The Role of Polybrominated Diphenyl Ethers in Thyroid Carcinogenesis: Is It a Weak Hypothesis or a Hidden Reality? From Facts to New Perspectives
Abstract
1. Background
2. Thyroid Cancer: Etiology and Risk Factors Involved
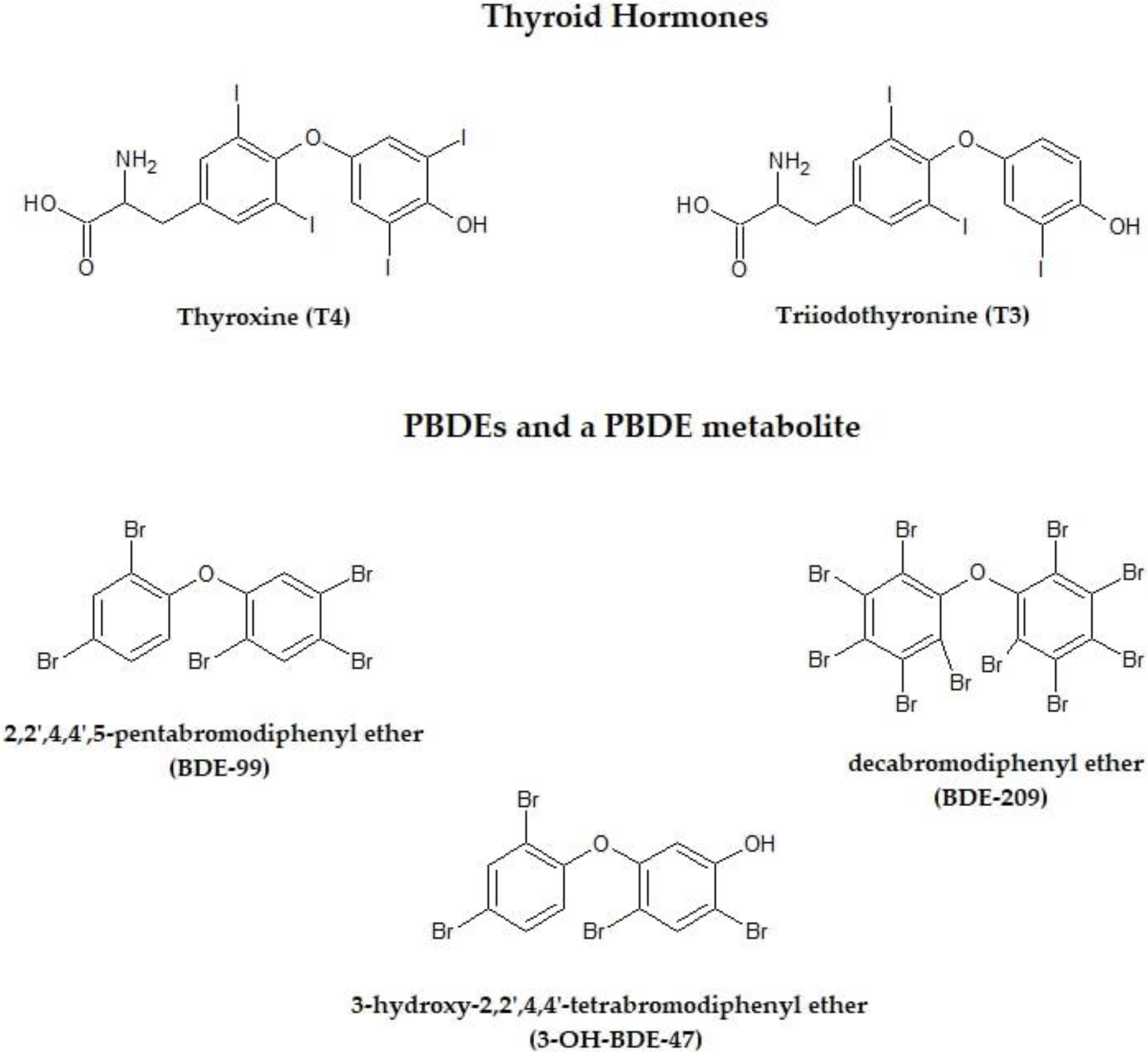
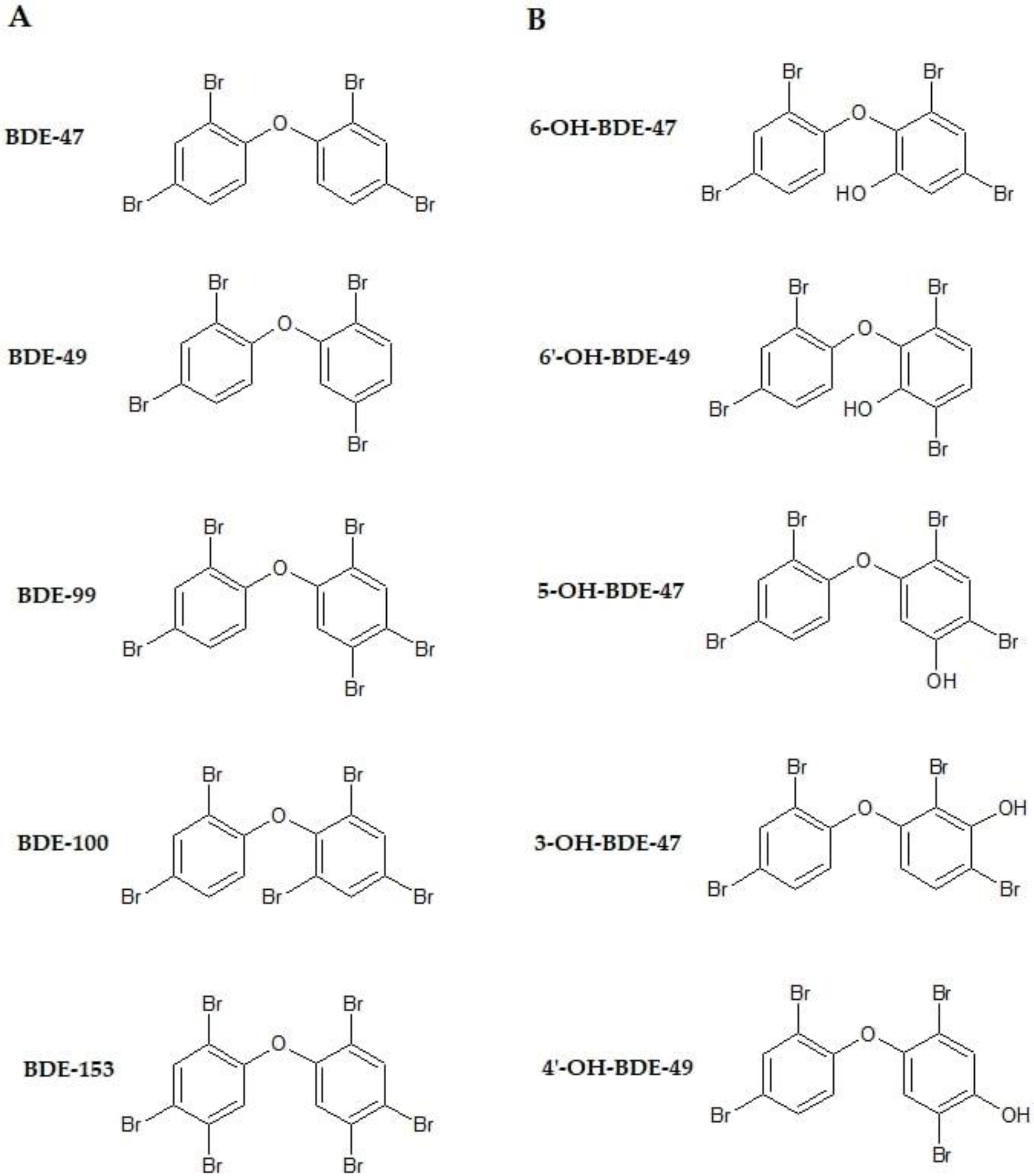
3. Effects of Polybrominated Diphenyl Ethers on Thyroid Gland
3.1. Experimental Studies
3.2. Human Studies
4. Polybrominated Diphenyl Ethers and Thyroid Cancer: Epidemiological Evidence
5. Discussion and Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Darnerud, P.O.; Eriksen, G.S.; Jóhannesson, T.; Larsen, P.B.; Viluksela, M. Polybrominated Diphenyl Ethers: Occurrence, Dietary Exposure, and Toxicology. Environ. Health Perspect. 2001, 109, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.A.; Laessig, R.H.; Reed, K.D. Polybrominated Diphenyl Ethers (PBDEs): New Pollutants–Old Diseases. Clin. Med. Res. 2003, 1, 281–290. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Technical Fact Sheet–Polybrominated Diphenyl Ethers (PBDEs); EPA 505-F-17–015; Office of Land and Emergency Management (5016P): Washington, DC, USA, November 2017.
