Effects of Horticultural Therapy on Asian Older Adults: A Randomized Controlled Trial
Abstract
1. Introduction
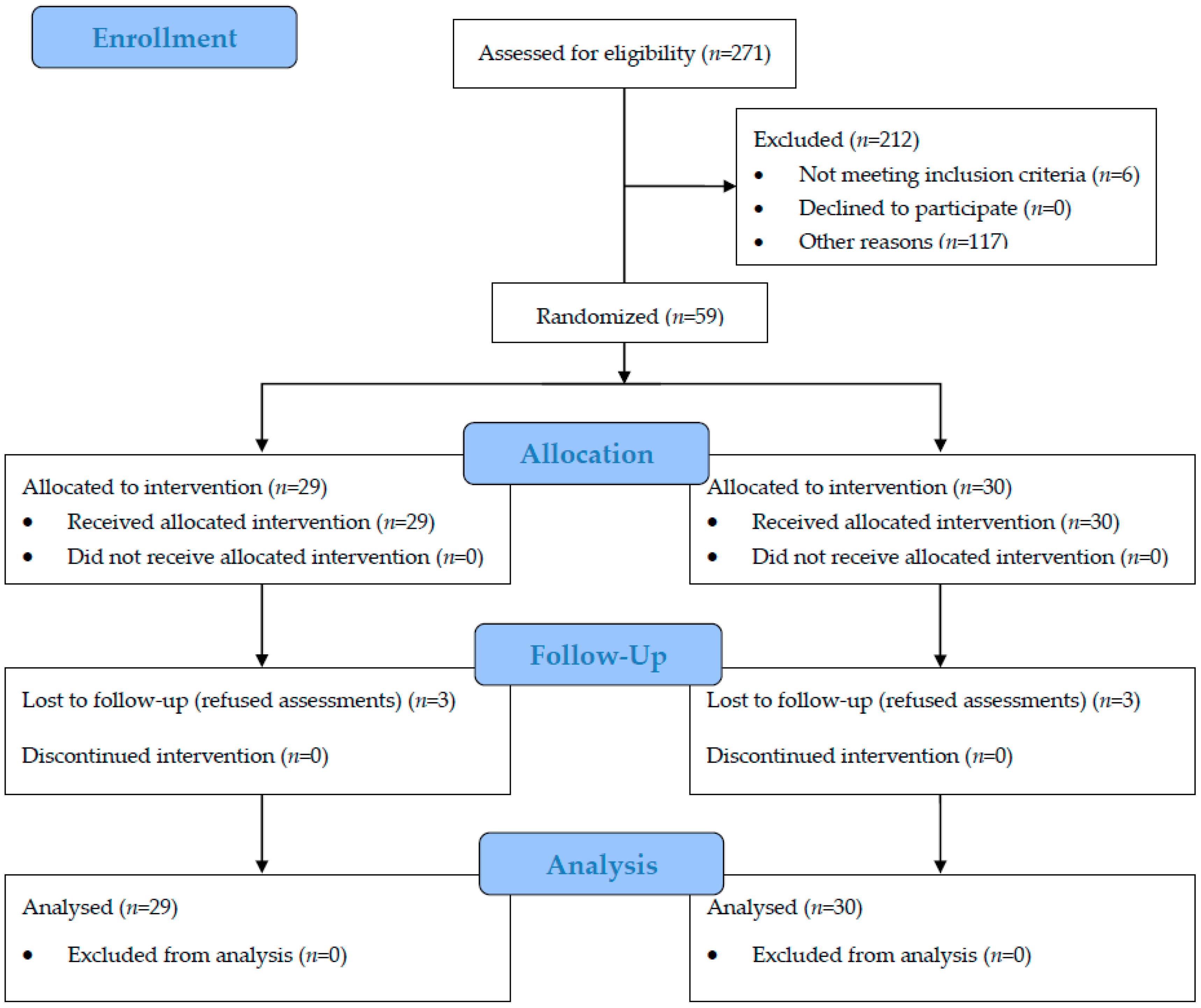
2. Materials and Methods
2.1. Participants
2.2. The Interventions
2.3. Outcome Measurements
2.4. Biological Measures
2.5. Psychosocial Measures
2.6. Statistical Analyses
3. Results
3.1. Baseline Data
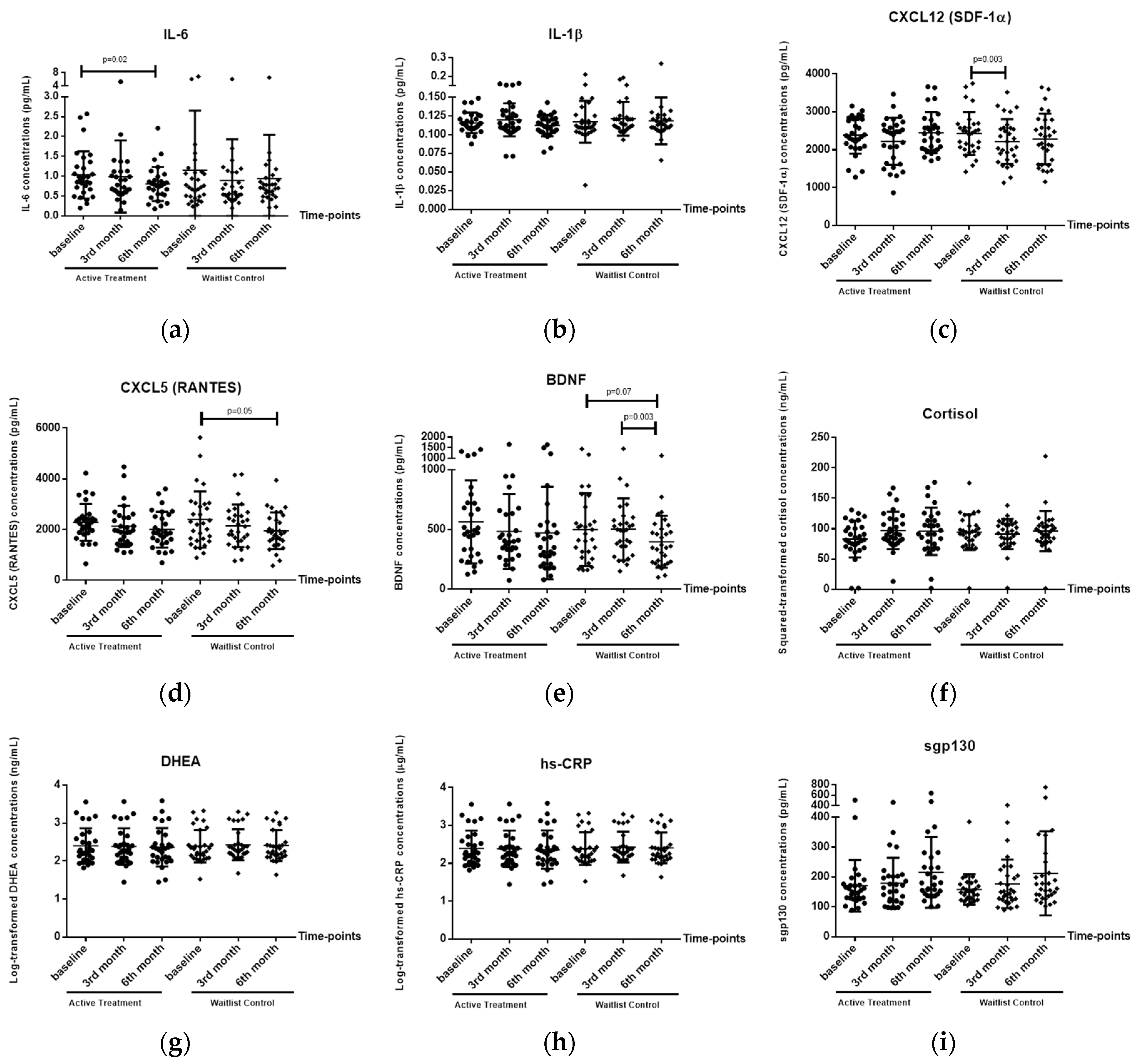
3.2. Effects of HT Intervention on Biological Markers
3.2.1. Plasma IL-6 Levels
3.2.2. Plasma CXCL12 (SDF-1α) Levels
3.2.3. Plasma CXCL5 (RANTES) Levels
3.2.4. Plasma BDNF Levels
3.2.5. Plasma IL-1β, Cortisol, DHEA, hs-CRP and Sgp-130 Levels
3.3. Effects of HT Intervention on Psychological Well-Being
3.3.1. Positive Relations with Others
3.3.2. Cognitive Function, Depression, Anxiety and Psychological Well-Being
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Puri, B.; Hall, A.; Ho, R. Revision Notes in Psychiatry; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Yao, Y.F.; Chen, K.M. Effects of horticulture therapy on nursing home older adults in southern Taiwan. Qual. Life Res. 2017, 26, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Blake, M.; Mitchell, G. Horticultural therapy in dementia care: A literature review. Nurs. Stand. 2016, 30, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Lin, C.Y.; Li, Y.C. Planting Hope in Loss and Grief: Self-Care Applications of Horticultural Therapy for Grief Caregivers in Taiwan. Death Stud. 2014, 38, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Reed, C. The Origins, Development and Perceived Effectiveness of Horticulture-Based Therapy in Victoria. Ph.D. Thesis, Deakin Univeristy, Geelong, Australia, 2015. [Google Scholar]
- Connell, B.R.; Sanford, J.A.; Lewis, D. Therapeutic effects of an outdoor activity program on nursing home residents with dementia. J. Hous. Elder. 2007, 21, 194–209. [Google Scholar] [CrossRef]
- MPtSc, M.L.V.; Lehmann, S.; Aeschlimann, A. Horticultural therapy for patients with chronic musculoskeletal pain: Results of a pilot study. Altern. Ther. Health Med. 2012, 18, 44. [Google Scholar]
- Wichrowski, M.; Whiteson, J.; Haas, F.; Mola, A.; Rey, M.J. Effects of horticultural therapy on mood and heart rate in patients participating in an inpatient cardiopulmonary rehabilitation program. J. Cardiopulm. Rehabil. Prev. 2005, 25, 270–274. [Google Scholar] [CrossRef]
