Using Calcination Remediation to Stabilize Heavy Metals and Simultaneously Remove Polycyclic Aromatic Hydrocarbons in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Contaminated Soils
2.3. Calcination Experiments
2.4. Analysis of Available Contents of Heavy Metals in Soils
2.5. Analysis of PAHs in Soils
2.6. Statistical Analysis
3. Results and Discussion
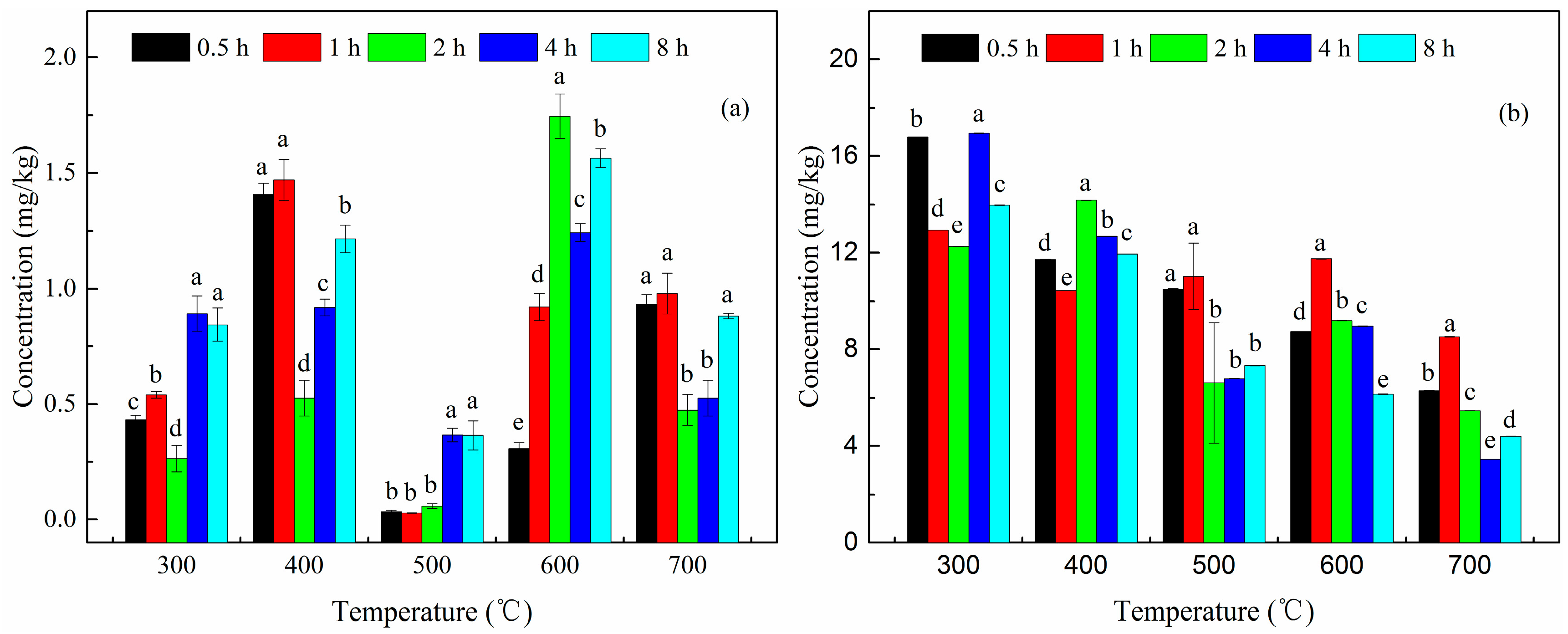
3.1. Stabilization of Heavy Metals in Soil
3.2. Removal of PAHs in Soil
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gao, Y.Z.; Xiong, W.; Ling, W.T.; Xu, J.M. Sorption of phenanthrene by contaminated soils with heavy metals. Chemosphere 2006, 65, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Sun, M.M.; Kengara, F.O.; Wang, J.T.; Ni, N.; Wang, L.; Song, Y.; Yang, X.L.; Li, H.X.; Hu, F.; et al. Evaluation of soil washing process with carboxymethyl-β-cyclodextrin and carboxymethyl chitosan for recovery of PAHs/heavy metals/fluorine from metallurgic plant site. J. Environ. Sci. 2014, 26, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, M.D.; Nagpal, V.; Guigard, S.E. Remediation of contaminated soils using supercritical fluid extraction: A review (1994–2004). Environ. Technol. Lett. 2005, 26, 1013–1032. [Google Scholar] [CrossRef] [PubMed]
- Colacicco, A.; Gioannis, G.D.; Muntoni, A.; Pettinao, E.; Polettini, A.; Pomi, R. Enhanced electrokinetic treatment of marine sediments contaminated by heavy metals and PAHs. Chemosphere 2010, 81, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Cassina, L.; Tassi, E.; Pedron, F.; Petruzzelli, G.; Barbafieri, M. Using a plant hormone and a thioligand to improve phytoremediation of Hg-contaminated soil from a petrochemical plant. J. Hazard. Mater. 2012, 231–232, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Z.; Yang, Y.; Ling, W.T.; Kong, H.L.; Zhu, X.Z. Gradient distribution of root exudates and polycyclic aromatic hydrocarbons in rhizosphere soil. Soil Sci. Soc. Am. J. 2011, 75, 1694–1703. [Google Scholar] [CrossRef]
- Ling, W.T.; Gao, Y.Z. Promoted dissipation of phenanthrene and pyrene in soils by amaranth (Amaranthus tricolor L.). Environ. Geol. 2004, 46, 553–560. [Google Scholar] [CrossRef]
- Busto, Y.; Cabrera, X.; Tack, F.M.G.; Verloo, M.G. Potential of thermal treatment for decontamination of mercury containing wastes from chlor-alkali industry. J. Hazard. Mater. 2011, 186, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Yen, J. On-site mercury-contaminated soils remediation by using thermal desorption technology. J. Hazard. Mater. 2006, 128, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Taube, F.; Pommer, L.; Larsson, T.; Shchukarev, A.; Nordi, A. Soil remediation-mercury speciation in soil and vapor phase during thermal treatment. Water Air Soil Pollut. 2008, 193, 155–163. [Google Scholar] [CrossRef]
