Abstract
This study aimed to integrate and analyze the existing studies and to explore research trends and hotspots related to the effects of xenobiotics on glucose metabolism in male testes. All articles were retrieved from the PubMed database, from an inception date up to 10 June 2017. CiteSpace software (version 5.1.R8 SE) was used for the co-word cluster analysis. A total of 165 eligible publications were included in this study. In 1949–1959, only two articles were published. After 1960, the number of articles increased steadily. These articles were published in 97 journals, in particular, in the Indian Journal of Experimental Biology (11 articles, 6.7%). Most of the authors (87.0%) only published one article. Only a few established research teams, mostly from the USA, worked consistently in this field. The main xenobiotics that had been studied were medicine and common environmental pollutants, e.g., gossypol, cadmium, di-n-butyl phthalate, and alpha-chlorohydrin. The hotspot keywords were Sertoli cell, lactate dehydrogenase, 6-phosphate dehydrogenase, oxidative stress, and glucose metabolism. The focus of research had been changed overtime. This is the first bibliometric study between xenobiotics and glucose metabolism in the male testes. The findings suggest that environmental pollutants have become a huge concern, and related research should be strengthened.
1. Introduction
Many agricultural and industrial chemicals introduced to the environment are deleterious to the development and reproductive systems of humans and animals [1]. Xenobiotics are chemicals foreign to the human system. They include a wide range of chemicals in our environment, e.g., persistent organic compounds, pesticides, heavy metals, organic solvents, medicine, and tobacco smoke. They are also defined as organic and inorganic compounds, which are produced by human beings and gradually introduced to the environment [2]. Continuous exposure to xenobiotics may have cumulative effects that lead to reproductive disorders [3]. For example, xenobiotics such as glyphosate are potentially toxic to sperm motility [4].
The testicles are a pair of organs that essentially perform two functions: sex steroid hormone biosynthesis and production of spermatozoa [5]. Glucose metabolism in the testes is critical for normal spermatogenesis and fertility [5,6]. Xenobiotics can affect the glucose metabolism at multiple levels, directly or indirectly, and impair spermatogenesis irreversibly [3,5,7].
Bibliometrics has been utilized to evaluate scientific output and the importance of scientific studies [8,9]. It is effective and useful in evaluating the scientific productions and research trends in a specific research field by word cluster analysis. Furthermore, it can identify the intellectual structures and research fronts by analyzing the most cited words.
In this study, a bibliometric analysis of xenobiotics on glucose metabolism in male testis research was carried out based on articles retrieved from the PubMed database, from an inception date to 10 June 2017. We presented publication trend by year, geographic regions, and most influential authors. We aimed to identify the intellectual structure, research trends, and hotspots of the effects of xenobiotics on glucose metabolism in male testes.
2. Methods and Materials
2.1. Search Strategy
PubMed database was searched from inception date up to 10 June 2017. Only studies in English were included. An auxiliary manual retrieval was performed to prevent missing studies. The keyword retrieval strategy was as follows: (1) nutrient or nutrition; (2) heavy metal; (3) organic pollutant or organic compound or organic chemical or organic solvent or benzene or cyanide or phenol; (4) pesticide; (5) terms (1) or (2) or (3) or (4); (6) testis or testes or testicle; (7) carbohydrate metabolism or glucose metabolism or metabolism of carbohydrates; and (8) terms (5) and (6) and (7).
2.2. Selected Criteria and Data Extraction
Three authors (Jun Yu, Jiantao Sun, and Yu Wu) conducted the search independently. After removing the duplicates and news reports by EndNote X8, 2408 records were obtained. These data were extracted from the eligible studies according to the following criteria: (1) focused on male testis (including humans or animals, tissues or cells); (2) the contents of the study were about xenobiotics and glucose metabolism; and (3) written in English. The exclusion criteria included the following: (1) similar objective results in the same study or in the same institution at different time and (2) duplicates and news reports. Meanwhile, references were examined manually to identify any of the missing articles. If the full text of the included articles could not be obtained directly from the databases, we used the document delivery service from Wuhan University Library or directly contacted the author via email. The screening and review strategy is illustrated in Figure 1.