- Li, Q. Effect of forest bathing trips on human immune function. Environ. Health Prev. Med. 2010, 15, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Söderback, I.; Söderström, M.; Schälander, E. Horticultural therapy: The ‘healing garden’and gardening in rehabilitation measures at Danderyd Hospital Rehabilitation Clinic, Sweden. Pediatr. Rehabil. 2004, 7, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Noone, S.; Innes, A.; Kelly, F.; Mayers, A. ‘The nourishing soil of the soul: The role of horticultural therapy in promoting well-being in community-dwelling people with dementia. Dementia 2017, 16, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Kamioka, H.; Tsutani, K.; Yamada, M.; Park, H.; Okuizumi, H.; Honda, T.; Okada, S.; Park, S.J.; Kitayuguchi, J.; Abe, T.; et al. Effectiveness of horticultural therapy: A systematic review of randomized controlled trials. Complement. Ther. Med. 2014, 22, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ho, R.C.M.; Mak, A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J. Affect. Disord. 2012, 139, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Garbers, C.; Thaiss, W.; Jones, G.W.; Waetzig, G.H.; Lorenzen, I.; Guilhot, F.; Lissilaa, R.; Ferlin, W.G.; Grötzinger, J.; Jones, S.A.; et al. Inhibition of classic signaling is a novel function of soluble glycoprotein 130 (sgp130), which is controlled by the ratio of interleukin 6 and soluble interleukin 6 receptor. J. Biol. Chem. 2011, 286, 42959–42970. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, I.M.; Golabkesh, Z.; Källberg, H.; Leander, K.; de Faire, U.; Gigante, B. Circulating levels of interleukin 6 soluble receptor and its natural antagonist, sgp130, and the risk of myocardial infarction. Atherosclerosis 2015, 240, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Laske, C.; Stellos, K.; Eschweiler, G.W.; Leyhe, T.; Gawaz, M. Decreased CXCL12 (SDF-1) plasma levels in early Alzheimer’s disease: A contribution to a deficient hematopoietic brain support? J. Alzheimer’s Dis. 2008, 15, 83–95. [Google Scholar] [CrossRef]
- Weinstein, G.; Beiser, A.S.; Choi, S.H.; Preis, S.R.; Chen, T.C.; Vorgas, D.; Au, R.; Pikula, A.; Wolf, P.A.; DeStefano, A.L.; et al. Serum brain-derived neurotrophic factor and the risk for dementia: The Framingham Heart Study. JAMA Neurol. 