- Danon-Schaffer, M.N.; Mahecha-Botero, A.; Grace, J.R.; Ikonomou, M. Mass balance evaluation of polybrominated diphenyl ethers in landfill leachate and potential for transfer from e-waste. Sci. Total Environ. 2013, 461–462, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Linares, V.; Bellés, M.; Domingo, J.L. Human exposure to PBDE and critical evaluation of health hazards. Arch. Toxicol. 2015, 89, 335–356. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, M.; Vorkamp, K.; Thomsen, M.; Knudsen, L.E. Human internal and external exposure to PBDEs—A review of levels and sources. Int. J. Hyg. Environ. Health 2009, 212, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Lanphear, B.P.; Bellinger, D.; Axelrad, D.A.; McPartland, J.; Sutton, P.; Davidson, L.; Daniels, N.; Sen, S.; Woodruff, T.J. Developmental PBDE Exposure and IQ/ADHD in Childhood: A Systematic Review and Meta-analysis. Environ. Health Perspect. 2017, 125, 086001. [Google Scholar] [CrossRef] [PubMed]
- Mazdai, A.; Dodder, N.G.; Abernathy, M.P.; Hites, R.A.; Bigsby, R.M. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ. Health Perspect. 2003, 111, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Hites, R.A. Polybrominated Diphenyl Ethers in the Environment and in People: A Meta-Analysis of Concentrations. Environ. Sci. Technol. 2004, 38, 945–956. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Polybrominated Diphenyl Ethers (PBDEs) Action Plan Summary. Available online: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/polybrominated-diphenyl-ethers-pbdes (accessed on 18 April 2018).
- Fischer, D.; Hooper, K.; Athanasiadou, M.; Athanassiadis, I.; Bergman, A. Children Show Highest Levels of Polybrominated Diphenyl Ethers in a California Family of Four: A Case Study. Environ. Health Perspect. 2006, 114, 1581–1584. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kannan, K.; Moon, H.B. Assessment of exposure to polybrominated diphenyl ethers (PBDEs) via seafood consumption and dust ingestion in Korea. Sci. Total Environ. 2013, 443, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Fängström, B.; Athanassiadis, I.; Odsjö, T.; Norén, K.; Bergman, A. Temporal trends of polybrominated diphenyl ethers and hexabromocyclododecane in milk from Stockholm mothers, 1980–2004. Mol. Nutr. Food Res. 2008, 52, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Schecter, A.; Pavuk, M.; Päpke, O.; Ryan, J.J.; Birnbaum, L.; Rosen, R. Polybrominated diphenyl ethers (PBDEs) in U.S. mothers’ milk. Environ. Health Perspect. 2003, 111, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Fürst, P. Dioxins, polychlorinated biphenyls and other organohalogen compounds in human milk. Levels, correlations, trends and exposure through breastfeeding. Mol. Nutr. Food Res. 2006, 50, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K.; Sosa, J.A.; Stapleton, H.M. Do flame retardant chemicals increase the risk for thyroid dysregulation and cancer? Curr. Opin. Oncol. 2017, 29, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ren, X.; Ren, B.; Luo, Z.; Zhu, R. Life-cycle exposure to BDE-47 results in thyroid endocrine disruption to adults and offsprings of zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 2016, 48, 157–167. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.A. A perspective on the potential health risks of PBDEs. Chemosphere 2002, 46, 745–755. [Google Scholar] [CrossRef]
- Huang, H.; Rusiecki, J.; Zhao, N.; Chen, Y.; Ma, S.; Yu, H.; Ward, M.H.; Udelsman, R.; Zhang, Y. Thyroid-Stimulating Hormone, Thyroid Hormones, and Risk of Papillary Thyroid Cancer: A Nested Case-Control Study. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Olaleye, O.; Ekrikpo, U.; Moorthy, R.; Lyne, O.; Wiseberg, J.; Black, M.; Mitchell, D. Increasing incidence of differentiated thyroid cancer in South East England: 1987–2006. Eur. Arch. Otorhinolaryngol. 2011, 268, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2015. Int. J. Cancer 2012, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- James, B.C.; Mitchell, J.M.; Jeon, H.D.; Vasilottos, N.; Grogan, R.H.; Aschebrook-Kilfoy, B. An update in international trends in incidence rates of thyroid cancer, 1973–2007. Cancer Causes Control. 2018, 29, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Pellegriti, G.; Frasca, F.; Regalbuto, C.; Squatrito, S.; Vigneri, R. Worldwide Increasing Incidence of Thyroid Cancer: Update on Epidemiology and Risk Factors. J. Cancer Epidemiol. 2013, 2013, 965212. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.S.; Davies, L.; Nixon, I.J.; Palmer, F.L.; Wang, L.Y.; Patel, S.G.; Ganly, I.; Wong, R.J.; Tuttle, R.M.; Morris, L.G. Increasing diagnosis of subclinical thyroid cancers leads to spurious improvements in survival rates. Cancer 2015, 121, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.K.; Little, M.P.; Lubin, J.H.; Brenner, A.V.; Wells, S.A., Jr.; Sigurdson, A.J.; Nikiforov, Y.E. The Increase in Thyroid Cancer Incidence During the Last Four Decades is Accompanied by a High Frequency of BRAF Mutations and a Sharp Increase in RAS Mutations. J. Clin. Endocrinol. MeTab. 2014, 99, E276–E285. [Google Scholar] [CrossRef] [PubMed]
- Sipos, J.A.; Mazzaferri, E.L. Thyroid Cancer Epidemiology and Prognostic Variables. Clin. Oncol. (R. Coll. Radiol.) 2010, 22, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Dal Maso, L.; Panato, C.; Franceschi, S.; Serraino, D.; Buzzoni, C.; Busco, S.; Ferretti, S.; Torrisi, A.; Falcini, F.; Zorzi, M.; et al. The impact of overdiagnosis on thyroid cancer epidemic in Italy, 1998–2012. Eur. J. Cancer 2018, 94, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Welch, H.G. Current thyroid cancer trends in the United States. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Jemal, A.; Ward, E.M. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 2009, 115, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.G.; Gale, S.; Zoeller, R.T.; Spengler, J.D.; Birnbaum, L.; McNeely, E. PBDE flame retardants, thyroid disease, and menopausal status in U.S. women. Environ. Health. 2016, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Derwahl, M.; Nicula, D. Estrogen and its role in thyroid cancer. Endocr. Relat. Cancer 2014, 21, T273–T283. [Google Scholar] [CrossRef] [PubMed]
