (69.35) 42 (22.58) 15 (8.06) |
| Inhaled antibiotic | No Yes Unknown | 72 (38.71) 82 (44.09) 32 (17.20) | 118 (63.44) 59 (31.72) 9 (4.84) | |
| Inhaled bronchodilators | No Yes Unknown | 56 (30.11) 95 (51.08) 35 (18.82) | 78 (41.94) 93 (50.00) 15 (8.06) | |
| Use of oxygen | No Yes Unknown | 70 (37.63) 83 (44.62) 33 (17.74) | 169 (90.86) 4 (2.15) 13 (6.99) | |
| Use of rhDNase | No Yes Unknown | 58 (31.18) 103 (55.38) 25 (13.44) | 74 (39.78) 107 (57.53) 5 (2.69) | |
| Use of macrolide | No Yes Unknown | 75 (40.32) 74 (39.78) 37 (19.89) | 120 (64.52) 51 (27.42) 15 (8.06) | |
| Use of ursodeoxycholic acid | No Yes Unknown | 68 (36.56) 87 (46.77) 31 (16.67) | 99 (53.23) 82 (44.09) 5 (2.69) | |
| Use of pancreatic enzymes | No Yes Unknown | 13 (6.99) 146 (78.49) 27 (14.52) | 22 (11.83) 160 (86.02) 4 (2.15) | |
| Complication | ABPA | No Yes Unknown | 137 (73.66) 25 (13.44) 24 (12.90) | 160 (86.02) 16 (8.60) 10 (5.38) |
| CFRD | No Yes Unknown | 120 (64.52) 45 (24.19) 21 (11.29) | 168 (90.32) 13 (6.99) 5 (2.69) | |
| Pneumothorax requiring chest drain | No Yes Unknown | 154 (82.80) 11 (5.91) 21 (11.29) | 179 (96.24) 1 (0.54) 6 (3.23) | |
| Liver disease | No Yes Unknown | 95 (51.08) 64 (34.41) 27 (14.52) | 145 (77.96) 25 (13.44) 16 (8.60) | |
| Haemoptysis | No Yes Unknown | 145 (77.96) 17 (9.14) 24 (12.90) | 176 (94.62) 1 (0.54) 9 (4.84) | |
| Occurrence of malignancy | No Yes Unknown | 158 (84.95) 1 (0.54) 27 (14.52) | 173 (93.01) 0 (0.00) 13 (6.99) | |