2014, 71, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Moraes, H.; Deslandes, A.; Maciel-Pinheiro, Pd.T.; Corrêa, H.; Laks, J. Cortisol, DHEA, and depression in the elderly: The influence of physical capacity. Arq. de Neuro-Psiquiatria 2016, 74, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Cao, Y.; Wang, B.; Wang, S.; Chen, Z.; Wang, J.; Xing, W.; Ren, X.; Lv, X.; Dong, J.; et al. The salutary influence of forest bathing on elderly patients with chronic heart failure. Int. J. Environ. Res. Public Health 2017, 14, 368. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, H.; Ikei, H.; Song, C.; Kobayashi, M.; Takamatsu, A.; Miura, T.; Kagawa, T.; Li, Q.; Kumeda, S.; Imai, M.; et al. Physiological and psychological effects of forest therapy on middle-aged males with high-normal blood pressure. Int. J. Environ. Res. Public Health 2015, 12, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, H.; Ikei, H.; Song, C.; Kobayashi, M.; Miura, T.; Kagawa, T.; Li, Q.; Kumeda, S.; Imai, M.; Miyazaki, Y. Physiological and psychological effects of a forest therapy program on middle-aged females. Int. J. Environ. Res. Public Health 2015, 12, 15222–15232. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.Y.; Ho, R.C.M.; Mahendran, R.; Ng, K.S.; Wai-San, T.W.; Rawtaer, I. Effects of horticultural therapy on elderly’ health: Protocol of a randomized controlled trial. BMC Geriatr. 2017, 17, 192. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Andiappan, A.K.; Lee, B.; Ho, R.; Lim, T.K.; Kuan, W.S. Neuropeptide Y associated with asthma in young adults. Neuropeptides 2016, 59, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Peppe, A.; Carlesimo, G.A.; Serafini, F.; Zabberoni, S.; Barban, F. A pilot study on the effect of cognitive training on BDNF serum levels in individuals with Parkinson’s disease. Front. Hum. Neurosci. 2015, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- De Britto Rosa, N.M.; de Queiroz, B.Z.; Pereira, D.S.; di Sabatino Santos, M.L.A.; Oliveira, D.M.G.; Pereira, L.S.M. Interleukin-6 plasma levels and socioeconomic status in Brazilian elderly community-dwelling women. Arch. Gerontol. Geriatr. 2011, 53, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Elderkin-Thompson, V.; Irwin, M.R.; Hellemann, G.; Kumar, A. Interleukin-6 and memory functions of encoding and recall in healthy and depressed elderly adults. Am. J. Geriatr. Psychiatry 2012, 20, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Kozaki, K.; Sonohara, K.; Akishita, M.; Toba, K. Relationship between interleukin-6 and cerebral deep white matter and periventricular hyperintensity in elderly women. Geriatr. Gerontol. Int. 2011, 11, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Blum-Degena, D.; Müller, T.; Kuhn, W.; Gerlach, M.; Przuntek, H.; Riederer, P. Interleukin-1β and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci. Lett. 1995, 202, 17–20. [Google Scholar] [CrossRef]
