A Closer Look at the Bivariate Association between Ambient Air Pollution and Allergic Diseases: The Role of Spatial Analysis
Abstract
1. Introduction
2. Materials and Methods
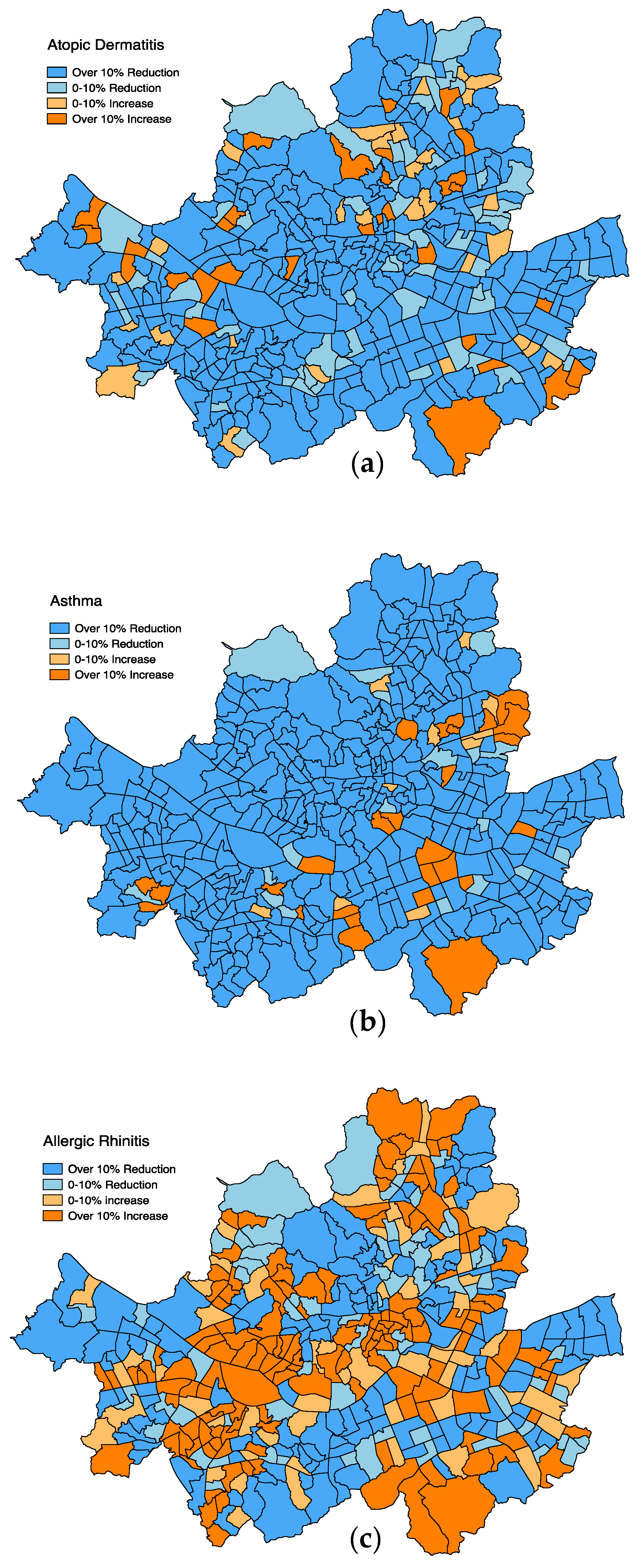
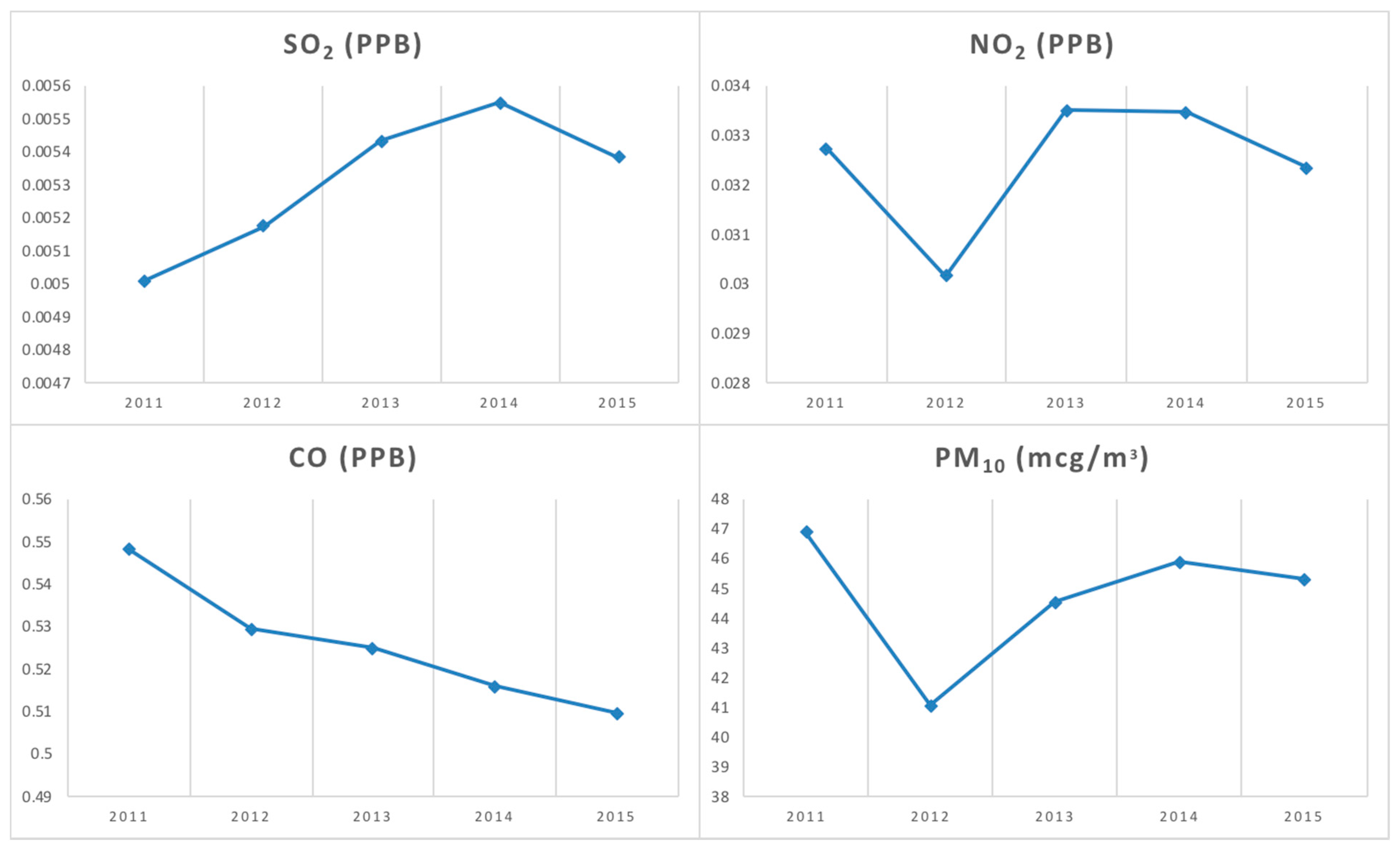
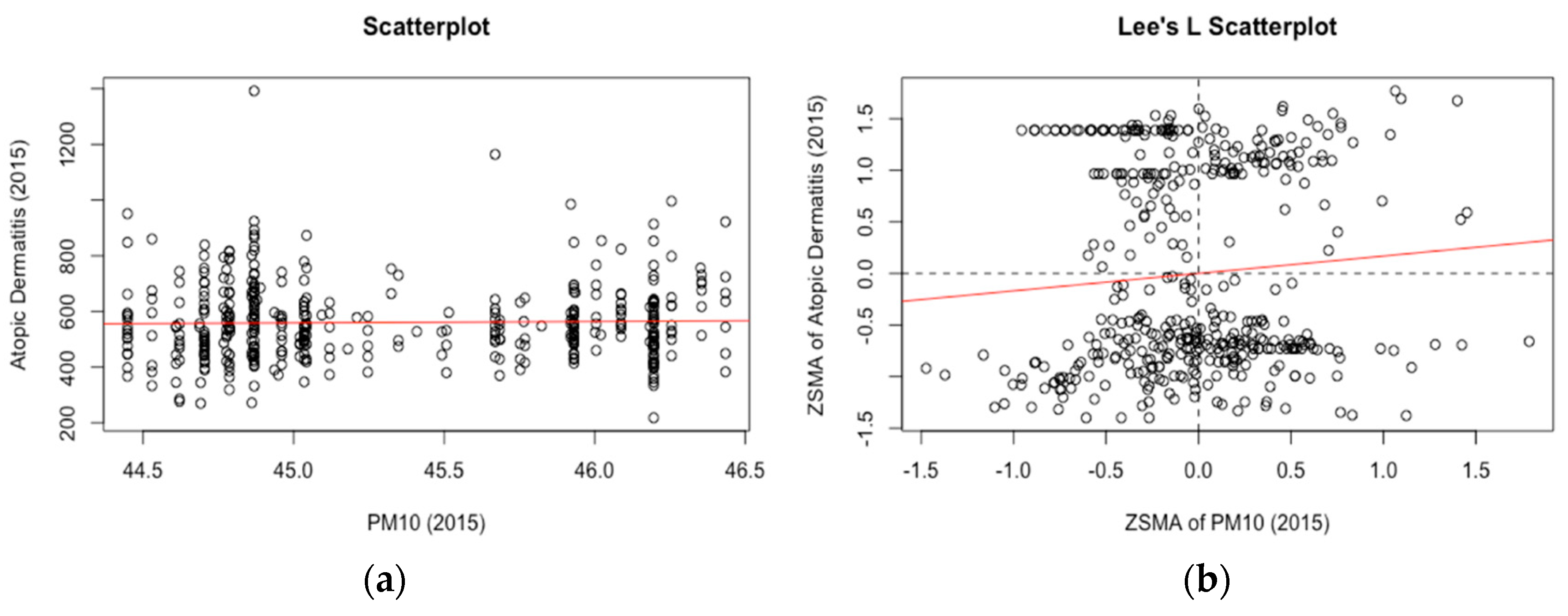
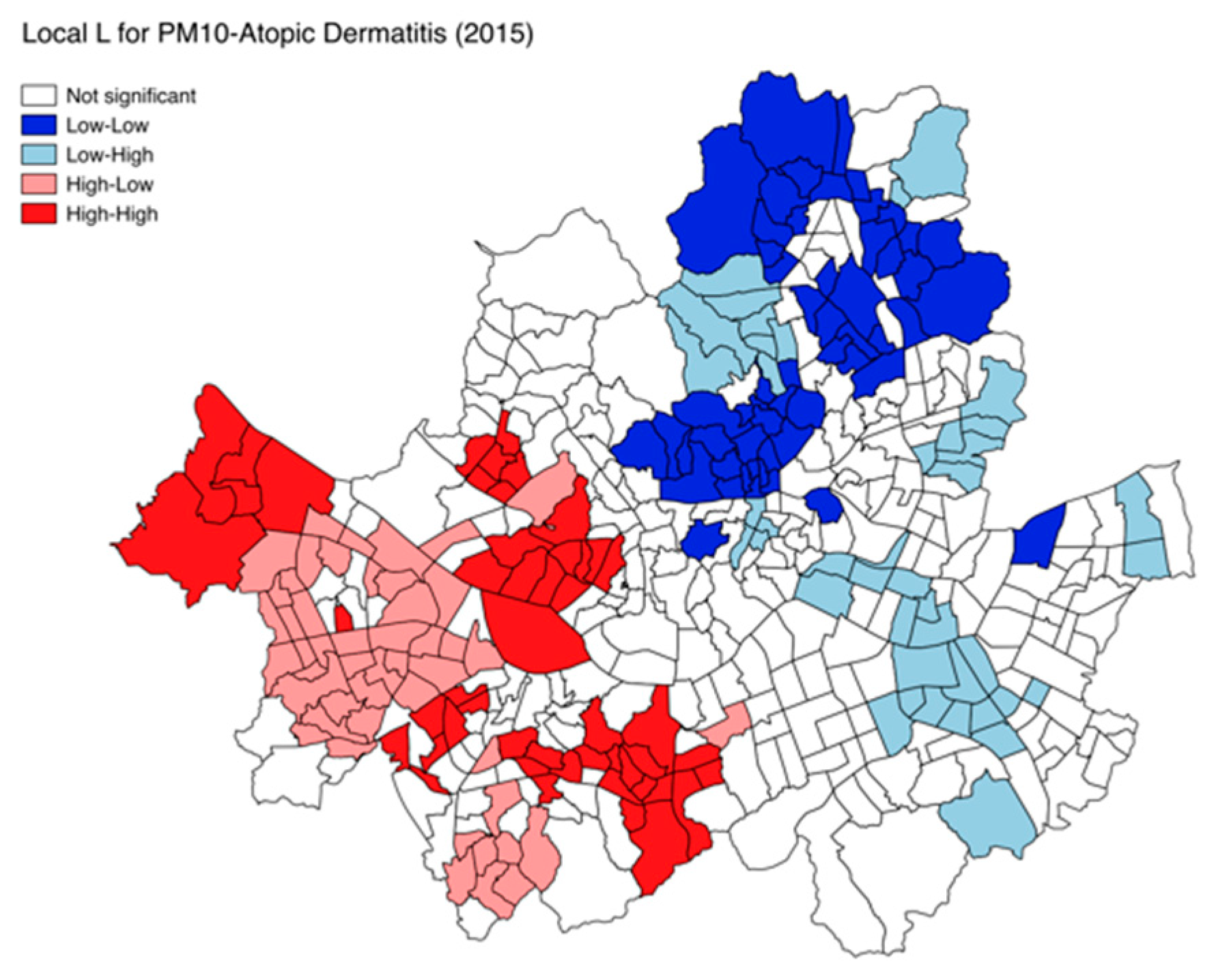
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Institute of Medicine. Clearing the Air: Asthma and Indoor Air Exposures; The National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Borish, L. Genetics of allergy and asthma. Ann. Allergy Asthma Immunol. 1999, 82, 413–426. [Google Scholar] [CrossRef]
- Prescott, S.; Tang, M. The australasian society of clinical immunology and allergy position statement: Summary of allergy prevention in children. Med. J. Aust. 2005, 182, 464–467. [Google Scholar] [PubMed]
- Bernard, S.M.; Samet, J.M.; Grambsch, A.; Ebi, K.L.; Romieu, I. The potential impacts of climate variability and change on air pollution-related health effects in the United States. Environ. Health Perspect. 2001, 109, 199–209. [Google Scholar] [PubMed]
- Wong, T.W.; Tam, W.; Yu, I.T.S.; Wun, Y.T.; Wong, A.H.; Wong, C.M. Association between air pollution and general practitioner visits for respiratory diseases in Hong Kong. Thorax 2006, 61, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Piantadosi, S.; Byar, D.P.; Green, S.B. The ecological fallacy. Am. J. Epidemiol. 1988, 127, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Openshaw, S. The Modifiable Areal Unit Problem; Geobooks: Norwich, UK, 1983. [Google Scholar]
