6 Hz Active Anticonvulsant Fluorinated N-Benzamide Enaminones and Their Inhibitory Neuronal Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.2. Biological Testing/Anticonvulsant Activity
2.3. Electrophysiology Studies—Slice Preparation
2.3.1. Electrophysiological Recording and Data Acquisition on the Slide Preparation
2.3.2. Chemicals and Solutions for MOB Recordings
2.4. ND7/23 Cell Culture Preparation
2.5. Electrophysiological Recording and Data Acquisition on Cell Cultures
3. Results
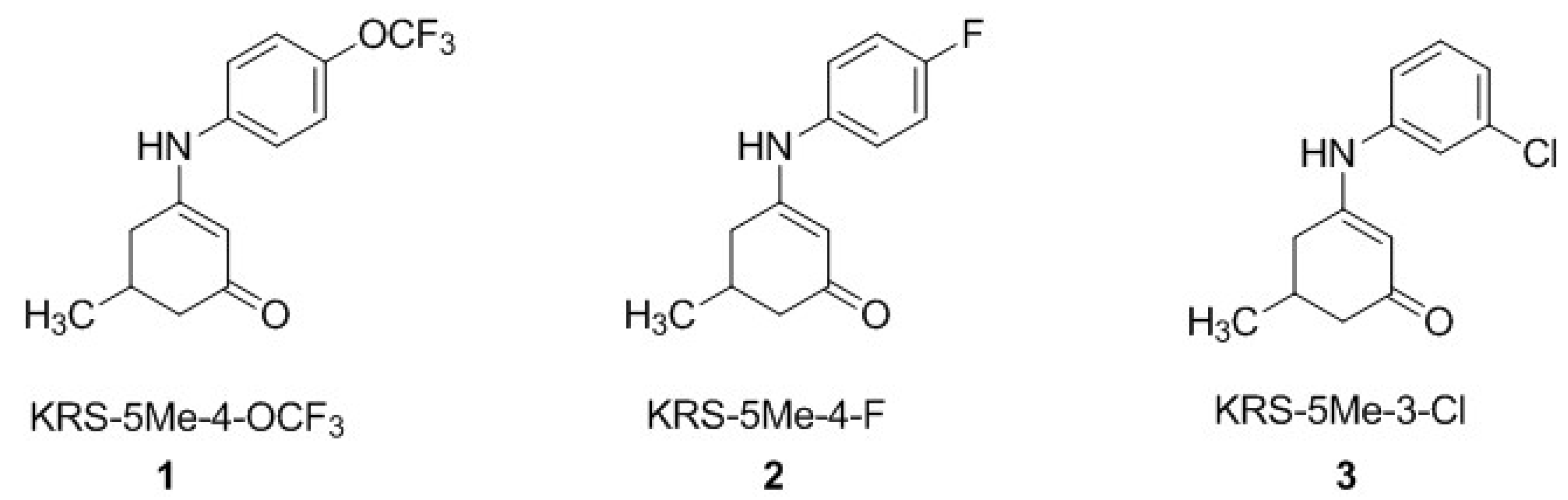
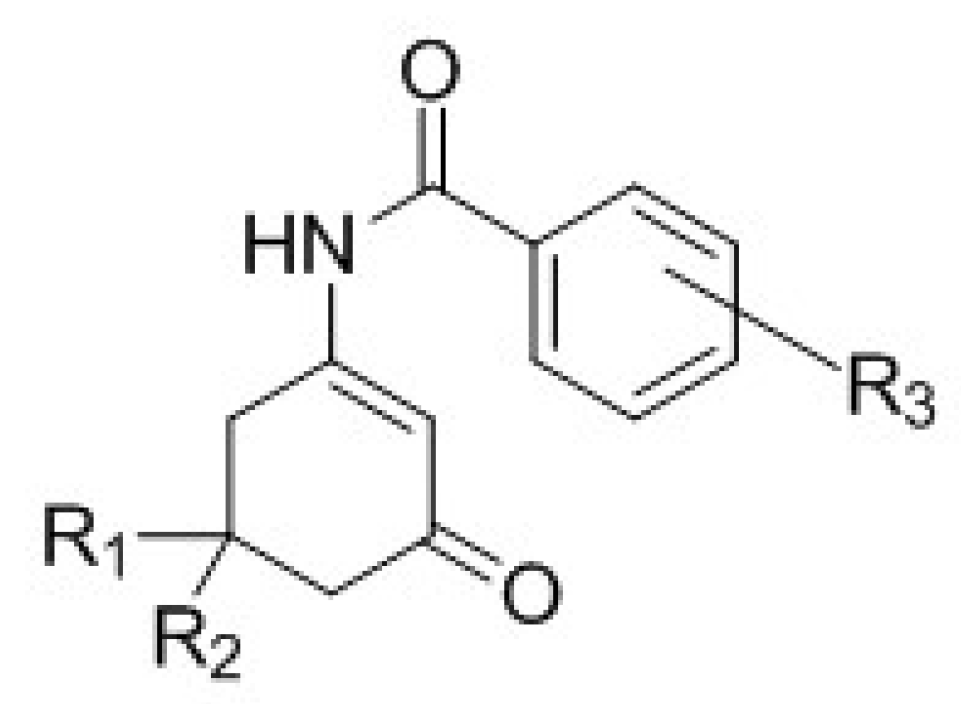
3.1. Chemistry
3.2. Pharmacology
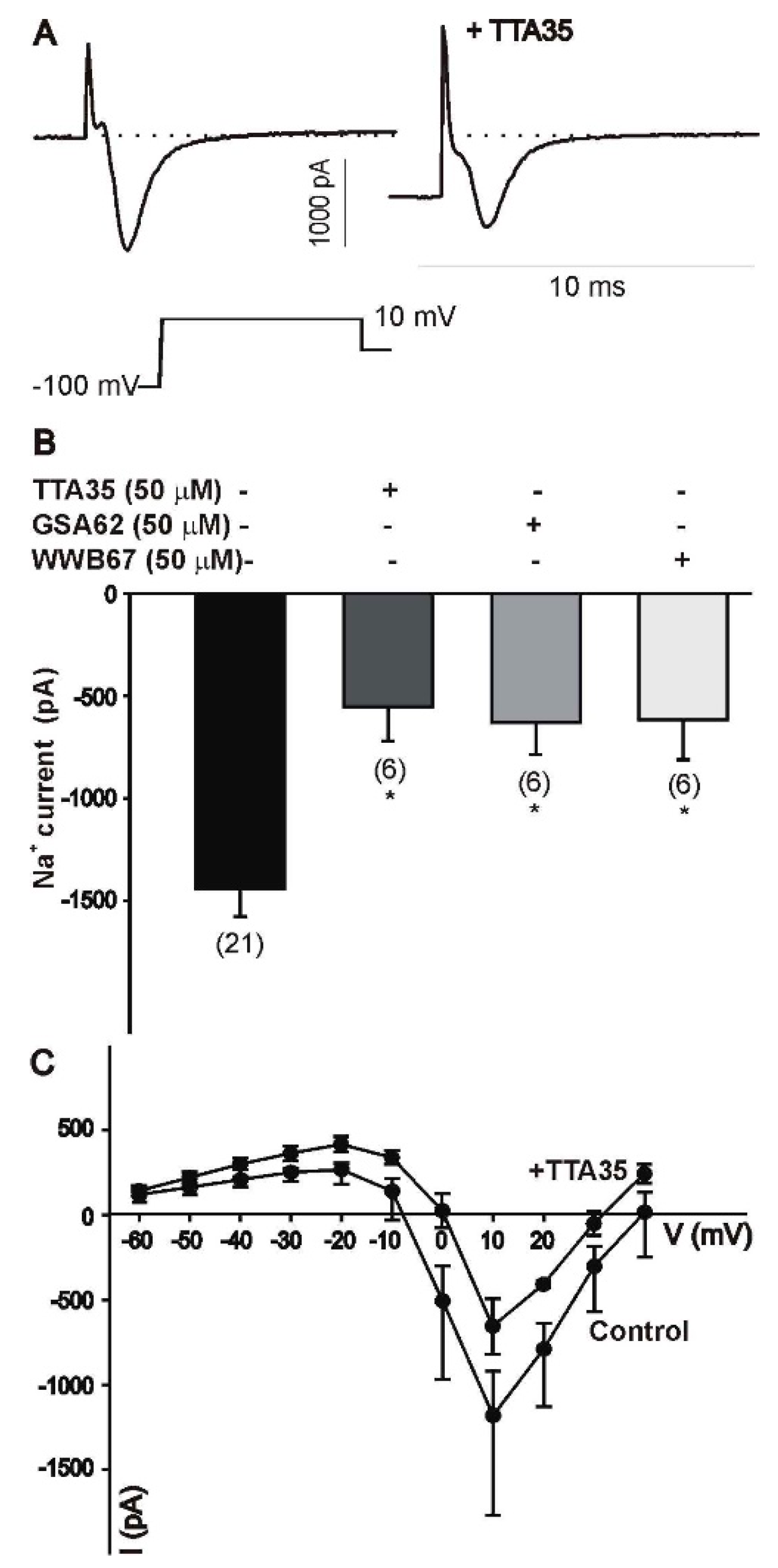
3.3. Cell Physiological Effects of Novel Enaminones
3.3.1. Mitral Cells
3.3.2. Cultured Cells
4. Discussion
4.1. Chemistry and Pharmacology
4.2. Physiology
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Citizens United for Research Epilepsy. What is Epilepsy? Available online: http://www.cureepilepsy.org/aboutepilepsy/facts.asp (accessed on 15 July 2017).