- Frasca, F.; Nucera, C.; Pellegriti, G.; Gangemi, P.; Attard, M.; Stella, M.; Loda, M.; Vella, V.; Giordano, C.; Trimarchi, F.; et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr. Relat. Cancer 2008, 15, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Marcello, M.A.; Malandrino, P.; Almeida, J.F.; Martins, M.B.; Cunha, L.L.; Bufalo, N.E.; Pellegriti, G.; Ward, L.S. The influence of the environment on the development of thyroid tumors: A new appraisal. Endocr. Relat. Cancer 2014, 21, T235–T254. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Viola, D.; Elisei, R.; Bendlova, B.; Yip, L.; Mian, C.; Vianello, F.; Tuttle, R.M.; et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2013, 309, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Pellegriti, G.; De Vathaire, F.; Scollo, C.; Attard, M.; Giordano, C.; Arena, S.; Dardanoni, G.; Frasca, F.; Malandrino, P.; Vermiglio, F.; et al. Papillary thyroid cancer incidence in the volcanic area of Sicily. J. Natl. Cancer Inst. 2009, 101, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Fallahi, P.; Antonelli, A.; Benvenga, S. Environmental Issues in Thyroid Diseases. Front. Endocrinol. 2017, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Vanadium pentoxide. In Cobalt in Hard Metals and Cobalt Sulfate, Gallium Arsenide, Indium Phosphide and Vanadium Pentoxide; IARC Press: Lyon, France, 2006; pp. 227–292. [Google Scholar]
- World Health Organization. WHO Handbook on Indoor Radon: A Public Health Perspective; World Health Organization: Geneva, France, 2009. [Google Scholar]
- Klaassen, C.S.; Liu, J.; Diwan, B.A. Metallothionein Protection of Cadmium Toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Buha, A.; Matovic, V.; Antonijevic, B.; Bulat, Z.; Curcic, M.; Renieri, E.A.; Tsatsakis, A.M.; Schweitzer, A.; Wallace, D. Overview of Cadmium Thyroid Disrupting Effects and Mechanisms. Int. J. Mol. Sci. 2018, 19, E1501. [Google Scholar] [CrossRef] [PubMed]
- Jancic, S.A.; Stosic, B.Z. Cadmium effects on the thyroid gland. Vitam. Horm. 2014, 94, 391–425. [Google Scholar] [CrossRef] [PubMed]
- Uetani, M.; Kobayashi, E.; Suwazono, Y.; Honda, R.; Nishijo, M.; Nakagawa, H.; Kido, T.; Nogawa, K. Tissue cadmium (Cd) concentrations of people living in a Cd polluted area, Japan. Biometals 2006, 19, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Yoshizuka, M.; Mori, N.; Hamasaki, K.; Tanaka, I.; Yokoyama, M.; Hara, K.; Doi, Y.; Umezu, Y.; Araki, H.; Sakamoto, Y.; et al. Cadmium toxicity in the thyroid gland of pregnant rats. Exp. Mol. Pathol. 1991, 55, 97–104. [Google Scholar] [CrossRef]
- Chung, H.K.; Nam, J.S.; Ahn, C.W.; Lee, Y.S.; Kim, K.R. Some Elements in Thyroid Tissue are Associated with More Advanced Stage of Thyroid Cancer in Korean Women. Biol. Trace Elem. Res. 2016, 171, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Boas, M.; Feldt-Rasmussen, U.; Main, K.M. Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 2012, 355, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.; Hernández, S.; Martinez-de-Mena, R.; Kolliker-Frers, R.; Obregón, M.J.; Kleiman de Pisarev, D.L. The role of type I and type II 5′ deiodinases on hexachlorobenzene-induced alteration of the hormonal thyroid status. Toxicology 2005, 207, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Scollon, E.J.; Carr, J.A.; Cobb, G.P. The effect of flight, fasting and p,p’-DDT on thyroid hormones and corticosterone in Gambel’s white-crowned sparrow, Zonotrichia leucophrys gambelli. Comp. Biochem. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 137, 179–189. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.D.; O’dea, M.F.; Moeckel, A.M.; Lerner, D.T.; Björnsson, B.T. Endocrine disruption of parr-smolt transformation and seawater tolerance of Atlantic salmon by 4-nonylphenol and 17β-estradiol. Gen. Comp. Endocrinol. 2005, 142, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cheng, Y.; Tang, Q.; Lin, S.; Li, Y.; Hu, X.; Nian, J.; Gu, H.; Lu, Y.; Tang, H.; et al. The association between prenatal exposure to organochlorine pesticides and thyroid hormone levels in newborns in Yancheng, China. Environ. Res. 2014, 29, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.; Koifman, R.J.; Sarcinelli, P.N.; Simões Rosa, A.C.; Clapauch, R.; Koifman, S. Long-term exposure to organochlorine pesticides and thyroid status in adults in a heavily contaminated area in Brazil. Environ. Res. 2013, 127, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.G.; Tang, Y.; Liu, Y.X.; Yuan, Y.; Zhao, B.Q.; Chao, X.J.; Zhu, B.Z. Low concentrations of bisphenol A suppress thyroid hormone receptor transcription through a nongenomic mechanism. Toxicol. Appl. Pharmacol. 2012, 259, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Yamauchi, K. In vitro and in vivo analysis of the thyroid disrupting activities of phenolic and phenol compounds in Xenopus laevis. Toxicol. Sci. 2005, 84, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Shen, O.X.; Wang, X.R.; Zhou, L.; Zhen, S.Q.; Chen, X.D. Anti-thyroid hormone activity of bisphenol A, tetrabromobisphenol A and tetrachlorobisphenol A in an improved reporter gene assay. Toxicol. In Vitro 2009, 23, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.E.; Webster, G.M.; Vuong, A.M.; Thomas Zoeller, R.; Chen, A.; Hoofnagle, A.N.; Calafat, A.M.; Karagas, M.R.; Yolton, K.; Lanphear, B.P.; et al. Gestational urinary bisphenol A and maternal and newborn thyroid hormone concentrations: The HOME Study. Environ. Res. 2015, 138, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lu, J.; Xu, M.; Xu, Y.; Li, M.; Liu, Y.; Tian, X.; Chen, Y.; Dai, M.; Wang, W.; et al. Urinary bisphenol A concentration and thyroid function in Chinese adults. Epidemiology 2013, 24, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Ferguson, K.K. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environ. Health Perspect. 2011, 119, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Calafat, A.M.; Hauser, R. Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ. Sci. Technol. 