- Bruno, F.; Cameletti, M.; Franco-Villoria, M.; Greco, F.; Ignaccolo, R.; Ippoliti, L.; Valentini, P.; Ventrucci, M. A survey on ecological regression for health hazard associated with air pollution. Spat. Stat. 2016, 18, 276–299. [Google Scholar] [CrossRef]
- Akinbami, L.J.; Schoendorf, K.C. Trends in childhood asthma: Prevalence, health care utilization, and mortality. Pediatrics 2002, 110, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, N.; Bell, N.; Dunn, J.R.; Oliver, L. Deprivation indices, population health and geography: An evaluation of the spatial effectiveness of indices at multiple scales. J. Urban Health 2007, 84, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Dark, S.J.; Bram, D. The modifiable areal unit problem (MAUP) in physical geography. Prog. Phys. Geogr. 2007, 31, 471–479. [Google Scholar]
- Gee, G.C.; Payne-Sturges, D.C. Environmental health disparities: A framework integrating psychosocial and environmental concepts. Environ. Health Perspect. 2004, 112, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Hales, S.; Weinstein, P.; Souares, Y.; Woodward, A. El niño and the dynamics of vectorborne disease transmission. Environ. Health Perspect. 1999, 107, 99–102. [Google Scholar] [PubMed]