- Salome, C.; Salome-Grosjean, E.; Park, K.; Marieux, P.; Swendiman, E.D.; Stables, J.; Kohn, H. Synthesis and anticonvulsant activities of (R)-N-(4′-substituted) benzyl 2-acetamido-3-methoxypropionamides. J. Med. Chem. 2010, 53, 1288–1305. [Google Scholar] [CrossRef] [PubMed]
- French, J.A.; Staley, B.A. AED treatment through different ages: As our brains change, should our drug choices also? Epilepsy Curr. 2012, 12, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Laxer, K.D.; Trinka, E.; Hirsh, L.J.; Cendes, F.; Langfitt, J.; Delanty, N.; Resnick, T.; Benbadis, S.R. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014, 37, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Hayat, M.J.; Zhang, R.; Hirsch, L.J.; Bazil, C.W.; Mendiratta, A.; Kato, K.; Javed, A.; Legge, A.W.; Buchsbaum, R.; et al. Drug-resistant epilepsy in adults: Outcome trajectories after failure of two medications. Epilepsia 2016, 57, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.M.; Harkless, J.; Butcher, J.R.; Scott, K.R.; Jackson-Ayotunde, L.P. Enaminones 11. An examination of some ethyl ester enaminone derivatives as anticonvulsant agents. Bioorg. Med. Chem. 2013, 21, 3272–3279. [Google Scholar] [CrossRef] [PubMed]
- Heinbockel, T.; Wang, Z.; Jackson-Ayotunde, P.L. Allosteric modulation of GABAA receptors by an anilino enaminone in an olfactory center of the mouse brain. Pharmaceuticals 2014, 7, 1069–1090. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, L.; Jackson, P.L.; Scott, R.K.; Heinbockel, T. A substituted anilino enaminone acts as a novel positive allosteric modulator of GABAA receptors in the mouse brain. J. Pharmacol. Exp. Ther. 2011, 336, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.L.; Hanson, C.D.; Farrell, A.K.; Raymond, B.J.; Stables, J.P.; Eddington, N.D.; Scott, K.R. Enaminones 12. An explanation of anticonvulsant activity and toxicity per Linus Pauling’s clathrate hypothesis. Eur. J. Med. Chem. 2012, 51, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.J.; Nicholson, J.M.; Bakare, O.; Butcher, R.J.; Wilson, T.L.; Scott, K.R. Enaminones 9. Further studies on the anticonvulsant activity and potential type IV phosphodiesterase inhibitory activity of substituted vinylic benzamides. Bioorg. Med. Chem. 2006, 14, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.L.; Scott, K.R.; Southerland, W.M.; Fang, Y. Enaminones 8. CoMFA and CoMSIA studies on some anticonvulsant enaminones. Bioorg. Med. Chem. 2009, 17, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Edafiogho, I.O.; Kombian, S.B.; Anathalaskshmi, K.V.V.; Salama, N.N.; Eddington, N.D.; Wilson, T.L.; Alexander, M.S.; Jackson, P.L.; Hanson, C.D.; Scott, K.R. Enaminones: Exploring additional therapeutic activities. J. Pharm. Sci. 2007, 96, 2509–2531. [Google Scholar] [CrossRef] [PubMed]
- Kehne, J.H.; Klein, B.D.; Raeissi, S.; Sharma, S. The National Institute of Neurological Disorders and Stroke (NINDS) Epilepsy Therapy Screening Program (ETSP). Neurochem. Res. 2017, 42, 1894–1903. [Google Scholar] [CrossRef]
- National Institute of Neurological Disorders and Stroke. The 6 Hz “Psychomotor” Seizure Test. Available online: https://panache.ninds.nih.gov/m_6hztest.aspx (accessed on 6 June 2016).