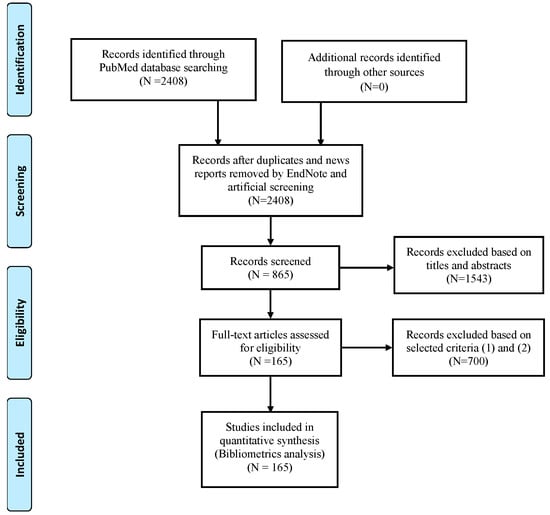
Figure 1.
Flow diagram of study selection based on PRISMA 2009 guidelines [10] (PRISMA is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses).
The collection of relevant data was extracted from the eligible studies. The main information included the title, year, corresponding author and his/her affiliation and country, journal title, xenobiotics, and experimental materials.
2.3. Analysis Methods
A descriptive analysis was used to present the characteristics of the included studies by publication years, countries, journals, and research teams.
To identify the intellectual structure and impacted works in the research field of xenobiotics on glucose metabolism in male testes, analyses were carried out in CiteSpace software (version 5.1.R8 SE). CiteSpace software (version 5.1.R8 SE) is a free Java-based application that was founded by Chaomei Chen (http://cluster.ischool.drexel.edu/~cchen/citespace/download/).
3. Results
As shown in Figure 1, 2408 publications were retrieved. After screening for article titles and abstracts, 865 publications remained. Finally, 165 eligible publications were included in this study based on these selected criteria formulated in advance (Table S1).
3.1. Characteristics of the Selected Studies
3.1.1. Publication Years
Figure 2a shows the number of publication of 165 articles by year. In 1949–1959, only two articles were published [10,11]. After 1960, the number of articles increased steadily from 15 in the 1960s to 34 in the 1980s. In the 1990s, the number declined to 22 papers and then increased to 34 in the first two decades of the new millennium.
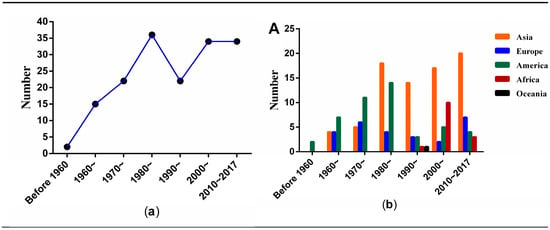
Figure 2.
The distribution of 165 included articles: (a) by publication year; (b) by continents and year.
According to the affiliation or country of the corresponding author, authors from five continents (Asia, America, Europe, Africa, and Oceania) contributed to this field (Figure 2b). Authors from Asia published approximately half of these papers (N = 78, 47.3%), followed by America (N = 46, 27.9%) and Europe (N = 26, 15.8%). American scholars were the first to start the research in this field. The first article was from the USA in 1949. However, after 1980, the number of articles from Asia exceeded the publications from America and Europe. Meanwhile, authors from Oceania only published one article in 1997 [12].
3.1.2. Distribution of Articles by Countries, Journals, and Authors
The authors of the 165 articles were from 29 countries. As listed in Table 1, the top five countries are India, America, China, Egypt, and Portugal. Indian authors published approximately one third of these articles (N = 54, 32.7%). Authors in the USA published a total of 33 articles (20%) and ranked second. China, a developing country, had a steadily increasing article number in basic research and ranked third.

Table 1.
The 5 most productive countries for publications of effects of xenobiotics on glucose metabolism in testes.
The 165 articles were published in 97 journals. Only five journals published no less than five articles. They were the Indian Journal of Experimental Biology (10 articles [13,14,15,16,17,18,19,20,21,22]), Biology of Reproduction (7 articles [23,24,25,26,27,28,29]), Journal of Reproduction and Fertility (6 articles [30,31,32,33,34,35]), Endocrinology (5 articles [10,36,37,38,39]), and International Journal of Andrology (5 articles [40,41,42,43,44]). A total of 26 journals (26.9%) published only 2–4 articles, and 66 journals (68.0%) published only one article. Therefore, no single journal is dominant over the other.