- Heron, G.; Van Zutphen, M.; Christensen, T.H.; Enfield, C.G. Soil heating for Enhanced remediation of chlorinated solvents: a laboratory study on resistive heating and vapor extraction in a silty, low-permeable soil contaminated with trichloroethylene. Environ. Sci. Technol. 1998, 32, 1474–1481. [Google Scholar] [CrossRef]
- Wang, Y.P.; Huang, J.; Gao, Y.Z. Use of experimental data and the application of a kinetic model to determine the subcellular distribution of Zn/Cd/Ni/Cu over time in Indian mustard. RSC Adv. 2013, 3, 12423–12431. [Google Scholar] [CrossRef]
- Kong, H.L.; Sun, R.Z.; Gao, Y.; Sun, B.Q. Elution of polycyclic aromatic hydrocarbons in soil columns using low-molecular-weight organic acids. Soil Sci. Soc. Am. J. 2013, 77, 72–82. [Google Scholar] [CrossRef]
- Wei, Y.L.; Yang, Y.W.; Cheng, N. Study of thermally immobilized cu in analogue minerals of contaminated soils. Environ. Sci. Technol. 2001, 35, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Spalding, B.P. Fixation of radionuclides in soil and minerals by heating. Environ. Sci. Technol. 2001, 35, 4327–4333. [Google Scholar] [CrossRef] [PubMed]
- Obrador, A.; Rico, M.I.; Alvarez, J.M.; Novillo, J. Influence of thermal treatment on sequential extraction and leaching behaviour of trace metals in a contaminated sewage sludge. Bioresour. Technol. 2001, 76, 259–264. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Zhang, S. The mechanisms of heavy metal immobilization by cementitious material treatments and thermal treatments: A review. J. Environ. Manag. 2017, 193, 410–422. [Google Scholar]
- Gan, S.; Lau, E.V.; Ng, H.K. Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2009, 172, 532–549. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, R.Z.; Xue, J.; Li, J.H. Generation and distribution of PAHs in the process of medical waste incineration. Waste Manag. 2013, 33, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Ives, P. Incineration at bayou bonfouca remediation project. Air Waste 2017, 44, 1195–1203. [Google Scholar]
- Renoldi, F.; Lietti, L.; Saponaro, S.; Bonomo, L.; Forzatti, P. Thermal desorption of a PAH-contaminated soil: A case study. Ecosyst. Sustain. Dev. IV 2003, 64, 1124–1132. [Google Scholar]

| PAHs | Molecular Weight (g/mol) | Boiling Point (°C) | Melting Point (°C) | LogKow | Water Solubility (mg/L) | Molecular Structure |
|---|---|---|---|---|---|---|
| Naphthalene | 128.18 | 217.9 | 80.5 | 3.37 | 31.00 |  |
| Fluoranthene | 202.25 | 384 | 111 | 4.58 | 0.26 | 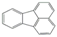 |
| Temperature (°C) | Time (h) | ||||
|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 4 | 8 | |
| 300 | 58.45 ± 6.85 c | 63.40 ± 9.27 c | 49.98 ± 5.78 c | 37.78 ± 2.00 c | 43.15 ± 2.61 c |
| 400 | 83.50 ± 3.73 b | 84.18 ± 2.98 b | 80.78 ± 3.69 b | 85.64 ± 1.58 b | 84.74 ± 4.18 b |
| 500 | 93.27 ± 0.69 a | 93.69 ± 5.86 a | 92.72 ± 0.42 a | 93.12 ± 0.39 a | 94.22 ± 0.19 a |
| 600 | 94.57 ± 0.19 a | 94.98 ± 0.43 a | 95.15 ± 0.42 a | 95.22 ± 0.73 a | 95.97 ± 0.70 a |
| 700 | 96.95 ± 1.75 a | 96.72 ± 0.46 a | 95.77 ± 1.35 a | 95.96 ± 0.29 a | 96.09 ± 0.66 a |
| Temperature (°C) | Time (h) | ||||