- Licastro, F.; Pedrini, S.; Caputo, L.; Annoni, G.; Davis, L.J.; Ferri, C. Increased plasma levels of interleukin-1, interleukin-6 and α-1-antichymotrypsin in patients with Alzheimer’s disease: Peripheral inflammation or signals from the brain? J. Neuroimmunol. 2000, 103, 97–102. [Google Scholar] [CrossRef]
- Da Cunha Nascimento, D.; de Sousa, N.M.F.; de Sousa Neto, I.V.; Tibana, R.A.; de Souza, V.C.; Vieira, D.C.L. Classification of pro-inflammatory status for interleukin-6 affects relative muscle strength in obese elderly women. Aging Clin. Exp. Res. 2015, 27, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, Y.; Wang, X.; Zou, X.; Zhang, D.; Wang, D. High serum interleukin-6 level is associated with increased risk of delirium in elderly patients after noncardiac surgery: A prospective cohort study. Chin. Med. J. 2012, 126, 3621–3627. [Google Scholar]
- Wakuda, R.; Miki, C.; Kusunoki, M. Autoreactivity against interleukin 6 as a risk factor in elderly patients with colorectal carcinoma. Arch. Surg. 2001, 136, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Lafarge, S.; Dawe, D.; Lakhi, S.; Kumar, R.; Morales, C. Association of interleukin-6 and interleukin-8 with poor prognosis in elderly patients with chronic lymphocytic leukemia. Leuk. Lymphoma 2012, 53, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, M.; Amici, G.; Ongaro, F.; Gajo, G.; De Angeli, S.; Forloni, G. Associations of the plasma interleukin 6 (IL-6) levels with disability and mortality in the elderly in the Treviso Longeva (Trelong) study. Arch. Gerontol. Geriatr. 2007, 44, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Onder, G.; Liperoti, R.; Russo, A.; Carter, C.; Capoluongo, E. Interleukin-6, C-Reactive Protein, and Tumor Necrosis Factor-Alpha as Predictors of Mortality in Frail, Community-Living Elderly Individuals. J. Am. Geriatr. Soc. 2011, 59, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Leyhe, T.; Hoffmann, N.; Stransky, E.; Laske, C. Increase of SCF plasma concentration during donepezil treatment of patients with early Alzheimer’s disease. Int. J. Neuropsychol. 