The 165 articles were published from 138 research teams. The top three productive authors for publications are shown in Table 2. The five authors published three articles or more. Satya P. Srivastava was the most productive author with five articles published [21,22,45,46,47]. The following three scientists were from the USA: Mannfred A. Hollinger [33,48,49,50] and Syed Husain [51,52,53,54], who published four articles, and Peter F. Hall [22,37,38], who published three articles. Pedro F. Oliveira from Portugal also published three articles [6,55,56]. For the remaining authors, the majority had only one article, indicating that only a few stable research teams were working in this field and mainly in the USA.

Table 2.
The 5 most productive authors for publications of effects of xenobiotics on glucose metabolism in testes.
3.2. Main Research Topics
3.2.1. Experimental Subjects and Xenobiotic Distribution
The 165 studies were conducted based on the population, animal models, and isolated tissues or cells. The animal models were the most used model to evaluate the association of xenobiotics on glucose metabolism in the testes, accounting for 84.8% of the articles. A total of 195 xenobiotics had been examined. Similar to the trend of publication years, the types of xenobiotics studied increased steadily (Figure 3a). All xenobiotics can be divided into six types: medicines, persistent organic pollutants (POPs), nutrients, heavy metals, pesticides, and others. Figure 3b illustrates that the most studied xenobiotics were medicines. Further analysis showed that several chemicals, e.g., gossypol, cadmium, di-n-butyl phthalate, alpha-chlorohydrin, cyclophosphamide, and delta-9-tetrahydrocannabinol, were frequently studied. Notably, cadmium, di-n-butyl phthalate, and alpha-chlorohydrin are all common environmental pollutants. Gossypol is a male contraceptive and also a residue in cottonseed oil. The findings implied that the effects of environmental pollutants on glucose metabolism in the testes are becoming a hotspot.
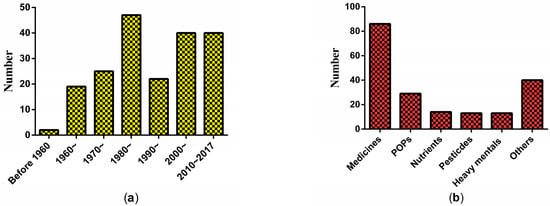
Figure 3.
The distribution of xenobiotics studied related to glucose metabolism in testes: (a) by publication year; (b) types.
3.2.2. Intellectual Structure and Hotspot Analysis
Network and cluster of co-words were applied to explore the intellectual structure and hotspot by CiteSpace software. From the map of co-words (Figure 4), 103 nodes and 263 connections were present between the keywords. The co-word clusters with more frequency and favorable silhouette presented large nodes. The top seven high-frequency clusters were Sertoli cell (SC), lactate dehydrogenase (LDH), 6-phosphate dehydrogenase, oxidative stress, rat testis, male rat, and glucose metabolism. The top seven favorable silhouette clusters were SC, LDH, glucose metabolism, 6-phosphate dehydrogenase, oxidative stress, gamma-glutamyl transpeptidase, and germ cell (Table 3). SC and LDH were the most used words by examining both the frequency and silhouette of co-word clustering. The other three hotspot keywords (6-phosphate dehydrogenase, oxidative stress, and glucose metabolism) were also significantly mentioned in these articles.
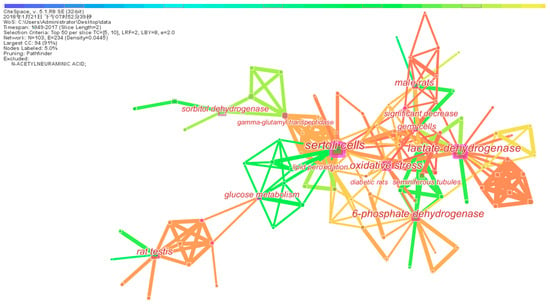
Figure 4.
A network of co-words of 165 articles.

Table 3.
The top 7 co-word clusters with high frequency and silhouette in articles examining effects of xenobiotics on glucose metabolism in testes.