2010, 44, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, J.; Gunier, R.B.; Bradman, A.; Holland, N.T.; Calafat, A.M.; Eskenazi, B.; Harley, K.G. Maternal urinary bisphenol Aa during pregnancy and maternal and neonatal thyroid function in the CHAMACOS study. Environ Health Perspect. 2013, 121, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Boas, M.; Frederiksen, H.; Feldt-Rasmussen, U.; Skakkebæk, N.E.; Hegedüs, L.; Hilsted, L.; Juul, A.; Main, K.M. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ. Health Perspect. 2010, 118, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, F.; Wang, Y.; Ning, Y.; Yang, J.Y.; Zhou, Y.K. Exposure to phthalates in children aged 5–7 years: Associations with thyroid function and insulin-like growth factors. Sci. Total Environ. 2017, 579, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, A.; Nishiyama, N.; Sugiyama, S.; Yamauchi, K. The effect of endocrine disrupting chemicals on thyroid hormone binding to Japanese quail transthyretin and thyroid hormone receptor. Gen. Comp. Endocrinol. 2003, 134, 36–43. [Google Scholar] [CrossRef]
- Shimada, N.; Yamauchi, K. Characteristics of 3,5,3′-triiodothyronine (T3)-uptake system of tadpole red blood cells: effect of endocrine-disrupting chemicals on cellular T3 response. J. Endocrinol. 2004, 183, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.; Shimada, N.; Miyoshi, H.; Yamauchi, K. Detection of thyroid system-disrupting chemicals using in vitro and in vivo screening assays in Xenopus laevis. Toxicol. Sci. 2005, 88, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, J.; Rylander, L.; Rignell-Hydbom, A.; Lindh, C.H.; Jönsson, B.A.; Giwercman, A. Prenatal phthalate exposure and reproductive function in young men. Environ. Res. 2015, 138, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.B.; Chuang, C.J.; Su, P.H.; Sun, C.W.; Wang, C.J.; Wu, M.T.; Wang, S.L. Prenatal and Childhood Exposure to Phthalate Diesters and Thyroid Function in a 9-Year Follow-up Birth Cohort Study: Taiwan Maternal and Infant Cohort Study. Epidemiology 2017, 28 (Suppl. 1), S10–S18. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.I.; Chen, M.H.; Lien, G.W.; Chen, S.; Lin, J.C.; Fang, C.C.; Chen, P.C. Effects of Gender on the Association of Urinary Phthalate Metabolites with Thyroid Hormones in Children: A Prospective Cohort Study in Taiwan. Int. J. Environ. Res. Public Health 2017, 14, E123. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Ji, M.; Bao, R.; Yu, H.; Wang, Y.; Hou, P.; Zhang, Y.; Shan, Z.; Teng, W.; Xing, M. Association of High Iodine Intake with the T1799A BRAF Mutation in Papillary Thyroid Cancer. J. Clin. Endocrinol. MeTable 2009, 94, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Knobel, M.; Medeiros-Neto, G. Relevance of iodine intake as a reputed predisposing factor for thyroid cancer. Arq. Bras. Endocrinol. Metab. 2007, 51, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Blount, B.C.; Pirkle, J.L.; Osterloh, J.D.; Valentin-Blasini, L.; Caldwell, K.L. Urinary perchlorate and thyroid hormone levels in adolescent and adult men and women living in the United States. Environ. Health Perspect. 2006, 114, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.; Watters, K.F.; Carpenter, A.D.; Ladenson, P.W.; Cooper, D.S.; Ding, E.L. Thyrotropin and Thyroid Cancer Diagnosis: A Systematic Review and Dose-Response Meta-Analysis. J. Clin. Endocrinol. MeTable 2012, 97, 2682–2692. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.; Santisteban, P. TSH-activated signaling pathways in thyroid tumorigenesis. Mol. Cell Endocrinol. 2003, 213, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Golbert, L.; de Cristo, A.P.; Faccin, C.S.; Farenzena, M.; Folgierini, H.; Graudenz, M.S.; Maia, A.L. Serum TSH levels as a predictor of malignancy in thyroid nodules: A prospective study. PLoS ONE 2017, 12, e0188123. [Google Scholar] [CrossRef] [PubMed]
- Fiore, E.; Vitti, P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J. Clin. Endocrinol. MeTab. 2012, 97, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Jang, H.W.; Ahn, H.S.; Ahn, S.; Park, S.Y.; Oh, Y.L.; Hahn, S.Y.; Shin, J.H.; Kim, J.H.; Kim, J.S.; et al. High Serum TSH Level Is Associated with Progression of Papillary Thyroid Microcarcinoma During Active Surveillance. J. Clin. Endocrinol. MeTab. 2018, 103, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Arena, S.; Latina, A.; Baratta, R.; Burgio, G.; Gullo, D.; Benvenga, S. Chronic lymphocytic thyroiditis: could it be influenced by a petrochemical complex? Data from a cytological study in South-Eastern Sicily. Eur. J. Endocrinol. 2015, 172, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Latina, A.; Gullo, D.; Trimarchi, F.; Benvenga, S. Hashimoto’s Thyroiditis: Similar and Dissimilar Characteristics in Neighboring Areas. Possible Implications for the Epidemiology of Thyroid Cancer. PLoS ONE 2013, 8, e55450. [Google Scholar] [CrossRef] [PubMed]
- Haymart, M.R.; Glinberg, S.L.; Liu, J.; Sippel, R.S.; Jaume, J.C.; Chen, H. Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin. Endocrinol. 2009, 71, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, G.L.; Han, X.; Zhu, C.; Kilfoy, B.A.; Zhu, Y.; Boyle, P.; Zheng, T. Do Polybrominated Diphenyl Ethers (PBDEs) Increase the Risk of Thyroid Cancer? Biosci. Hypotheses 2008, 1, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Noyes, P.D.; Stapleton, H.M. Toxicokinetics and thyroid hormone endocrine disruption in fish. Endocr. Disruptors 2014, 2, e29430. [Google Scholar] [CrossRef]
- Morck, A.; Hakk, H.; Orn, U.; Klasson Wehler, E. Decabromodiphenyl ether in the rat: absorption, distribution, metabolism, and excretion. Drug Metab. Dispos. 2003, 31, 900–907. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of Decabromodiphenyl Oxide (CAS No. 1163–19–5) in F344/N Rats and B6C3F1 Mice (Feed Studies); NTP Technical Report 309; Public Health Service, Department of Health and Human Services: Washington, DC, USA, 1986.
- Agency for Toxic Substances and Disease Registry. Toxicological profile for Polybrominated Diphenyl Ethers (PBDEs); U.S. Department of Health and Human Service, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, Georgia. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp207.pdf (accessed on 20 April 2018).