- Cocco, P.; Kazerouni, N.; Zahm, S.H. Cancer mortality and environmental exposure to dde in the United States. Environ. Health Perspect. 2000, 108, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Graber, L.K.; Asher, D.; Anandaraja, N.; Bopp, R.F.; Merrill, K.; Cullen, M.R.; Luboga, S.; Trasande, L. Childhood lead exposure after the phaseout of leaded gasoline: An ecological study of school-age children in Kampala, Uganda. Environ. Health Perspect. 2010, 118, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.; Tam, W.; Wong, T.; Lai, C.; Wong, G.; Leung, T.F.; Ng, S.; Hui, D. Effects of air pollution on asthma hospitalization rates in different age groups in Hong Kong. Clin. Exp. Allergy 2007, 37, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Kim, D.; Min, S.; Paul, C.; Yoo, Y.; Choung, J.T. Gis-based association between pm10 and allergic diseases in seoul: Implications for health and environmental policy. Allergy Asthma Immunol. Res. 2016, 8, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Holguin, F. Traffic, outdoor air pollution, and asthma. Immunol. Allergy Clin. N. Am. 2008, 28, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Ko, F.; Lau, T.; Li, S.; Hui, D.; Pang, S.; Leung, R.; Fok, T.; Lai, C. Temporal relationship between air pollution and hospital admissions for asthmatic children in Hong Kong. Clin. Exp. Allergy 