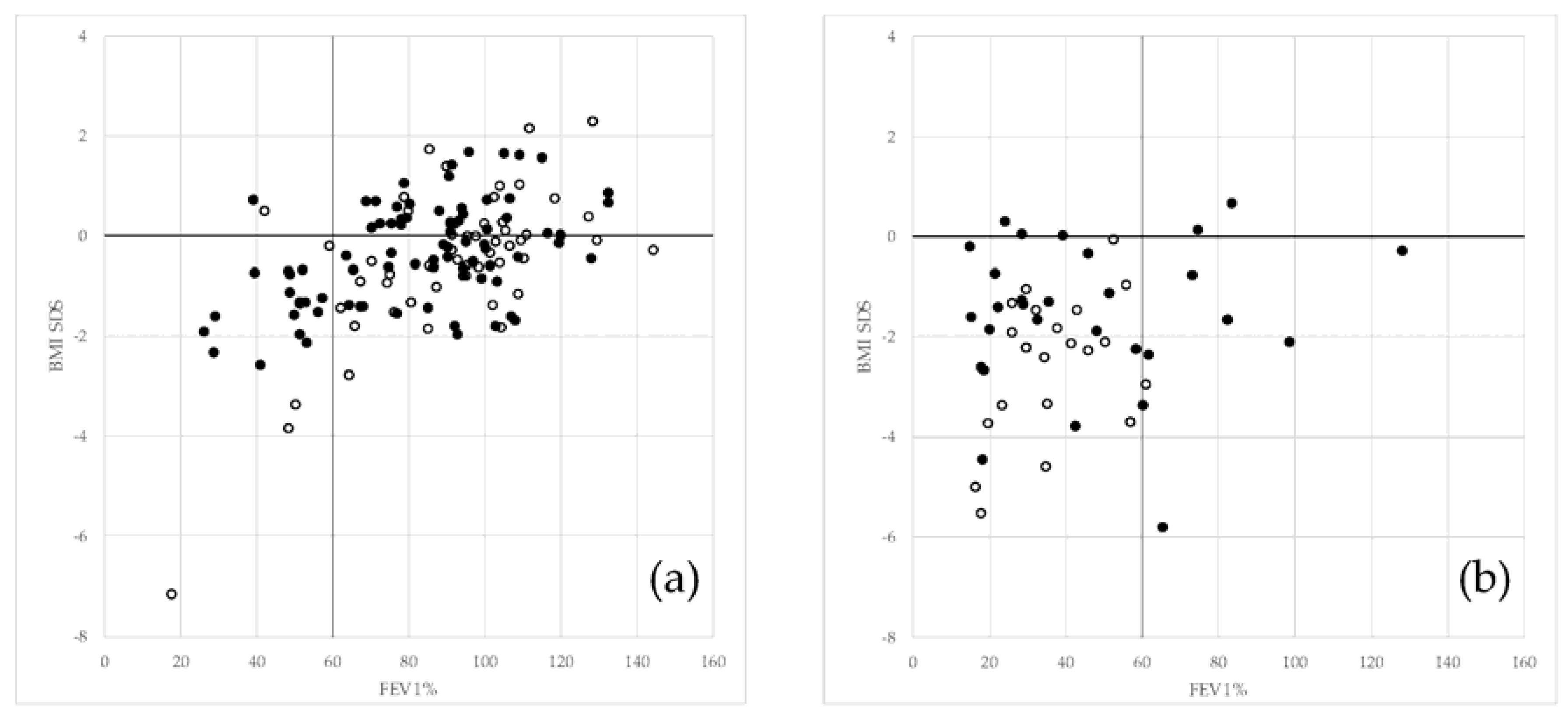
| Growth and lung function | BMI-SDS (only patients 2 years old or more) | <−2 ≥−2 Unknown | 45 (27.44) 49 (29.88) 70 (42.68) | 10 (6.13) 145 (88.96) 8 (4.91) |
| FEV1% * | <40 ≥40 Unknown | 28 (23.33) 22 (18.33) 70 (58.33) | 6 (4.03) 128 (85.91) 15 (10.07) |
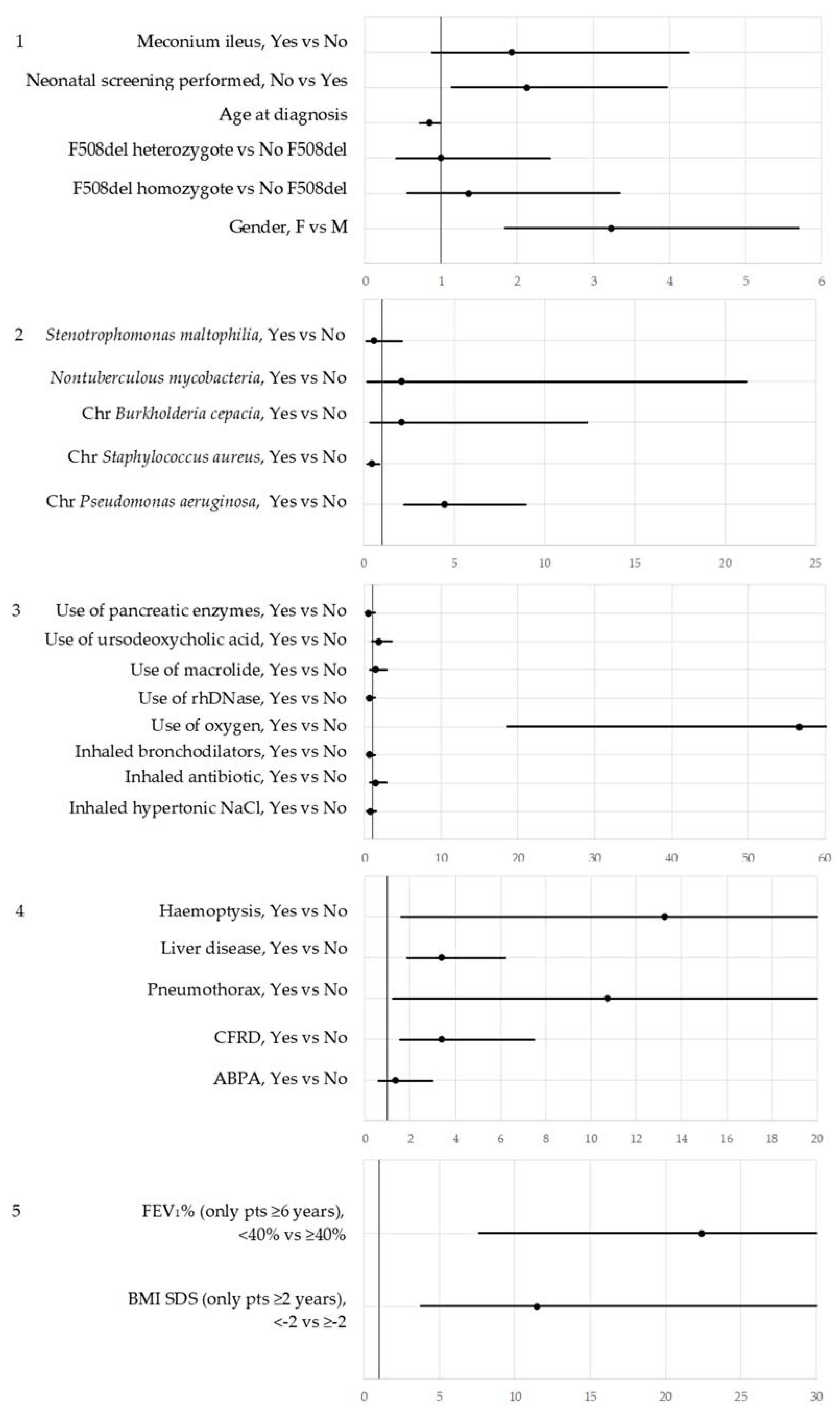
| OR | 95% CI | ||
|---|---|---|---|
| Model 6.1: 136 observations used | |||
| Gender: F vs. M | 5.626 | 1.878 | 16.853 |
| Neonatal screening: Not performed vs. Performed | 0.755 | 0.235 | 2.421 |
| Use of oxygen: Yes vs. No | 48.608 | 8.173 | 289.070 |
| CFRD: Yes vs. No | 3.646 | 0.832 | 15.991 |
| Liver disease: Yes vs. No | 1.935 | 0.673 | 5.568 |
| Chronic Pseudomonas aeruginosa: Yes vs. No | 2.512 | 0.852 | 7.410 |
| Chronic Staphylococcus aureus: Yes vs. No | 0.483 | 0.172 | 1.353 |
| Age at diagnosis | 0.835 | 0.604 | 1.153 |
| Model 6.2: 172 observations used | |||
| Gender: F vs. M | 4.118 | 1.211 | 14.005 |
| Use of oxygen: Yes vs. No | 20.787 | 4.336 | 99.668 |
| BMI-SDS (patients aged 2 or more): <−2 vs. ≥−2 | 7.975 | 2.403 | 26.461 |
| FEV1% *, <40 vs. ≥40 | 6.999 | 1.997 | 24.524 |
| Number of Ptients | ||||
|---|---|---|---|---|
| Income | Countries | total | total < 18 years | deaths < 18 years |
| Low/middle | Hungary, Lithuania, Republic of Macedonia *, Republic of Moldova, Romania *, Russian Federation, Serbia, Ukraine, Czech Republic, Greece, Israel, Italy, Latvia, Portugal, Slovakia *, Slovenia * and Spain | 12,867 | 7830 | 102 |