- Fowles, J.R.; Fairbrother, A.; Baecher-Steppan, L.; Kerkvliet, N.I. Immunologic and endocrine effects of the flame-retardant pentabromodiphenyl ether (DE-71) in C57BL/6J mice. Toxicology 1994, 86, 49–61. [Google Scholar] [CrossRef]
- Zhou, T.; Ross, D.G.; DeVito, M.J.; Crofton, K.M. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci. 2001, 61, 76–82. [Google Scholar] [CrossRef]
- Van der Ven, L.T.; van de Kuil, T.; Verhoef, A.; Leonards, P.E.; Slob, W.; Cantón, R.F.; Germer, S.; Hamers, T.; Visser, T.J.; Litens, S.; et al. A 28-day oral dose toxicity study enhanced to detect endocrine effects of a purified technical pentabromodiphenyl ether (pentaBDE) mixture in Wistar rats. Toxicology 2008, 245, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Stoker, T.M.; Laws, S.C.; Crofton, K.M.; Hedge, H.J.; Ferrell, J.M.; Cooper, R.L. Assessment of DE-71, a Commercial Polybrominated Diphenyl Ether (PBDE) Mixture, in the EDSP Male and Female Pubertal Protocols. Toxicol. Sci. 2004, 78, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Ernest, S.R.; Wade, M.G.; Lalancette, C.; Ma, Y.Q.; Berger, R.G.; Robaire, B.; Hales, B.F. Effects of chronic exposure to an environmentally relevant mixture of brominated flame retardants on the reproductive and thyroid, system in adult male rats. Toxicol. Sci. 2012, 127, 496–507. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Wang, A.; Niu, Q.; Guo, L.; Xia, T.; Chen, X. Toxic effect of PBDE-47 on thyroid development, learning, and memory, and the interaction between PBDE-47 and PCB153 that enhances toxicity in rats. Toxicol. Ind. Health 2011, 27, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Mulero, M.; Heredia, L.; Pujol, A.; Domingo, J.L.; Sánchez, D.J. Perinatal exposure to BDE-99 causes learning disorders and decreases serum thyroid hormone levels and BDNF gene expression in hippocampus in rat offspring. Toxicology 2013, 308, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Taylor, M.M.; DeVito, M.J.; Crofton, K.M. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol. Sci. 2002, 66, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.N.; Wanner, A.; Fidalgo-Neto, A.A.; Talsness, C.E.; Koerner, W.; Chahoud, I. Developmental exposure to low-dose PBDE-99: Tissue distribution and thyroid hormone levels. Toxicology 2007, 242, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Bowers, W.J.; Wall, P.M.; Nakai, J.S.; Yagminas, A.; Wade, M.; Li, N. Behavioral and thyroid effects of in utero and lactational exposure of Sprague-Dawley rats to the polybrominated diphenyl ether mixture DE71. Neurotoxicol. Teratol. 2015, 52, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, H.; Woo, G.H.; Inoue, K.; Takahashi, M.; Hirose, M.; Nishikawa, A.; Shibutani, M. Impaired oligodendroglial development by decabromodiphenyl ether in rat offspring after maternal exposure from mid-gestation through lactation. Reprod. Toxicol. 2011, 31, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.H.; Li, M.H.; Tsai, S.S.; Lee, C.W.; Pan, M.H.; Yao, W.J.; Hsu, P.C. Developmental exposure to decabromodiphenyl ether (PBDE 209): Effects on thyroid hormone and hepatic enzyme activity in male mouse offspring. Chemosphere 2008, 70, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Blake, C.A.; McCoy, G.L.; Hui, Y.Y.; LaVoie, H.A. Perinatal exposure to low-dose DE-71 increases serum thyroid hormones and gonadal osteopontin gene expression. Exp. Biol. Med. 2011, 236, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Harden, F.A.; Toms, L.M.; Norman, R.E. Health consequences of exposure to brominated flame retardants: A systematic review. Chemosphere 2014, 106, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, J. Invited commentary: Maternal plasma polybrominated diphenyl ethers and thyroid hormones—Challenges and opportunities. Am. J. Epidemiol. 2013, 178, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Hamers, T.; Kamstra, J.H.; Sonneveld, E.; Murk, A.J.; Visser, T.J.; Van Velzen, M.J.; Brouwer, A.; Bergman, A. Biotransformation of brominated flame retardants into potentially endocrine-disrupting metabolites, with special attention to 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47). Mol. Nutr. Food Res. 2008, 52, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xie, Q.; Li, X.; Li, N.; Chi, P.; Chen, J.; Wang, Z.; Hao, C. Hormone activity of hydroxylated polybrominated diphenyl ethers on human thyroid receptor-β: in vitro and in silico investigations. Environ. Health Perspect. 2010, 118, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Marsh, G.; Bergman, A.; Bladh, L.G.; Gillner, M.; Jakobsson, E. Synthesis of p-hydroxybromodiphenyl ethers and binding to the thyroid receptor. Organohal. Compd. 1998, 37, 305–308. [Google Scholar]
- Meerts, I.A.; Lilienthal, H.; Hoving, S.; Van Den Berg, J.H.; Weijers, B.M.; Bergman, A.; Koeman, J.H.; Brouwer, A. Developmental Exposure to 4-hydroxy-2,3,3′,4′,5-pentachlorobiphenyl (4-OH-CB107): Long-Term Effects on Brain Development, Behavior, and Brain Stem Auditory Evoked Potentials in Rats. Toxicol. Sci. 2004, 82, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Hurley, S.; Reynolds, P.; Goldberg, D.; Nelson, D.O.; Jeffrey, S.S.; Petreas, M. Adipose levels of polybrominated diphenyl ethers and risk of breast cancer. Breast Cancer Res. Treat. 2011, 129, 505–511. [Google Scholar] [CrossRef] [PubMed]
- El Majidi, N.; Bouchard, M.; Carrier, G. Systematic analysis of the relationship between standardized biological levels of polychlorinated biphenyls and thyroid function in pregnant women and newborns. Chemosphere 2014, 98, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Noyes, P.D.; Hinton, D.E.; Stapleton, H.M. Accumulation and debromination of decabromodiphenyl ether (BDE-209) in juvenile fathead minnows (Pimephales promelas) induces thyroid disruption and liver alterations. Toxicol. Sci. 2011, 122, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Marelli, F.; Persani, L. How zebrafish research has helped in understanding thyroid diseases. F1000Research 2017, 6, 2137. [Google Scholar] [CrossRef] [PubMed]
- Anelli, V.; Villefranc, J.A.; Chhangawala, S.; Martinez-McFaline, R.; Riva, E.; Nguyen, A.; Verma, A.; Bareja, R.; Chen, Z.; Scognamiglio, T.; et al. Oncogenic BRAF disrupts thyroid morphogenesis and function via twist expression. Elife 2017, 6, e20728. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, Y.; Zhang, S.; Song, N.; Xu, H.; Dang, Y.; Liu, C.; Giesy, J.P.; Yu, H. Prenatal transfer of decabromodiphenyl ether (BDE-209) results in disruption of the thyroid system and developmental toxicity in zebrafish offspring. Aquat Toxicol. 2017, 190, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yu, L.; Yang, L.; Zhou, B. Bioconcentration and metabolism of decabromodiphenyl ether (BDE-209) result in thyroid endocrine disruption in zebrafish larvae. Aquat Toxicol. 2012, 110, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Deng, J.; Shi, X.; Liu, C.; Yu, K.; Zhou, B. Exposure to DE-71 alters thyroid hormone levels and gene transcription in the hypothalamic-pituitary-thyroid axis of zebrafish larvae. Aquat. Toxicol. 2010, 97, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Kodavanti, P.R.; Coburn, C.G.; Moser, V.C.; MacPhail, R.C.; Fenton, S.E.; Stoker, T.E.; Rayner, J.L.; Kannan, K.; Birnbaum, L.S. Developmental exposure to a commercial PBDE mixture, DE-71: Neurobehavioral, hormonal, and reproductive effects. Toxicol. Sci. 2010, 116, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, T.H.; Choi, J.S.; Nabanata, P.; Kim, N.Y.; Ahn, M.Y.; Jung, K.K.; Kang, I.H.; Kim, T.S.; Kwack, S.J.; et al. Evaluation of liver and thyroid toxicity in Sprague-Dawley rats after exposure to polybrominated diphenyl ether BDE-209. J. Toxicol. Sci. 2010, 35, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.J.; Schramm, K.W.; Darnerud, P.O.; Aune, M.; Feicht, E.A.; Fried, K.; Henkelmann, B.; Lenoir, D.; Schmid, P.; McDonald, T.A. Terminal elimination half-lives (T1/2H) of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohal. Compd. 2004, 66, 3867–3871. [Google Scholar]