Timeline mapping of co-words was applied to further exhibit the research trends of this field. As illustrated in Figure 5, four noticeable turning points were observed in the term usage trend. Since 1972, “rat testis” was mostly used, as most of the studies were animal models. In the 1980s, the word “Sertoli cell” was introduced, as studies in isolated cells emerged in this field. In the late 1980s, more researchers began to study “lactate dehydrogenase” and “6-phosphate dehydrogenase” and this trend has changed into “oxidative stress” since 2006.
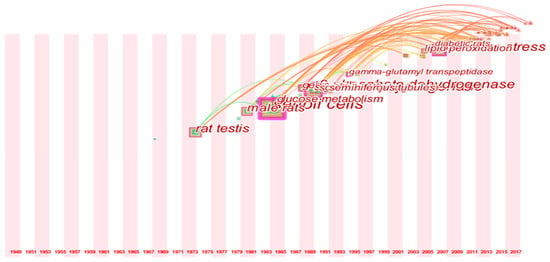
Figure 5.
Timeline view for co-words analysis.
3.2.3. Main Regulatory Pathways
In accordance with previous studies, oxidative stress was a key factor in the etiology of male infertility, as demonstrated by enhanced lipid peroxidation and antioxidant defense system [57,58,59]. Another regulatory pathway, apoptosis, was presented by the balance of proapoptotic factor and antiapoptotic factors [1]. More details are provided in Figure 6.
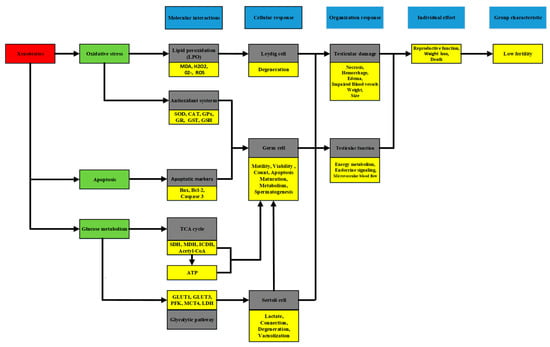
Figure 6.
The main regulatory pathways induced by xenobiotics. Malondialdehyde (MDA), Superoxide radical (O2−), Hydrogen peroxide (H2O2), Reactive oxygen species (ROS), Peroxisome proliferator-activated receptor-gamma (PPAR-γ), Superoxide dismutase (SOD), Catalase (CAT), Glutathione peroxidase (GPx), Glutathione-S-transferase (GST), Glutathione reductase (GR), Reduced glutathione (GSH), Succinate dehydrogenase (SDH), Malate dehydrogenase (MDH), Isocitrate dehydrogenase (ICDH), Phosphofructokinase (PFK), Lactate dehydrogenase (LDH).
In this study, the main changes induced by xenobiotics had more association with testicular glucose metabolism, and SCs metabolism was primarily affected [56,60]. Glycolysis was the main pathway for SCs to provide adequate energy substrate for themselves and germ cells. The expression of glucose transporter type 1 (GLUT1) and glucose transporter type 3 (GLUT3) was mainly distributed on the plasma membrane of SCs and was responsible for glucose transport [61]. For example, melatonin-exposed SCs presented higher GLUT1 expression [56], caffeine significantly increased the protein levels of GLUT1 and GLUT3 in human SCs [60], and both stimulated glucose uptake. Once glucose was transported into SC, the glycolytic pathway came into function, and phosphofructokinase was the rate-limiting control [62]. Glucose normally converted into lactate via LDH and transported across the plasma membrane to germ cells by specific monocarboxylate transporters (MCTs), particularly monocarboxylate transporter 1 (MCT1) and monocarboxylate transporter 4 (MCT4) [55]. The decline in their activities would lead to numerous abnormalities in SCs, including alterations in connection (the main component of the blood–testis barrier), cell degeneration, and vacuolization [63]. Thus, less lactate production and transportation was from SCs. Finally, it would cause defects in sperm maturation and spermatogenesis and even accelerated germ cell apoptosis. A study showed that testis exposed to xenobiotics have altered tricarboxylic acid (TCA) cycle due to testicular enzymes (SDH, Sorbitol dehydrogenase; MDH, Malate dehydrogenase; ICDH, Isocitrate dehydrogenase) in the mitochondrial fractions [59]. The pachytene spermatocyte maturation of the germinal epithelium was associated with SDH. The decreased SDH activity was mainly attributed to the reduced aerobic oxidation of acetyl CoA and ATP [64]. ATP served as an energy substance, and the loss of sperm motility was possibly correlated with low generation of ATP by xenobiotic-induced mitochondrial impairment [65].