2009, 12, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Parachikova, A.; Cotman, C. Reduced CXCL12/CXCR4 results in impaired learning and is downregulated in a mouse model of Alzheimer disease. Neurobiol. Dis. 2007, 28, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, Y.S.; Khan, M.A.; Brunk, E.; Martin-Cook, K.; Weiner, M.F. Global brain hypoperfusion and oxygenation in amnestic mild cognitive impairment. Alzheimer’s Dement. 2014, 10, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Appay, V.; Rowland-Jones, S.L. RANTES: A versatile and controversial chemokine. Trends Immunol. 2001, 22, 83–87. [Google Scholar] [CrossRef]
- Fiala, M.; Lin, J.; Ringman, J.; Kermani-Arab, V.; Tsao, G.; Patel, A. Ineffective phagocytosis of amyloid-β by macrophages of Alzheimer’s disease patients. J. Alzheimer’s Dis. 2005, 7, 221–232. [Google Scholar] [CrossRef]
- Reale, M.; Patruno, A.; De Lutiis, M.A.; Pesce, M.; Felaco, M.; Di Giannantonio, M. Dysregulation of chemo-cytokine production in schizophrenic patients versus healthy controls. BMC Neurosci. 2011, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Grassi-Oliveira, R.; Brieztke, E.; Teixeira, A.; Pezzi, J.C.; Zanini, M.; Lopes, R.P. Peripheral chemokine levels in women with recurrent major depression with suicidal ideation. Rev. Bras. de Psiquiatria 2012, 34, 71–75. [Google Scholar] [CrossRef]
- Eugenin, E.; D’aversa, T.; Lopez, L.; Calderon, T.; Berman, J.W. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J. Neurochem. 2003, 85, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Thirumangalakudi, L.; Grammas, P. RANTES upregulation in the Alzheimer’s disease brain: A possible neuroprotective role. Neurobiol. Aging 2010, 31, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Makizako, H.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front. Aging Neurosci. 2014, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Coelho, F.; Pereira, D.; Lustosa, L.; Silva, J.; Dias, J.; Dias, R. Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch. Gerontol. Geriatr. 2012, 54, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Forti, L.N.; Njemini, R.; Beyer, I.; Eelbode, E.; Meeusen, R.; Mets, T. Strength training reduces circulating interleukin-6 but not brain-derived neurotrophic factor in community-dwelling elderly individuals. Age 2014, 36, 9704. [Google Scholar] [CrossRef] [PubMed]