- Von Meyerinck, L.; Hufnagel, B.; Schmoldt, A.; Benthe, H.F. Induction of rat liver microsomal cytochrome P-450 by the pentabromo diphenyl ether Bromkal 70 and half-lives of its components in the adipose tissue. Toxicology 1990, 61, 259–274. [Google Scholar] [CrossRef]
- Allen, J.G.; Stapleton, H.M.; Vallarino, J.; McNeely, E.; McClean, M.D.; Harrad, S.J.; Rauert, C.B.; Spengler, J.D. Exposure to flame retardant chemicals on commercial airplanes. Environ. Health 2013, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, H.M.; Kelly, S.M.; Allen, J.G.; Mcclean, M.D.; Webster, T.F. Measurement of polybrominated diphenyl ethers on hand wipes: estimating exposure from hand-to-mouth contact. Environ. Sci. Technol. 2008, 42, 3329–3334. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Restrepo, B.; Kannan, K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere 2009, 76, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Thomas, G.O.; Jones, K.C.; Qu, W.; Sheng, G.; Martin, F.L.; Fu, J. Exposure of electronics dismantling workers to polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in South China. Environ. Sci. Technol. 2007, 41, 5647–5653. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, M.; Jin, J.; Wang, Y.; Bu, Y.; Xu, M.; Yang, X.; Liu, A. Concentrations and trends of halogenated flame retardants in the pooled serum of residents of Laizhou Bay, China. Environ. Toxicol. Chem. 2013, 32, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, H.M.; Eagle, S.; Sjödin, A.; Webster, T.F. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ. Health Perspect. 2012, 120, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Turyk, M.E.; Persky, V.W.; Imm, P.; Knobeloch, L.; Chatterton, R.; Anderson, H.A. Hormone disruption by PBDEs in adult male sport fish consumers. Environ. Health Perspect. 2008, 116, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, H.M.; Eagle, S.; Anthopolos, R.; Wolkin, A.; Miranda, M.L. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ. Health Perspect. 2011, 119, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Johnson, P.I.; Camann, D.; Hauser, R. Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in men. Sci. Total Environ. 2009, 407, 3425–3429. [Google Scholar] [CrossRef] [PubMed]
- Zota, A.R.; Park, J.S.; Wang, Y.; Petreas, M.; Zoeller, R.T.; Woodruff, T.J. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ. Sci. Technol. 2011, 45, 7896–7905. [Google Scholar] [CrossRef] [PubMed]
- Abdelouahab, N.; Langlois, M.F.; Lavoie, L.; Corbin, F.; Pasquier, J.C.; Takser, L. Maternal and cord-blood thyroid hormone levels and exposure to polybrominated diphenyl ethers and polychlorinated biphenyls during early pregnancy. Am. J. Epidemiol. 2013, 178, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Makey, C.M.; McClean, M.D.; Braverman, L.E.; Pearce, E.N.; He, X.M.; Sjödin, A.; Weinberg, J.M.; Webster, T.F. Polybrominated Diphenyl Ether Exposure and Thyroid Function Tests in North American Adults. Environ. Health Perspect. 2016, 124, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Bloom, M.; Spliethoff, H.; Vena, J.; Shaver, S.; Addink, R.; Eadon, G. Environmental exposure to PBDEs and thyroid function among New York anglers. Environ. Toxicol. Pharmacol. 2008, 25, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, J.; Harley, K.G.; Bradman, A.; Gharbi, M.; Sjödin, A.; Eskenazi, B. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ. Health Perspect. 2010, 118, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Herbstman, J.B.; Sjödin, A.; Apelberg, B.J.; Witter, F.R.; Halden, R.U.; Patterson, D.G.; Panny, S.R.; Needham, L.L.; Goldman, L.R. Birth delivery mode modifies the associations between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ. Health Perspect. 2008, 116, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Yu, J.; Chen, L.; Wang, C.; Zhou, Y.; Hu, Y.; Shi, R.; Zhang, Y.; Cui, C.; Gao, Y.; et al. Polybrominated diphenyl ethers (PBDEs) and thyroid hormones in cord blood. Environ. Pollut. 2017, 229, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Stagnaro-Green, A.; Abalovich, M.; Alexander, E.; Azizi, F.; Mestman, J.; Negro, R.; Nixon, A.; Pearce, E.N.; Soldin, O.P.; Sullivan, S.; et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011, 21, 1081–1125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, H.; Li, J.; Shan, Z.; Teng, W.; Teng, X. The Correlation between Polybrominated Diphenyl Ethers (PBDEs) and Thyroid Hormones in the General Population: A Meta-Analysis. PLoS ONE 2015, 10, e0126989. [Google Scholar] [CrossRef] [PubMed]
- Almstrup, K.; Fernández, M.F.; Petersen, J.H.; Olea, N.; Skakkebaek, N.E.; Leffers, H. Dual effects of phytoestrogens result in U-shaped dose–response curves. Environ Health Perspect. 2002, 110, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Ahn, N.S.; Hu, H.; Park, J.S.; Park, J.S.; Kim, J.S.; An, S.; Kong, G.; Aruoma, O.I.; Lee, Y.S.; Kang, K.S. Molecular mechanisms of the 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced inverted U-shaped dose responsiveness in anchorage independent growth and cell proliferation of human breast epithelial cells with stem cell characteristics. Mutat. Res. 2005, 579, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, J.; Harley, K.G.; Bradman, A.; Sjödin, A.; Eskenazi, B. Prenatal exposure to polybrominated diphenyl ether flame retardants and neonatal thyroid-stimulating hormone levels in the CHAMACOS study. Am. J. Epidemiol. 2011, 174, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Hardell, L.; Bavel, B.; Lindström, G.; Eriksson, M.; Carlberg, M. In utero exposure to persistent organic pollutants in relation to testicular cancer risk. Int. J. Androl. 2006, 29, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Hardell, K.; Carlberg, M.; Hardell, L.; Björnfoth, H.; Ericson Jogsten, I.; Eriksson, M.; Van Bavel, B.; Lindström, G. Concentrations of organohalogen compounds and titres of antibodies to Epstein-Barr virus antigens and the risk for non-Hodgkin lymphoma. Oncol. Rep. 2009, 21, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Aschebrook-Kilfoy, B.; DellaValle, C.T.; Purdue, M.; Kim, C.; Zhang, Y.; Sjodin, A.; Ward, M.H. Polybrominated diphenyl ethers and thyroid cancer risk in the Prostate, Colorectal, Lung, and Ovarian Cancer Screening Trial cohort. Am. J. Epidemiol. 