Therefore, the balance loss in the main regulatory pathways would result in testicular damage and testicular dysfunction. The testicular damage included impaired vessels, changes in testicular size and weight with irreversible edema, hemorrhage, and necrosis. The testicular dysfunction induced by xenobiotics in energy metabolism, endocrine signaling, and microvascular blood flow would lead to body weight loss and reproductive problems, even death in individual effect. Finally, group characteristic presented low fertility.
4. Discussion
In this article, we presented the characteristics and research hotspot of the selected studies in the field of xenobiotic effects on glucose metabolism in male testes from 1949 to 2017. The first article was from the USA in 1949, but most published authors in this field were from Asia. Thus, developing countries, e.g., India and China, were more interested in researching this field.
Furthermore, the most studied xenobiotics were mainly medicine. The most frequently studied medicine was gossypol, a contraceptive causing infertility in humans and animals [66]. Gossypol was also a residue in cottonseed oil, and its side effects in male reproductive health should not be ignored [62,67]. Further analysis showed that cadmium, di-n-butyl phthalate, and alpha-chlorohydrin were in the top four chemicals studied, and these are common environmental pollutants. With the number of xenobiotics increasingly introduced to the environment, their impact on glucose metabolism dysfunction in male testes is becoming an important topic of research.
CiteSpace software was used to analyze research hotspots and emerging trends. Based on the co-word cluster analysis, hotspot keywords were Sertoli cell, lactate dehydrogenase, 6-phosphate dehydrogenase, oxidative stress, and glucose metabolism. The word with the highest frequency and the greatest silhouette was SCs. SCs are responsible for providing energy and nutritional support to germ cell development [5]. Germ cell development has specific metabolic requirements, preferentially using lactate as a substrate for ATP production. SCs produce lactate via the metabolism of various substrates, preferentially glucose. This is why SCs were the most valuable target for studying the deleterious effect of xenobiotics [1]. Meanwhile, some enzymes such as LDH and 6-phosphate dehydrogenase, which are associated with glucose metabolism, and ATP were also an active research area in recently published papers.
From timeline mapping of co-words, the focus of the research has changed over time. Before 1980, the effect of exogenous chemicals on testes was the key point; after 1980, the adverse effect of xenobiotics on glucose metabolism was studied in testicular cells [59,68]. In 1989, the research focus had shifted to enzymes related to testicular cell glucose metabolism, such as LDH and 6-phosphate dehydrogenase. Subsequently, the mechanism of how exogenous chemicals disrupted the glucose metabolism of testicular cells was also studied. Since 2006, one of the mechanisms, oxidative stress, has become a hotspot. Previous studies found that oxidative stress directly or indirectly interfered with enzyme activity and affected testicular carbohydrate metabolism [69,70,71].
Glucose metabolism was primarily affected by xenobiotics in the testes, including the process of TCA cycle and glycolytic pathway. The molecular interactions changed the specific markers during the process, resulting in alterations in the function and structure of Leydig cells, germ cells, and SCs to ameliorate the injury. Further testicular damage and dysfunction decreased fertility.
However, a few limitations must be considered for the bibliometric assessment. First, quantifying multiple literature problems is difficult. In particular, the literature system is very complex and unstable enough that we cannot obtain enough and effective information to reveal the macroscopic rule of the literature. Second, our articles were only from the PubMed databases. Third, bibliometrics depend on the support of mathematical and statistical techniques. Thus, some information may not have been analyzed.
5. Conclusions
To our knowledge, this study is the first bibliometric assessment of xenobiotics on glucose metabolism literature from 1949 to 2017. The number of published articles increased rapidly, especially in Asia. Medicines and environmental pollutants were the main xenobiotics studied. SCs were the most used models in these studies, and most studies focused on the glucose metabolism related to enzymes. Because environmental pollution is a huge concern and has a great impact on the human reproductive system, further research in this area will undoubtedly take place.
Supplementary Materials
The following are available online at http://www.mdpi.com/1660-4601/15/8/1590/s1, Table S1: The list of 165 eligible publications.