- Gomes, W.F.; Lacerda, A.C.R.; Mendonça, V.A.; Arrieiro, A.N.; Fonseca, S.F.; Amorim, M.R. Effect of exercise on the plasma BDNF levels in elderly women with knee osteoarthritis. Rheumatol. Int. 2014, 34, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.E.; Kang, E.B. The effects of senior brain health exercise program on basic physical fitness, cognitive function and BDNF of elderly women-a feasibility study. J. Exerc. Nutr. Biochem. 2016, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Lennartsson, A.K.; Kushnir, M.M.; Bergquist, J.; Jonsdottir, I.H. DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biol. Psychol. 2012, 90, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, B.J.; Hsu, F.C.; Brinkley, T.J.; Church, T.; Goodpaster, B.H.; Kritchevsky, S.B. Exercise training and Plasma C-reactive Protein and Interleukin-6 in elderly people. J. Am. Geriatr. Soc. 2008, 56, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Liu, X.; Jiang, H.; Pan, F.; Ho, C.S.; Ho, R.C. The Effects of High-fat-diet Combined with Chronic Unpredictable Mild Stress on Depression-like Behavior and Leptin/LepRb in Male Rats. Sci. Rep. 2016, 6, 35239. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y. A forest bathing trip increases human natural killer activity and expression of anti-cancer proteins in female subjects. J. Biol. Regul. Homeost. Agents 2008, 22, 45–55. [Google Scholar] [PubMed]
| Date (dd/mm/yyyy) | Session | Topic | Activity | Venue |
|---|---|---|---|---|
| Weekly Sessions | ||||
| 14/05/2015 | 1 | Introduction | 1. TaRA familiarisation 2. Group formation 7/group 3. Indoor Gardening basics | TaRA@JP |
| 21/05/2015 | 2 | Introduction | 1. Garden familiarisation 2. Vegetables growing brief 3. Sowing vegetables seedlings | Chinese Garden |
| 28/05/2015 | 3 | Wetland Walk | 1. Park amenities familiarization 2. Interpretive walk 3. Reflection | Sungei Buloh Reserve |
| 04/06/2015 | 4 | Introduction | 1. Briefing for vegetables maintenance 2. Weeding and fertilizing vegetables plot | Chinese Garden |
| 11/06/2015 | 5 | Nurturing | 1. Briefing on pressed flowers 2. Material preparation 3. Make pressed flowers card | TaRA@JP |
| 18/06/2015 | 6 | Nurturing | 1. Garden maintenance briefing 2. Weeding, pruning, mulching Arden/vegetables | Chinese Garden |
| 25/06/2015 | 7 | Colour Walk | 1. Park amenities familiarization 2. Interpretive walk 3. Reflection | Singapore Botanical Gardens |
| 02/07/2015 | 8 | Nurturing | 1. Vegetables maintenance 2. Compost making brief 3. Make compost | Chinese Garden |
| 09/07/2015 | 9 | Harvest and Cook | 1. Harvest vegetables 2. Hands on preparation for food 3. Sharing of cooked vegetables | TaRA@JP |