| High | Austria, Belgium, Denmark, France, Germany, Ireland, The Netherlands, Sweden, Switzerland * and United Kingdom | 31,237 | 16,586 | 107 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolin, A.; Bossi, A.; Cirilli, N.; Kashirskaya, N.; Padoan, R. Cystic Fibrosis Mortality in Childhood. Data from European Cystic Fibrosis Society Patient Registry. Int. J. Environ. Res. Public Health 2018, 15, 2020. https://doi.org/10.3390/ijerph15092020
Zolin A, Bossi A, Cirilli N, Kashirskaya N, Padoan R. Cystic Fibrosis Mortality in Childhood. Data from European Cystic Fibrosis Society Patient Registry. International Journal of Environmental Research and Public Health. 2018; 15(9):2020. https://doi.org/10.3390/ijerph15092020
Chicago/Turabian StyleZolin, Anna, Anna Bossi, Natalia Cirilli, Nataliya Kashirskaya, and Rita Padoan. 2018. "Cystic Fibrosis Mortality in Childhood. Data from European Cystic Fibrosis Society Patient Registry" International Journal of Environmental Research and Public Health 15, no. 9: 2020. https://doi.org/10.3390/ijerph15092020
APA StyleZolin, A., Bossi, A., Cirilli, N., Kashirskaya, N., & Padoan, R. (2018). Cystic Fibrosis Mortality in Childhood. Data from European Cystic Fibrosis Society Patient Registry. International Journal of Environmental Research and Public Health, 15(9), 2020. https://doi.org/10.3390/ijerph15092020