Author Contributions
The study question was conceptualized by J.Y., J.S., Y.W.; Pubmed database was set by Y.M., Z.H.; Analyses were conducted by X.Z.; manuscript was wrote by Y.F., G.Y.; the manuscript was substantively revised by C.W. All authors read and approved the final manuscript.
Funding
The project was supported by The National Natural Science Foundation of China (No. 81773471) and The National Science Foundation for Post-doctoral Scientists of China (No. 2015M582279).
Acknowledgments
The authors thank all members in C.W.’s laboratory at Wuhan University for their valuable assistances for comments of the manuscript and Chunzhe Duan in The University of South Florida College of Medicine for revision of manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alves, M.G.; Neuhaus-Oliveira, A.; Moreira, P.I.; Socorro, S.; Oliveira, P.F. Exposure to 2,4-dichlorophenoxyacetic acid alters glucose metabolism in immature rat Sertoli cells. Reprod. Toxicol. 2013, 38, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Iovdijova, A.; Bencko, V. Potential risk of exposure to selected xenobiotic residues and their fate in the food chain—Part I: Classification of xenobiotics. Ann. Agric. Environ. Med. 2010, 17, 183–192. [Google Scholar] [PubMed]
- Aly, H.A.; Azhar, A.S. Methoxychlor induced biochemical alterations and disruption of spermatogenesis in adult rats. Reprod. Toxicol. 2013, 40, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Anifandis, G.; Katsanaki, K.; Lagodonti, G.; Messini, C.; Simopoulou, M.; Dafopoulos, K.; Daponte, A. The Effect of Glyphosate on Human Sperm Motility and Sperm DNA Fragmentation. Int. J. Environ. Res. Public Health 2018, 15, 1117. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.; Alves, M.G.; Rato, L.; Laurentino, S.; Silva, J.; Sá, R.; Barros, A.; Sousa, M.; Carvalho, R.A.; Cavaco, J.E.; et al. Effect of insulin deprivation on metabolism and metabolism-associated gene transcript levels of in vitro cultured human Sertoli cells. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.; Aktas, C.; Erboga, M. Curcumin attenuates testicular damage, apoptotic germ cell death, and oxidative stress in streptozotocin-induced diabetic rats. Mol. Nutr. Food Res. 2013, 57, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Mund, M.; Kloft, B.; Bundschuh, M.; Klingelhoefer, D.; Groneberg, D.A.; Gerber, A. Global Research on Smoking and Pregnancy—A Scientometric and Gender Analysis. Int. J. Environ. Res. Public Health 2014, 11, 5792–5806. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Fu, H.; Ho, Y. Research trends and hotspots related to ammonia oxidation based on bibliometric analysis. Environ. Sci. Pollut. Res. 2017, 24, 20409–20421. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Paul, H.E.; Paul, M.F.; Kopko, F.; Bender, R.C.; Everett, G. Carbohydrate metabolism studies on the testis of rats fed certain nitrofurans. Endocrinology 1953, 53, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Miller, Z.B.; Davison, C.; Smith, P.K. The effect of podophyllotoxin on tissue metabolism and enzyme systems. J. Exp. Med. 1949, 90, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Boukhliq, R.; Martin, G.B.; White, C.L.; Blackberry, M.A.; Murray, P.J. Role of glucose, fatty acids and protein in regulation of testicular growth and secretion of gonadotrophin, prolactin, somatotrophin and insulin in the mature ram. Reprod. Fertil. Dev. 1997, 9, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Bedwal, R.S.; Edwards, M.S.; Katoch, M.; Bahuguna, A.; Dewan, R. Histological and biochemical changes in testis of zinc deficient BALB/c strain of mice. Indian J. Exp. Biol. 1994, 32, 243–247. [Google Scholar] [PubMed]
- Bhargava, S.K. Effects of plumbagin on reproductive function of male dog. Indian J. Exp. Biol. 1984, 22, 153–156. [Google Scholar] [PubMed]
- Chinoy, N.J.; Seethalakshmi, L. Effects of drugs on the reproductive physiology of male albino rats: Part II—Nicotine & morphine. Indian J. Exp. Biol. 1978, 16, 323–325. [Google Scholar] [PubMed]