| 16/07/2015 | 10 | Harvest and Cook | 1. Seed sowing 2. Herbal plant brief 3. Herbal plants propagation | Chinese Garden |
| 23/07/2015 | 11 | Festive Walk | 1. Park amenities familiarization 2. Interpretive walk 3. Reflection | Gardens by the Bay (Cloud Forest/flower dome) |
| 30/07/2015 | 12 | Harvest and Cook | 1. Community Garden tour 2. Plant care tips for herbs and plants that will be brought home 3. Reflection | Chinese Garden |
| Monthly Sessions | ||||
| 20/08/2015 | 1 | Healing Walk | 1. Park amenities familiarization 2. Interpretive walk 3. Reflection | Botanical Garden—Healing Garden |
| 17/09/2015 | 2 | Gardening | 1. Briefing 2. Create a culinary garden 3. Maintenance tips | Chinese garden—Culinary garden creation |
| 15/10/2015 | 3 | Nature Walk | 1. Park amenities familiarization 2. Interpretive walk 3. Reflection | Gardens by the Bay (Cloud Forest/flower dome) |
| Variables | Active Treatment (N = 29) | Waitlist Control (N = 30) | Test (p-Value) | ||
|---|---|---|---|---|---|
| Mean (SD) | N (%) | Mean (SD) | N (%) | ||
| Age | 67.21 (4.52) | 67.00 (4.18) | t = 0.18 (0.86) | ||
| Gender | |||||
| Male | 6 (20.7%) | 7 (23.30) | χ2 = 0.60 (0.81) | ||
| Female | 23 (79.3%) | 23 (76.7%) | |||
| Years of Formal Education | 7.34 (3.89) | 7.23 (3.47) | t = 0.12 (0.91) | ||
| BP (systolic), mmHg | 131.93 (18.52) | 130.90 (14.56) | t = 0.24 (0.81) | ||
| BP (diastolic), mmHg | 73.93 (11.35) | 73.27 (9.14) | t = 0.25 (0.81) | ||
| Pulse rate, BPM | 70.10 (10.84) | 67.97 (10.24) | t = 0.77 (0.44) | ||
| BMI, kg/m2 | 24.37 (3.99) | 23.34 (3.31) | t = 1.08 (0.28) | ||
| Ethnicity | |||||
| Chinese | 27 (93.1%) | 30 (100%) | F = 2.14 (0.24) | ||
| Indian | 2 (6.9%) | 0 (0%) | |||
| Others | 0 (0%) | 0 (0%) | |||
| Employment Status | |||||
| Retired | 9 (31%) | 15 (50%) | F = 4.29 (0.33) | ||
| Self-employed | 1 (3.4%) | 0 (0%) | |||
| Full-time worker | 0 (0%) | 1 (3.3%) | |||
| Part-time worker | 8 (27.6%) | 7 (23.3%) | |||
| Housewife | 11 (37.9%) | 7 (23.3%) | |||
| Marital Status | |||||
| Never married | 0 (0%) | 2 (6.7%) | F = 3.05 (0.39) | ||
| Currently Married | 23 (79.3%) | 20 (66.7%) | |||
| Divorced | 3 (10.3%) | 2 (6.7%) | |||
| Widowed | 3 (10.3%) | 6 (20%) | |||
| Park Visitor | |||||
| Yes | 16 (55.2%) | 15 (50%) | χ2 = 0.16 (0.69) | ||
| No | 13 (44.8%) | 15 (50%) | |||
| Perform regular gardening works | |||||
| Yes | 16 (55.2%) | 13 (43.3%) | χ2 = 0.83 (0.36) | ||
| No | 13 (44.8%) | 17 (56.7%) | |||
| MoCA | 26.34 (2.19) | 26.60 (2.31) | t = −0.44 (0.67) | ||