- Anderson, A.J.; Nicholson, J.M.; Bakare, O.; Butcher, R.J.; Scott, K.R. A base-catalyzed solution-phase parallel synthesis of substituted vinylic benzamide from 3-amino-2-cyclohexanones. J. Comb. Chem. 2004, 6, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Stables, J.P.; Kupferberg, H.J. The NIH Anticonvulsant Drug Development (ADD) Program: Preclinical anticonvulsant screening. In Molecular and Cellular Targets for Anti-Epileptic Drugs; Avanzini, G., Tanganelli, P., Avoli, M., Eds.; John Libbey: London, UK, 1997; p. 191. [Google Scholar]
- White, H.S.; Bender, A.S.; Swinyard, E.A. Effect of the selective N-methyl-d-aspartate receptor agonist 3-(2-carboxypiperazin-4-yl) propyl-1-phosphonic acid on [3H] flunitrazepam binding. Eur. J. Pharmacol. 1988, 147, 149–151. [Google Scholar] [CrossRef]
- Barton, M.E.; Klein, B.D.; Wolf, H.H.; White, H.S. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001, 47, 217–227. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Hsia, S.C.; Martin-Caraballo, M. Regulation of T-type Ca2+ channel expression by herpes simplex virus-1 infection in sensory-like ND7 cells. J. Neurovirol. 2017, 23, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Friary, R.J.; Gilligan, J.M.; Szajewski, R.P.; Falci, K.J.; Franck, R.W. Heterocyclic syntheses via the intramolecular acylation of enamines derived from amino acids. J. Org. Chem. 1973, 38, 3487–3490. [Google Scholar] [CrossRef]
- Ennis, M.; Hamilton, K.A.; Hayar, A. Neurochemistry of the main olfactory system. In Handbook of Neurochemistry and Molecular Neurobiology, 3rd ed.; Lajtha, A., Johnson, D.A., Eds.; Springer: Heidelberg, Germany, 2007; Volume 20, pp. 137–204. [Google Scholar]
- Shepherd, G.W.; Chen, W.R.; Greer, C.A. Olfactory bulb. In The Synaptic Organization of the Brain; Shepherd, G.M., Ed.; Oxford University Press: New York, NY, USA, 2004; pp. 165–216. [Google Scholar]
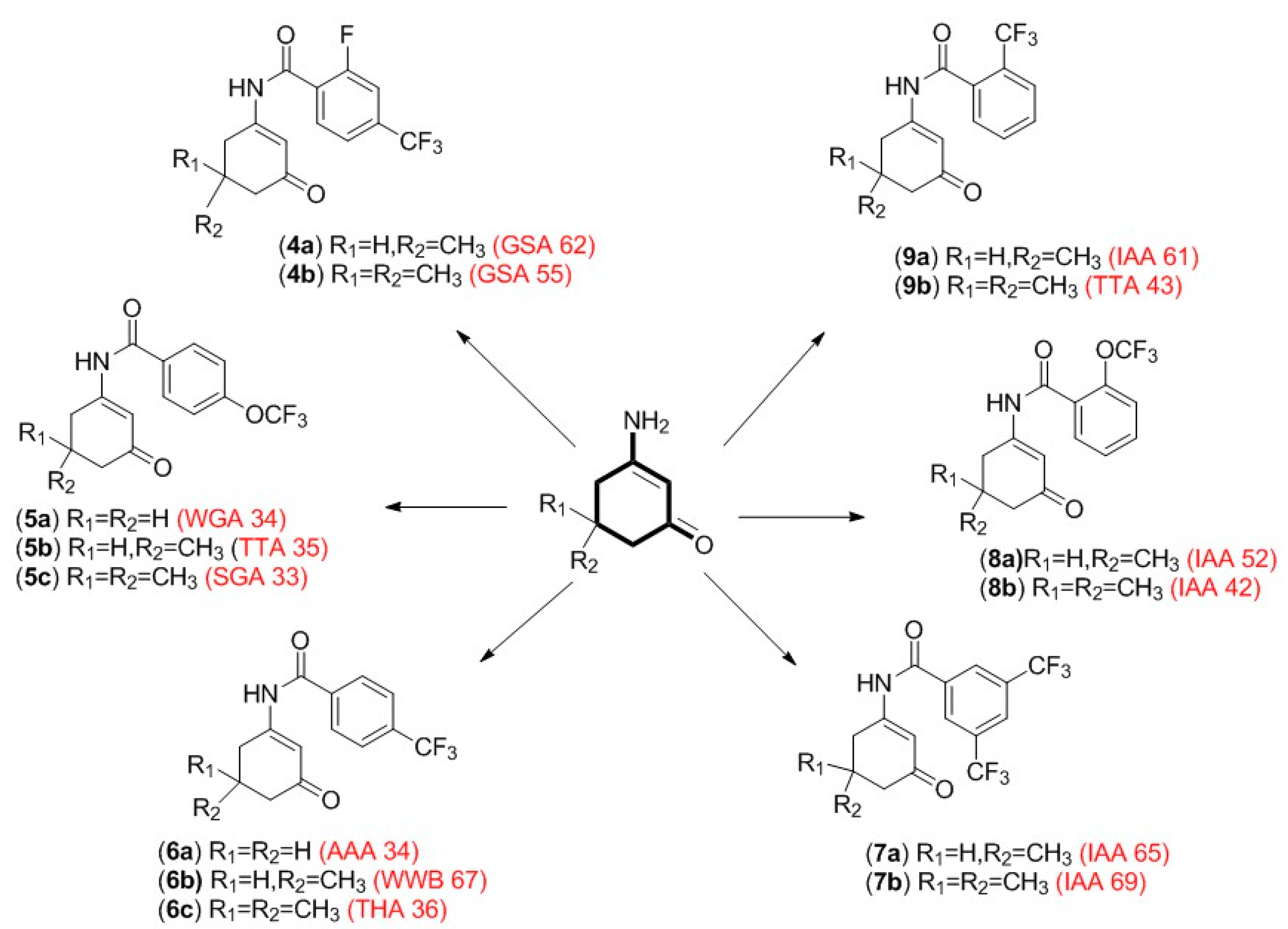


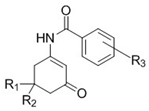
| Compound | R1 | R2 | R3 | Yield (%) | MP °C |