- Chinoy, N.J.; Seethalakshmi, L. Effects of drugs on the reproductive physiology of male albino rats: Part I—Central depressants & analgesic-antipyretics. Indian J. Exp. Biol. 1978, 16, 316–322. [Google Scholar] [PubMed]
- Jehan, Q.; Kamboj, V.P.; Kar, A.B. Effect of progesterone on biochemical composition of rat seminiferous tubules. Indian J. Exp. Biol. 1970, 8, 68–70. [Google Scholar] [PubMed]
- Sarkar, S.N.; Majumdar, A.C.; Chattopadhyay, S.K. Effect of isoproturon on male reproductive system: Clinical, histological and histoenzyonological studies in rats. Indian J. Exp. Biol. 1997, 35, 133. [Google Scholar] [PubMed]
- Shrivastava, S.; Jadon, A.; Shukla, S.; Mathur, R. Chelation therapy and vanadium: Effect on reproductive organs in rats. Indian J. Exp. Biol. 2007, 45, 515–523. [Google Scholar] [PubMed]
- Sinha, S.; Mathur, R.S. Effect of steroidal fraction of seeds of Abrus precatorius Linn. on rat testis. Indian J. Exp. Biol. 1990, 28, 752–756. [Google Scholar] [PubMed]
- Srivastava, S.; Seth, P.K.; Srivastava, S.P. Effect of styrene on testicular enzymes of growing rat. Indian J. Exp. Biol. 1992, 30, 399–401. [Google Scholar] [PubMed]
- Srivastava, S.; Singh, G.B.; Srivastava, S.P.; Seth, P.K. Testicular toxicity of di-n-butyl phthalate in adult rats: Effect on marker enzymes of spermatogenesis. Indian J. Exp. Biol. 1990, 28, 67–70. [Google Scholar] [PubMed]
- Mita, M.; Hall, P.F. Metabolism of round spermatids from rats: Lactate as the preferred substrate. Biol. Reprod. 1982, 26, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Nehar, D.; Mauduit, C.; Boussouar, F.A.; Benahmed, M. Interleukin 1α Stimulates Lactate Dehydrogenase A Expression and Lactate Production in Cultured Porcine Sertoli Cells1. Biol. Reprod. 1998, 59, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Ravault, J.P.; Courot, M.; Garnier, D.; Pelletier, J.; Terqui, M. Effect of 2-bromo-alpha-ergocryptine (CB 154) on plasma prolactin, LH and testosterone levels, accessory reproductive glands and spermatogenesis in lambs during puberty. Biol. Reprod. 1977, 17, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.; Borriero, L.; Tanphaichitr, N.; Bellve, A.R.; Benos, D.J. Energy metabolism of cultured TM4 cells and the action of gossypol. Biol. Reprod. 1986, 34, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Storey, B.T. Energy metabolism of spermatozoa. IV. Effect of calcium on respiration of mature epididymal sperm of the rabbit. Biol. Reprod. 1975, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vera, C.N.; Gomes, W.R.; Vandemark, N.L. The effects of steroid and gonadotrophic hormones in vitro on the metabolic activity of normal and cryptorchid rat testicular tissues. Biol. Reprod. 1970, 2, 376–386. [Google Scholar] [CrossRef]
- Voglmayr, J.K. Alpha-chlorohydrin-induced changes in the distribution of free myo-inositol and prostaglandin F2alpha, and synthesis of phosphatidylinositol in the rat epididymis. Biol. Reprod. 1974, 11, 593. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.M.; Dacheux, J.L.; Waites, G.M. Effects of alpha-chlorohydrin on the metabolism of testicular and epididymal spermatozoa of rams. J. Reprod. Fertil. 1976, 48, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Grootegoed, J.A.; Jansen, R.; van der Molen, H.J. Effect of glucose on ATP dephosphorylation in rat spermatids. J. Reprod. Fertil. 1986, 77, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Harkonen, M.; Kormano, M. Acute cadmium-induced changes in the energy metabolism of the rat testis. J. Reprod. Fertil. 1970, 21, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, M.A.; Davis, J.R. Effect of nitrofurazone on the aerobic metabolism of uniformly labelled [14C]glucose in tissue slices of rat testes. J. Reprod. Fertil. 1969, 19, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Moonsammy, G.I.; Stewart, M.A. Hexose phosphate levels in testes of galactose-fed rats. J. Reprod. Fertil. 