2015, 181, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K.; Lorenzo, A.; Butt, C.M.; Hammel, S.C.; Henderson, B.B.; Roman, S.A.; Scheri, R.P.; Stapleton, H.M.; Sosa, J.A. Exposure to flame retardant chemicals and occurrence and severity of papillary thyroid cancer: A case-control study. Environ. Int. 2017, 107, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, G.; Li, J.; Zhao, H.; Wang, Y.; Chen, J.; Zhao, H. Association of polybrominated diphenylethers (PBDEs) and hydroxylated metabolites (OH-PBDEs) serum levels with thyroid function in thyroid cancer patients. Environ. Res. 2017, 159, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, M.M.; Heusinkveld, H.J.; Bergman, A.; van den Berg, M.; Westerink, R.H. Bromination pattern of hydroxylated metabolites of BDE-47 affects their potency to release calcium from intracellular stores in PC12 cells. Environ. Health Perspect. 2010, 118, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Meerts, I.A.; van Zanden, J.J.; Luijks, E.A.; van Leeuwen-Bol, I.; Marsh, G.; Jakobsson, E.; Bergman, A.; Brouwer, A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol. Sci. 2000, 56, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.J.; McClean, M.D.; Fraser, A.J.; Weinberg, J.; Stapleton, H.M.; Sjödin, A.; Webster, T.F. Exposure to PBDEs in the office environment: Evaluating the relationships between dust, handwipes, and serum. Environ. Health Perspect. 2011, 119, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Kusky, T.M. 2003 Volcanic eruptions. In Geological Hazards: A Source Book; Kusky, T.M., Ed.; Greenwood Publishing Group: Westport, CT, USA, 2003; pp. 49–74. [Google Scholar]
- Vigneri, R.; Malandrino, P.; Gianì, F.; Russo, M.; Vigneri, P. Heavy metals in the volcanic environment and thyroid cancer. Mol. Cell. Endocrinol. 2017, 457, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Zona, A.; Beccaloni, E.; Carere, M.; Comba, P. Incidence of Breast, Prostate, Testicular, and Thyroid Cancer in Italian Contaminated Sites with Presence of Substances with Endocrine Disrupting Properties. Int. J. Environ. Res. Public Health 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Species | Dose/Duration | Effects Observed | Reference |
|---|---|---|---|---|
| DE-71 | Female C57BL/6 mice | Acute single doses: 0, 0.8, 4.0, 20, 100, 500 mg/kg by diet; 1 day Subchronic daily doses: 0, 250, 500, or 1000 mg/kg/day by diet; 14 days | Lower TT4 levels except at 100 mg/kg dose Decrease of TT4 and FT4 levels in a dose-dependent manner. | [84] |
| DE-71, DE-79, DE-83R | Female Long-Evans rats | 0, 0.3, 1, 3, 10, 30, 60, 100, or 300 mg/kg/day by diet 14 days | Dose-dependent depletion of TT4 following exposures of DE-71 and DE-79 TT4 was decreased a maximum of 80% for DE-71 for the 300 mg/kg dose and 70% for DE-79 for 100 mg/kg dose TT3 was decreased of 30% for the 300 mg/kg dose and 25% for DE-79 for 100 mg/kg dose | [85] |
| DE-71 | Primiparous Long-Evans rats | 0, 1, 10, or 30 mg/kg/day by diet; GD6-PND22 | Reduction of TT4 in dose-dependent manner in fetuses on GD20 Reduction of TT4 in GD20 and GD22 dams exposed at 30 mg/kg/day On PND 4 and PND 14 significant dose-dependent decreases of TT4 at 10 and 30 mg/kg/day doses No significant effects on TT3 in either the dams or offspring. | [91] |
| DE-71 | Wistar rats (RIVM/Cpb:WU) of both sexes | 0, 0.27, 0.82, 2.47, 7.4, 22.2, 66.7 or 200 mg/kg/day by diet 5 days | Lower TT4 levels No effects on circulating TT3 | [86] |
| BDE-99 | Wistar rats | 0.06 or 0.3 mg/kg by diet GD6 | Lower T4 and T3 levels in exposed dams at 0.3 mg/kg. Lower T4 levels in male and female offspring on PND 22 at 0.3 mg/kg dose. Lower FT4 levels in female pups on PND22 (0.06 mg/kg) | [92] |
| DE-71 | Male and female Wistar rats | 0, 3, 30, or 60 mg/kg/day by diet PND 23–53 and 23–28 in males; PND 22–41 and 21–26 in females | Lower TT4 levels in the 30 and 60 mg/kg dose groups following the 5-day and 21-day exposures in females. Lower TT4 levels at 3, 30, and 60 mg/kg doses in 31-day exposed males. Lower TT3 and higher TSH levels in 30 and 60 mg/kg doses in the 31-day exposed males. | [87] |
| DE-71 | Pregnant Long-Evans rats | 0, 1.7, 10.2, or 30.6 mg/kg/day by diet GD6-PND21 | In dams, lower TT4 levels in the 10.2 and 30.6 mg/kg dose groups In both male and female offspring, age-dependent decrease in TT4 levels at 30.6 mg/kg/day dose No significant effects for maternal TT3 levels in dams and offspring | [111] |
| BDE-47 | Pregnant Sprague–Dawley rats | BDE-47 (0, 1, 5, 10 mg/kg) and/or PCB153 (5 mg/kg) by diet PND10 | Lower T4 levels in the 5 mg/kg BDE-47 + 5 mg/g PCB153 group compared to the 5 mg/kg BDE-47 group Lower T4 levels in the 10 mg/kg BDE-47 + 5 mg/kg PCB153 group compared to the 5 mg/kg BDE-47 group No significant alterations for T3 and TSH levels | [89] |
| 52.1% DE-71, 0.4% DE-79, 44.2% decaBDE-209, | Adult male Sprague Dawley rats | 0, 0.02, 0.2, 2, or 20 mg/kg/day by diet 70 days | Lower T4 levels in the 20 mg/kg dose group No significant effects on TSH levels | [88] |
| BDE-99 | Male and female Sprague Dawley rat | 0, 1 or 2 mg/kg/day by diet GD6-PND21 | Lower FT4, T4 and T3 levels in the 2 mg/kg dose group | [90] |
| DE-71 | Pregnant Sprague–Dawley rats | 0, 0.3, 3.0 or 30 mg/kg/day by diet GD1-PND21 | Lower T3 and T4 levels in dams only in the 30 mg/kg dose group. In male and female pups 3.0 and 30 mg/kg doses decreased T4 and TSH levels at PND21 In male and female pups only 30 mg/kg doses decreased T3 levels | [93] |
| DE-71 | Male and female rats (CD®IGS) | 0.06 mg/kg/day by diet GD1.5-PND20 (except the day of parturition) | Greater TT4 and TT3 levels in pregnant F1 offspring (GD14.5). No significant effects on TT3 and TT4 levels in the F0 mothers and pups | [96] |
| BDE-209 | Pregnant Sprague–Dawley rats | 0, 10, 100, 1000 mg/kg/day by diet PND10-PND42 | Lower T3 and T4 levels in male offspring at the highest dose | [94] |
| BDE-209 | Adult male Sprague Dawley rats | 0, 100, 300, 600 mg/kg/day by diet PND10-PND42 | Lower T3 levels only in the 300 and 600 mg/kg BDE209 groups. Higher TSH levels in the 300 and 600 mg/kg dose groups | [112] |
| BDE-209 | Adult male and female CD-1 mice | 0, 10, 500, or 1500 mg/kg/day by diet GD0-GD17 | No significant changes in T4 levels in male offspring. Significant reduction of T3 levels 20.6% for 10 mg/kg and 20.7% for 1500 mg/kg group) in male offspring at PND71 | [95] |
| Study Design | Country | Study Sample | Sample Size (N) | Age (Years) | Main Results | Exposure Assessment Matrix/Chemical Concentration | Confounders | Reference |
|---|---|---|---|---|---|---|---|---|
| Prospective cohort | USA | Great Lakes anglers (non-Hispanic White) | 36 | 29–45 | No statistically significant associations among nine PBDE congeners or their sum, and either TSH or FT4 | Serum blood BDE-47: median 7.9 ng/g lipid Sum PBDE: median 15 ng/g lipid LOD range: 0.002–0.028 ng/g lipid | (1), (14), physician-diagnosed goiter or thyroid condition, use of thyroid-active pharmaceuticals at the time of blood donation, having ever worked with or near plastics | [127] |