2001, 31, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.; Griffiths, R.; Ayres, J. Temporal association between hospital admissions for asthma in birmingham and ambient levels of sulphur dioxide and smoke. Thorax 1994, 49, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Kimes, D.; Levine, E.; Timmins, S.; Weiss, S.R.; Bollinger, M.E.; Blaisdell, C. Temporal dynamics of emergency department and hospital admissions of pediatric asthmatics. Environ. Res. 2004, 94, 7–17. [Google Scholar] [CrossRef]
- Griffith, D.A. Spatial Autocorrelation: A Primer; Association of American Geographers: Washington, DC, USA, 1987. [Google Scholar]
- Haining, R. Bivariate correlation with spatial data. Geogr. Anal. 1991, 23, 210–227. [Google Scholar] [CrossRef]
- Park, Y.M.; Kim, Y. A spatially filtered multilevel model to account for spatial dependency: Application to self-rated health status in South Korea. Int. J. Health Geogr. 2014, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P. Spatial autocorrelation: Trouble or new paradigm? Ecology 1993, 74, 1659–1673. [Google Scholar] [CrossRef]
- Shankardass, K.; Jerrett, M.; Dell, S.; Foty, R.; Stieb, D. Spatial analysis of exposure to traffic-related air pollution at birth and childhood atopic asthma in Toronto, Ontario. Health Place 2015, 34, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Crighton, E.J.; Feng, J.; Gershon, A.; Guan, J.; To, T. A spatial analysis of asthma prevalence in Ontario. Can. J. Public Health 2012, 103, 384–389. [Google Scholar]