|---|---|---|---|---|---|
| 4a | H | CH3 | 2−F, 4−CF3 | 46 | 170–172 |
| 4b | CH3 | CH3 | 2−F, 4−CF3 | 50 | 177–179 |
| 5a | H | H | 4−OCF3 | 54 | 157–158 |
| 5b | H | CH3 | 4−OCF3 | 32 | 186–187 |
| 5c | CH3 | CH3 | 4−OCF3 | 43 | 170–171 |
| 6a | H | H | 4−CF3 | 63 | 197–198 |
| 6b | H | CH3 | 4−CF3 | 48 | 201–203 |
| 6c | CH3 | CH3 | 4−CF3 | 56 | 197–200 |
| 7a | H | CH3 | 3,5−CF3 | 34 | 186–188 |
| 7b | CH3 | CH3 | 3,5−CF3 | 14 | 149–151 |
| 8a | H | CH3 | 2−OCF3 | 34 | 142–143 |
| 8b | CH3 | CH3 | 2−OCF3 | 42 | 153–155 |
| 9a | H | CH3 | 2−CF3 | 21 | 180–181 |
| 9b | CH3 | CH3 | 2−CF3 | 28 | 195–197 |
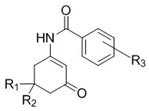
| Compound | R1 | R2 | R3 | Dose (mg/kg) | Pretreatment Times (h) a | Tox b | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.50 | 1 | 2 | 4 | ||||||
| WWB 67 (6b) | CH3 | H | 4−CF3 | 100 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| 150 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | ||||
| 200 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | ||||
| THA 36 (6c) | CH3 | CH3 | 4−CF3 | 150 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| 200 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | ||||
| TTA 35 (5b) | CH3 | H | 4−OCF3 | 150 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| 300 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | ||||
| SGA 33 (5c) | CH3 | CH3 | 4−OCF3 | 150 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| 300 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | ||||
| GSA 62 (4a) | CH3 | H | 2−F, 4−CF3 | 30 | 0/4 | 0/4 | 0/4 | 1/4 | 0/4 | 0/4 |
| 100 | 0/4 | 0/4 | 0/4 | 2/4 | 0/4 | 0/4 | ||||
| 300 | 0/4 | 2/4 | 0/4 | 4/4 | 0/4 | 0/4 | ||||
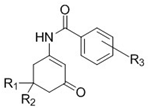
| Compound | R1 | R2 | R3 | Dose (mg/kg) | Pretreatment Times (h) a | Tox b | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.50 | 1 | 2 | 4 | ||||||
| WWB 67 (6b) | CH3 | H | 4−CF3 | 100 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| 150 | 2/4 | 4/4 | 4/4 | 2/4 | 1/4 | 0/4 | ||||