| Retrospective cohort | USA | Pregnant women. Blood samples collected at 27.3 ± 3.1 weeks’ gestation | 270 | 18–45 | None of the five PBDE congeners or their sum significantly associated with FT4 and TT4 concentrations. All PBDE congeners (BDE-28, 47, 99, 100, 153) significantly inversely associated with TSH (10.9–18.7% decrease in TSH for a 10-fold increase in serum concentration of individual congeners). | Serum blood Sum PBDE: GM 26.5 ng/g lipid, median 25.2 ng/g lipid LOD range: 0.2–1.6 ng/g lipid | (1), (2), (3), (4), (5), (6), (7), (8), (9) at the time of blood collection, (14), drug consumption during pregnancy, blood lead, serum PCB, organochlorine pesticide concentrations | [128] |
| Retrospective cohort | USA | Pregnant women. Blood samples collected at 27 3 ± 3 weeks’ weeks’ gestation (n = 209) and at 40 ± 2 weeks’ gestation (n=80). TSH levels measured in infants within 24 h after birth | 289 | 18–45 | No statistically significant associations between maternal total serum PBDE concentrations and neonatal TSH levels | Serum blood Sum PBDE: GM 28,0 ng/g lipid, median 25.4 ng/g lipid LOD range: 0.2–2.6 ng/g lipid | (1), (2), (3), (6), (7), (9), (10), (11),(12), (13) duration of residence in US, serum levels of total PCBs, HCB, DDT, and DDE | [135] |
| Cross-sectional | USA | Women with singleton deliveries. Umbilical cord blood collected at delivery | 92 | 14–43 | For infants born by spontaneous, vaginal, unassisted deliveries, BDE-47 significantly correlated with increased TSH levels in cord blood. PBDEs showed a negative association (not statically significant) with FT4 and TT4. | Serum blood BDE-47: mean 14.4 ng/g lipid, median 13.8 ng/g lipid Median LOD: 1.3 ng/g lipid | (1), (2), (3), (4), (8), (9), (10), (13), maternal socioeconomic status; history of STDs, hypertension, diabetes, and anemia | [129] |
| Prospective cohort | USA | Great Lakes fish consumers (adult males) | 308 | 30–59 | ΣPBDEs significantly and positively associated with TT4, FT4, urinary T4, rT3, and albumin-bound T4, and was negatively associated with TSH and TT3 Similar results for BDE-47, the dominant PBDE congener ΣPBDEs positively related to the percentage of T4 bound to albumin and inversely related to the percentage of T4 bound to TBG | Serum blood Sum PBDE: GM 27.7 g/g lipid, median 38 ng/g lipid LOD range: 0.025-0.15 ng/g lipid | (1), (4), (5), (14), medication use, Great Lakes sport fish meals in the past year, sport fish meals in the past year, ΣPCBs, DDE, years consuming sport fish meals, years consuming Great Lakes sport fish meals, HA1c level, levels of testosterone, SHBG, and SHBG-bound testosterone | [121] |
| Prospective cohort | USA | Pregnant women (80% non-Hispanic black) Blood samples collected at >34 weeks’ gestation | 137 | 18–39 | TT4 positively and significantly correlated with BDE-47, 99, 100, and ΣPBDEs FT4 positively and significantly associated with BDE-47, 153, and ΣPBDEs No significant association between TSH, TT3 or FT3 and PBDEs | Serum blood: BDE-47: GM 16.5 ng/g lipid | (1), (2), (4), (8), (9) | [122] |
| Cross-sectional | USA | Pregnant women. Blood samples collected prior to second trimester pregnancy termination | 25 | 16–45 | Positive significant association between ΣPBDE5 and TSH levels Slightly negative association between BDE-28 and FT4 Individual OH-PBDEs and their sum positively associated with TSH Relationships between OH-PBDEs and TT4 and FT4 null except for 6-OH-BDE-47 (not significant inverse association) | Serum blood: Sum PBDE: GM 85.8 ng/g lipids; BDE-47: GM 47.1 ng/g lipid | (1), (2), (8), type of health insurance | [124] |
| Prospective cohort | USA | Men from an infertility clinic. Blood and house dust samples | 24 | 18–54 | Positive association of PBDEs with FT4. | House dust: BDE-47: GM 577 ng/g BDE-99: GM 809 ng/g BDE-100:GM 220 ng/g LOD: 83 ng/g | (1), (14) | [123] |
| Prospective cohort | Canada | Pregnant women. Blood samples collected at <20 weeks’ gestation for analysis of PBDEs and THs. Maternal blood and umbilical cord blood collected at delivery for TH analyses | 387 | 17–40 | At <20 weeks’ gestation TT4 and TT3 were negatively related to BDE-47, BDE-99, and ΣPBDE. Serum TSH was not related to PBDEs. A positive relationship was observed between FT4 and PBDE-47, PBDE-99, and ΣPBDE and between FT3 and PBDE-99 and ΣPBDE At delivery, maternal TT4 decreased in relation to BDE-99. A negative association was observed between FT3 and BDE-47. No relationships were observed between TT3, TSH and PBDE congeners. In umbilical-cord blood, TT4 and FT4 levels decreased in relation to BDE-47, BDE-99, and ΣPBDE | Serum blood Sum PBDE: median 30.92 ng/g lipid BDE-47: median 21.47 ng/g lipid | (1), (4), (5), (9), (13), (14), (15), (16), blood selenium, blood mercury, medication use, familial history of hypothyroidism, and occupational and recreational exposures to chemicals | (130] |
| Prospective cohort | USA | 26 male and 25 female adult office workers Serum samples at approximately six-month intervals from January 2010 to May 2011. Urinary samples | 51 | 20–≥60 | Significant, inverse associations between PBDEs (BDE-28, 47, 99, 100, 153) and serum TT4. Associations of PBDEs with TSH positive but small and not statistically significant. Any important associations between PBDEs and FT4 or TT3. | Serum blood Sum PBDE: Sample 1 GM 22 ng/g lipid; sample 2 GM 23 ng/g lipid; sample 3 GM 19 ng/g lipid LOD range: 0.2–0.8 ng/g lipid | (1), (14), (15), (16), sex, oral contraceptives, urinary perchlorate, urinary thiocyanate, urinary specific gravity | (131] |
| Prospective cohort | China | Pregnant women. Cord blood samples collected immediately post-delivery | 123 | ≤25–≥35 | BDE-99 and Σ4PBDEs (the sum of BDE-47, 99, 100, and 153) were associated with increased TT4 levels. | Cord blood (n = 106): BDE-47: GM 4.34 ng/g lipid, median 3.96 ng/g lipid BDE-99: GM 9.90 ng/g lipid, median 15.85 ng/g lipid | (1), (4), (8), (9) (10), (14) | (132] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorini, F.; Iervasi, G.; Coi, A.; Pitto, L.; Bianchi, F. The Role of Polybrominated Diphenyl Ethers in Thyroid Carcinogenesis: Is It a Weak Hypothesis or a Hidden Reality? From Facts to New Perspectives. Int. J. Environ. Res. Public Health 2018, 15, 1834. https://doi.org/10.3390/ijerph15091834
Gorini F, Iervasi G, Coi A, Pitto L, Bianchi F. The Role of Polybrominated Diphenyl Ethers in Thyroid Carcinogenesis: Is It a Weak Hypothesis or a Hidden Reality? From Facts to New Perspectives. International Journal of Environmental Research and Public Health. 2018; 15(9):1834. https://doi.org/10.3390/ijerph15091834
Chicago/Turabian StyleGorini, Francesca, Giorgio Iervasi, Alessio Coi, Letizia Pitto, and Fabrizio Bianchi. 2018. "The Role of Polybrominated Diphenyl Ethers in Thyroid Carcinogenesis: Is It a Weak Hypothesis or a Hidden Reality? From Facts to New Perspectives" International Journal of Environmental Research and Public Health 15, no. 9: 1834. https://doi.org/10.3390/ijerph15091834
APA StyleGorini, F., Iervasi, G., Coi, A., Pitto, L., & Bianchi, F. (2018). The Role of Polybrominated Diphenyl Ethers in Thyroid Carcinogenesis: Is It a Weak Hypothesis or a Hidden Reality? From Facts to New Perspectives. International Journal of Environmental Research and Public Health, 15(9), 1834. https://doi.org/10.3390/ijerph15091834