- Kim, B.-K.; Kim, J.-Y.; Kang, M.-K.; Yang, M.-S.; Park, H.-W.; Min, K.-U.; Cho, S.-H.; Kang, H.-R. Allergies are still on the rise? A 6-year nationwide population-based study in Korea. Allergol. Int. 2016, 65, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yang, H.; Kim, M.; Kim, J.; Ahn, K. Is the prevalence of atopic dermatitis in korean children decreasing?: Analysis of the national statistics data, 2009–2014. Asian Pac. J. Allergy Immunol. 2017, 35, 144–149. [Google Scholar] [PubMed]
- Ghim, Y.S.; Moon, K.-C.; Lee, S.; Kim, Y.P. Visibility trends in Korea during the past two decades. J. Air Waste Manag. Assoc. 2005, 55, 73–82. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Environmental Research. Annual Report of Air Quality in Korea 2015. Available online: http://webbook.me.go.kr/DLi-File/NIER/09/022/5618423.pdf (accessed on 13 September 2017).
- Leem, J.-H.; Kaplan, B.M.; Shim, Y.K.; Pohl, H.R.; Gotway, C.A.; Bullard, S.M.; Rogers, J.F.; Smith, M.M.; Tylenda, C.A. Exposures to air pollutants during pregnancy and preterm delivery. Environ. Health Perspect. 2006, 114, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environment. What Is Fine Dust? Available online: http://m.me.go.kr/issue/finedust/ebook.pdf (accessed on 13 September 2017).