|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 4 | 8 | |
| 300 | 93.25 ± 1.61 c | 94.04 ± 2.07 b | 92.37 ± 0.82 c | 90.61 ± 0.40 e | 93.00 ± 0.59 c |
| 400 | 96.18 ± 0.39 b | 96.39 ± 0.38 a | 96.34 ± 0.24 bc | 96.41 ± 0.30 d | 97.05 ± 0.60 ab |
| 500 | 97.40 ± 0.27 ab | 97.44 ± 0.22 a | 97.16 ± 0.19 b | 97.42 ± 0.15 b | 97.63 ± 0.03 a |
| 600 | 97.90 ± 0.07 a | 97.92 ± 0.20 a | 98.06 ± 0.20 a | 98.25 ± 0.11 a | 98.27 ± 0.03 a |
| 700 | 98.41 ± 0.37 a | 97.68 ± 0.49 a | 97.05 ± 0.22 b | 96.92 ± 0.08 c | 96.90 ± 0.13 b |
| Temperature (°C) | Time (h) | ||||
|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 4 | 8 | |
| 300 | 99.30 ± 0.03 c | 99.12 ± 0.03 b | 99.57 ± 0.10 b | 98.55 ± 0.13 c | 98.63 ± 0.12 b |
| 400 | 97.72 ± 0.08 e | 97.62 ± 0.14 d | 99.15 ± 0.13 c | 98.51 ± 0.06 c | 98.03 ± 0.10 c |
| 500 | 99.94 ± 0.05 a | 99.95 ± 0.05 a | 99.91 ± 0.02 a | 99.41 ± 0.05 a | 99.41 ± 0.10 a |
| 600 | 99.50 ± 0.04 b | 98.51 ± 0.09 c | 97.17 ± 0.16 d | 97.98 ± 0.07 d | 97.46 ± 0.08 d |
| 700 | 98.49 ± 0.07 d | 98.41 ± 0.14 c | 99.23 ± 0.11 c | 99.15 ± 0.13 b | 98.57 ± 0.04 b |
| Temperature (°C) | Time (h) | ||||
|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 4 | 8 | |
| 300 | 94.75 ± 0.05 e | 95.95 ± 0.05 c | 96.17 ± 0.03 c | 94.70 ± 0.00 e | 95.63 ± 0.03 e |
| 400 | 96.34 ± 0.04 d | 96.74 ± 0.04 b | 95.57 ± 0.03 c | 96.03 ± 0.03 d | 96.27 ± 0.03 d |
| 500 | 96.72 ± 0.02 c | 96.55 ± 0.43 b | 97.93 ± 0.78 a | 97.88 ± 0.02 b | 97.71 ± 0.01 c |
| 600 | 97.27 ± 0.03 b | 96.33 ± 0.03 bc | 97.12 ± 0.02 b | 97.19 ± 0.01 c | 98.08 ± 0.02 b |
| 700 | 98.04 ± 0.03 a | 97.34 ± 0.04 a | 98.29 ± 0.01 a | 98.92 ± 0.02 a | 98.63 ± 0.03 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Hu, X.; He, Q.; Waigi, M.G.; Wang, J.; Ling, W. Using Calcination Remediation to Stabilize Heavy Metals and Simultaneously Remove Polycyclic Aromatic Hydrocarbons in Soil. Int. J. Environ. Res. Public Health 2018, 15, 1731. https://doi.org/10.3390/ijerph15081731
Wang P, Hu X, He Q, Waigi MG, Wang J, Ling W. Using Calcination Remediation to Stabilize Heavy Metals and Simultaneously Remove Polycyclic Aromatic Hydrocarbons in Soil. International Journal of Environmental Research and Public Health. 2018; 15(8):1731. https://doi.org/10.3390/ijerph15081731
Chicago/Turabian StyleWang, Peixin, Xiaojie Hu, Qianjia He, Michael Gatheru Waigi, Jian Wang, and Wanting Ling. 2018. "Using Calcination Remediation to Stabilize Heavy Metals and Simultaneously Remove Polycyclic Aromatic Hydrocarbons in Soil" International Journal of Environmental Research and Public Health 15, no. 8: 1731. https://doi.org/10.3390/ijerph15081731
APA StyleWang, P., Hu, X., He, Q., Waigi, M. G., Wang, J., & Ling, W. (2018). Using Calcination Remediation to Stabilize Heavy Metals and Simultaneously Remove Polycyclic Aromatic Hydrocarbons in Soil. International Journal of Environmental Research and Public Health, 15(8), 1731. https://doi.org/10.3390/ijerph15081731