| 200 | 0/4 | 3/8 | 1/4 | 1/4 | 0/4 | 0/4 | ||||
| THA 36 (6c) | CH3 | CH3 | 4−CF3 | 150 | 0/4 | 1/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| 200 | 0/4 | 1/4 | 0/4 | 0/4 | 0/4 | 0/4 | ||||
| TTA 35 (5b) | CH3 | H | 4−OCF3 | 150 | 0/4 | 3/4 | 1/4 | 1/4 | 0/4 | 0/4 |
| 300 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | ||||
| SGA 33 (5c) | CH3 | CH3 | 4−OCF3 | 150 | 0/4 | 1/4 | 1/4 | 3/4 | 0/4 | 0/4 |
| 300 | 0/4 | 1/4 | 4/4 | 3/4 | 2/4 | 0/4 | ||||
| GSA 62 (4a) | CH3 | H | 2−F, 4−CF3 | 30 | 0/4 | 0/4 | 0/4 | 1/4 | 0/4 | 0/4 |
| 100 | 0/4 | 0/4 | 0/4 | 1/4 | 0/4 | 0/4 | ||||
| 300 | 0/4 | 1/4 | 0/4 | 4/4 | 0/4 | 0/4 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaye, I.J.; Heinbockel, T.; Woods, J.; Wang, Z.; Martin-Caraballo, M.; Jackson-Ayotunde, P. 6 Hz Active Anticonvulsant Fluorinated N-Benzamide Enaminones and Their Inhibitory Neuronal Activity. Int. J. Environ. Res. Public Health 2018, 15, 1784. https://doi.org/10.3390/ijerph15081784
Amaye IJ, Heinbockel T, Woods J, Wang Z, Martin-Caraballo M, Jackson-Ayotunde P. 6 Hz Active Anticonvulsant Fluorinated N-Benzamide Enaminones and Their Inhibitory Neuronal Activity. International Journal of Environmental Research and Public Health. 2018; 15(8):1784. https://doi.org/10.3390/ijerph15081784
Chicago/Turabian StyleAmaye, Isis J., Thomas Heinbockel, Julia Woods, Zejun Wang, Miguel Martin-Caraballo, and Patrice Jackson-Ayotunde. 2018. "6 Hz Active Anticonvulsant Fluorinated N-Benzamide Enaminones and Their Inhibitory Neuronal Activity" International Journal of Environmental Research and Public Health 15, no. 8: 1784. https://doi.org/10.3390/ijerph15081784
APA StyleAmaye, I. J., Heinbockel, T., Woods, J., Wang, Z., Martin-Caraballo, M., & Jackson-Ayotunde, P. (2018). 6 Hz Active Anticonvulsant Fluorinated N-Benzamide Enaminones and Their Inhibitory Neuronal Activity. International Journal of Environmental Research and Public Health, 15(8), 1784. https://doi.org/10.3390/ijerph15081784