- Son, J.-Y.; Bell, M.L.; Lee, J.-T. Individual exposure to air pollution and lung function in Korea: Spatial analysis using multiple exposure approaches. Environ. Res. 2010, 110, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Hong, S.-J. Ambient air pollution and allergic diseases in children. Korean J. Pediatr. 2012, 55, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.S.; Hieu, N.C.; Kim, S.M.; Sohn, J.W.; Heo, J. Spatial autocorrelation of disease prevalence in south korea using 2012 community health survey data. J. Korean Soc. Surv. Geodesy Photogramm. Cartogr. 2016, 34, 253–262. [Google Scholar] [CrossRef]
- Hasegawa, K.; Tsugawa, Y.; Tsai, C.L.; Brown, D.F.; Camargo, C.A. Frequent utilization of the emergency department for acute exacerbation of chronic obstructive pulmonary disease. Respir. Res. 2014, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Steer, J.; Gibson, G.J.; Bourke, S.C. Predicting outcomes following hospitalization for acute exacerbations of copd. QJM Int. J. Med. 2010, 103, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ding, H.; Jiang, L.; Chen, S.; Zheng, J.; Qiu, M.; Zhou, Y.; Chen, Q.; Guan, W. Association between air pollutants and asthma emergency room visits and hospital admissions in time series studies: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0138146. [Google Scholar] [CrossRef] [PubMed]
- Romangnani, S. The increased prevalence of allergy and the hygiene hypothesis: Missing immune deviation, reduced immune suppression, or both? Immunology 2004, 112, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.M.; Paller, A.S. Atopic dermatitis and the atopic march. J. Allergy Clin. Immunol. 2003, 112, S118–S127. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J.; Han, Y.; Lee, B.; Choi, D.; Cheong, H.; Jeon, B.; Oh, I.; Bae, G.; Lee, J.; et al. Comparison of diverse estimation methods for personal exposure to air pollutants and associations with allergic symptoms: The allergy & gene-environment link (angel) study. Sci. Total Environ. 2017, 579, 1127–1136. [Google Scholar] [PubMed]
- Joseph, J.; Sharif, H.O.; Sunil, T.; Alamgir, H. Application of validation data for assessing spatial interpolation method for 8-h ozone or other sparsely monitored constituents. Environ. Pollut. 2013, 178, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Peuquet, D.J.; Duan, Y.; Whitsel, E.A.; Dou, J.; Smith, R.L. GIS approaches for the estimation of residential-level ambient PM concentrations. Environ. Health Perspect. 2006, 114, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Dormann, C.; McPherson, J.M.; Araujo, M.B.; Bivand, R.; Bolliger, J.; Carl, G.; Davies, R.G.; Hirzel, A.; Jetz, W.; Kissling, W.D.; et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: A review. Ecography 2007, 30, 609–628. [Google Scholar] [CrossRef]
- Hu, Z.; Rao, K. Particulate air pollution and chronic ischemic heart disease in the eastern United States: A county level ecological study using satellite aerosol data. Environ. Health 2009, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hendryx, M.; Fedorko, E.; Anesetti-Rothermel, A. A geographical information system-based analysis of cancer mortality and population exposure to coal mining activities in West Virginia, United States of America. Geospat. Health 2010, 4, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Anselin, L.; Syabri, I.; Kho, Y. Geoda: An introduction to spatial data analysis. Geogr. Anal. 2006, 38, 5–22. [Google Scholar] [CrossRef]
- Lee, S. Developing a bivariate spatial association measure: An integration of Pearson’s R and Moran’s I. J. Geogr. Syst. 2001, 3, 369–385. [Google Scholar] [CrossRef]
- Ngoc, L.; Park, D.; Lee, Y.; Lee, Y. Systematic review and meta-analysis of human skin diseases due to particulate matter. Int. J. Environ. Res. Public Health 2017, 14, 1458. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.; Zhang, W.; Williams, M.; Pengetnze, Y. Predicting asthma-related emergency department visits using big data. IEEE J. Biomed. Health Inform. 2015, 19, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Kim, H.; Kang, I.; Cheong, J.; Kim, S.; Kook, M.; Kim, B.; Lee, H.; Oh, J. Evaluation of the relationship between pollen count and the outbreak of allergic diseases. Pediatr. Allergy Respir. Dis. 2009, 19, 354–364. [Google Scholar]