| SDS | 44.69 (3.75) | 45.31 (5.33) | t = −0.51 (0.61) | ||
| SAS | 35.14 (2.24) | 34.23 (2.53) | t = 1.45 (0.15) | ||
| Ryff’s Scales of Psychological Well-Being | 28.14 (4.87) | 27.87 (6.56) | t = 1.85 (0.07) | ||
| Friendship Scale | 11.10 (3.84) | 12.07 (4.75) | t = −0.85 (0.40) | ||
| Satisfaction with Life Scale | 79.83 (6.30) | 76.47 (7.60) | t = 0.18 (0.86) | ||
| Biomarkers | p-Value for Repeated-Measured ANOVA (General Linear Model) | |
|---|---|---|
| Time | Time × Group Interaction | |
| IL-6 | 0.095 | 0.41 |
| IL-1β | 0.15 | 0.62 |
| CXCL12 (SDF-1α) | 0.007 ** | 0.20 |
| CXCL5 (RANTES) | 0.005 ** | 0.78 |
| BDNF | 0.02 * | 0.35 |
| Cortisol | 0.35 | 0.25 |
| DHEA | 0.46 | 0.22 |
| hs-CRP | 0.70 | 0.66 |
| Sgp-130 | 0.01 * | 0.88 |
| Psychometric Measures | p-Value for Repeated-Measured ANOVA (General Linear Model) | |
|---|---|---|
| Time | Time × Group Interaction | |
| MoCA | 0.004 ** | 0.13 |
| SDS | 0.53 | 0.68 |
| SAS | 0.03 * | 0.81 |
| Ryff’s Scales of Psychological Well-Being | 0.86 | 0.34 |
| Positive Relations with Others Sub-scale | 0.14 | <0.001 *** |
| Friendship Scale | 0.20 | 0.10 |
| Satisfaction with Life Scale | 0.13 | 0.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, K.S.T.; Sia, A.; Ng, M.K.W.; Tan, C.T.Y.; Chan, H.Y.; Tan, C.H.; Rawtaer, I.; Feng, L.; Mahendran, R.; Larbi, A.; et al. Effects of Horticultural Therapy on Asian Older Adults: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2018, 15, 1705. https://doi.org/10.3390/ijerph15081705
Ng KST, Sia A, Ng MKW, Tan CTY, Chan HY, Tan CH, Rawtaer I, Feng L, Mahendran R, Larbi A, et al. Effects of Horticultural Therapy on Asian Older Adults: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2018; 15(8):1705. https://doi.org/10.3390/ijerph15081705
Chicago/Turabian StyleNg, Kheng Siang Ted, Angelia Sia, Maxel K. W. Ng, Crystal T. Y. Tan, Hui Yu Chan, Chay Hoon Tan, Iris Rawtaer, Lei Feng, Rathi Mahendran, Anis Larbi, and et al. 2018. "Effects of Horticultural Therapy on Asian Older Adults: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 15, no. 8: 1705. https://doi.org/10.3390/ijerph15081705
APA StyleNg, K. S. T., Sia, A., Ng, M. K. W., Tan, C. T. Y., Chan, H. Y., Tan, C. H., Rawtaer, I., Feng, L., Mahendran, R., Larbi, A., Kua, E. H., & Ho, R. C. M. (2018). Effects of Horticultural Therapy on Asian Older Adults: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 15(8), 1705. https://doi.org/10.3390/ijerph15081705