- Kim, K.; Kim, K.; Lee, S.; Park, H.; Lee, Y.; Nahm, D.; Son, C.; Yang, D.; Roh, M.; Choi, P.; et al. Sensitization rates for inhalant allergens in patients with respiratory allergy in Busan. Korean J. Asthma Allergy Clin. Immunol. 2005, 25, 59–63. [Google Scholar]
- Shea, K.; Truckner, R.; Weber, R.; Peden, D. Climate change and allergic disease. J. Allergy Clin. Immunol. 2008, 122, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.; Alexis, N.; Bernstein, J.; Cohn, J.; Demain, J.; Horner, E.; Levetin, E.; Nei, A.; Phipatanakul, W. Climate change and our environment: The effect on respiratory and allergic disease. J. Allergy Clin. Immunol. Pract. 2013, 1, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, J.C.; Lumley, T.; Sheppard, L.; Koenig, J.; Shapiro, G. Effects of ambient air pollution on symptom severity and medication use in children with asthma. Ann. Allergy Asthma Immunol. 2003, 91, 346–353. [Google Scholar] [CrossRef]

| 2011 | 2012 | 2013 | 2014 | 2015 | ||
|---|---|---|---|---|---|---|
| Allergic disease prevalence | Allergic rhinitis (children under 12) | 0.260 | 0.295 | 0.291 | 0.284 | 0.290 |
| Asthma (children under 12) | 0.290 | 0.339 | 0.296 | 0.359 | 0.346 | |
| Atopic dermatitis (children under 12) | 0.329 | 0.322 | 0.262 | 0.254 | 0.164 | |
| Ambient air pollutants | SO2 | 0.844 | 0.957 | 0.984 | 0.982 | 0.872 |
| NO2 | 0.833 | 0.711 | 0.761 | 0.876 | 0.901 | |
| CO | 0.796 | 0.891 | 0.618 | 0.957 | 0.969 | |
| PM10 | 0.961 | 0.971 | 0.982 | 0.838 | 0.946 |
| Air Pollutants | Allergic Disease Prevalence | 2011 | 2012 | 2013 | 2014 | 2015 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pearson’s R | Lee’s Global L | Pearson’s R | Lee’s Global L | Pearson’s R | Lee’s Global L | Pearson’s R | Lee’s Global L | Pearson’s R | Lee’s Global L | ||
| SO2 | Allergic rhinitis | NS | NS | NS | NS | NS | NS | −0.1 | NS | NS | NS |
| Asthma | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Atopic dermatitis | NS | NS | NS | NS | NS | NS | −0.151 | NS | −0.114 | NS | |
| NO2 | Allergic rhinitis | −0.113 | NS | −0.118 | NS | NS | NS | NS | NS | NS | NS |
| Asthma | NS | NS | −0.11 | NS | 0.119 | 0.091 | NS | NS | NS | 0.054 | |
| Atopic dermatitis | NS | 0.063 | 0.132 | 0.112 | NS | 0.055 | 0.098 | 0.064 | NS | NS | |
| CO | Allergic rhinitis | NS | NS | −0.099 | NS | NS | 0.039 | NS | NS | NS | NS |
| Asthma | NS | NS | NS | NS | NS | NS | −0.151 | NS | −0.11 | NS | |
| Atopic dermatitis | 0.149 | 0.136 | 0.16 | 0.18 | NS | NS | NS | 0.059 | NS | 0.077 | |
| PM10 | Allergic rhinitis | −0.114 | NS | NS | NS | NS | NS | −0.143 | NS | NS | NS |
| Asthma | −0.174 | NS | −0.114 | NS | NS | NS | −0.139 | NS | −0.127 | NS | |
| Atopic dermatitis | 0.252 | 0.242 | NS | NS | NS | NS | NS | NS | NS | 0.045 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Seo, S.; Min, S.; Simoni, Z.; Kim, S.; Kim, M. A Closer Look at the Bivariate Association between Ambient Air Pollution and Allergic Diseases: The Role of Spatial Analysis. Int. J. Environ. Res. Public Health 2018, 15, 1625. https://doi.org/10.3390/ijerph15081625
Kim D, Seo S, Min S, Simoni Z, Kim S, Kim M. A Closer Look at the Bivariate Association between Ambient Air Pollution and Allergic Diseases: The Role of Spatial Analysis. International Journal of Environmental Research and Public Health. 2018; 15(8):1625. https://doi.org/10.3390/ijerph15081625
Chicago/Turabian StyleKim, Dohyeong, SungChul Seo, Soojin Min, Zachary Simoni, Seunghyun Kim, and Myoungkon Kim. 2018. "A Closer Look at the Bivariate Association between Ambient Air Pollution and Allergic Diseases: The Role of Spatial Analysis" International Journal of Environmental Research and Public Health 15, no. 8: 1625. https://doi.org/10.3390/ijerph15081625
APA StyleKim, D., Seo, S., Min, S., Simoni, Z., Kim, S., & Kim, M. (2018). A Closer Look at the Bivariate Association between Ambient Air Pollution and Allergic Diseases: The Role of Spatial Analysis. International Journal of Environmental Research and Public Health, 15(8), 1625. https://doi.org/10.3390/ijerph15081625