1971, 27, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.L.; Smith, F.A.; Garman, R.H. Effects of fluoroacetate on the testis of the rat. J. Reprod. Fertil. 1979, 56, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Free, M.J.; Vera, C.N.; Johnson, A.D.; Gomes, W.R. Metabolism of glucose-1-14C and glucose-6-14C by testis tissue from cryptorchid and testosterone propionate treated rabbits. Endocrinology 1968, 82, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Means, A.R.; Hall, P.F. Protein biosynthesis in the testis. II. Role of adenosine triphosphate (ATP) in stimulation by glucose. Endocrinology 1968, 83, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.; Borland, K.; Price, J.M.; Hall, P.F. The influence of insulin and insulin-like growth factor-I on hexose transport by Sertoli cells. Endocrinology 1985, 116, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.; Price, J.M.; Hall, P.F. Stimulation by follicle-stimulating hormone of synthesis of lactate by Sertoli cells from rat testis. Endocrinology 1982, 110, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Guraya, S.S. Effect of low doses of alpha chlorohydrin on the enzymes of glycolytic and phosphogluconate pathways in the rat testis and epididymis. Int. J. Androl. 1981, 4, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Meroni, S.B.; Riera, M.F.; Pellizzari, E.H.; Schteingart, H.F.; Cigorraga, S.B. Possible role of arachidonic acid in the regulation of lactate production in rat Sertoli cells. Int. J. Androl. 2003, 26, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Paz, G.; Carmon, A.; Homonnai, Z.T. Effect of alpha-chlorohydrin on metabolism and testosterone secretion by rat testicular interstitial cells. Int. J. Androl. 1985, 8, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.R.; Govindarajulu, P. Effect of prolactin inhibition by bromocriptine on testicular metabolism in the adult rat. Int. J. Androl. 1982, 5, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Shrilatha, B. Occurrence of oxidative impairments, response of antioxidant defences and associated biochemical perturbations in male reproductive milieu in the Streptozotocin-diabetic rat. Int. J. Androl. 2007, 30, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Prasad, A.K.; Srivastava, S.C.; Shankar, R.; Srivastava, S.P. Effect of oral administration of carbofuran on male reproductive system of rat. Hum. Exp. Toxicol. 2016, 14, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.K.; Pant, N.; Srivastava, S.C.; Kumar, R.; Srivastava, S.P. Effect of dermal application of hexachlorocyclohexane (HCH) on male reproductive system of rat. Hum. Exp. Toxicol. 1995, 14, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.P.; Srivastava, S.; Saxena, D.K.; Chandra, S.V.; Seth, P.K. Testicular effects of di-n-butyl phthalate (DBP): Biochemical and histopathological alterations. Arch. Toxicol. 1990, 64, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, M.A.; Davis, J.R. Effect of nitrofurazone on the incorporation of l-lysine-U-14C into protein of rat testis. Biochem. Pharmacol. 1966, 15, 1235–1237. [Google Scholar] [CrossRef]
- Hollinger, M.A. Effects of clomiphene on various biochemical reactions in the mouse testis in vitro. Biochem. Pharmacol. 1971, 20, 2959–2964. [Google Scholar] [CrossRef]
- Hollinger, M.A.; Hwang, F. Effect of puromycin and actinomycin D on glucose-stimulated protein and RNA labeling, in vitro, in rat testis. Biochim. Biophys. Acta 1972, 262, 336–343. [Google Scholar] [CrossRef]
- Husain, S. Effects of delta-9-tetrahydrocannabinol on in vitro energy substrate metabolism in mouse and rat testis. Physiol. Behav. 1989, 46, 65–68. [Google Scholar] [CrossRef]
- Husain, S. Energy substrate metabolism in testis of rats treated with delta-9-tetrahydrocannabinol (THC) and cocaine (COC). NIDA Res. Monogr. 1989, 95, 509–510. [Google Scholar] [PubMed]
- Husain, S.; Anwer, J. Characteristics of cocaine interaction with delta-9-tetrahydrocannabinol on glucose metabolism in the rat testis. Pharmacol. Biochem. Behav. 1991, 40, 625–628. [Google Scholar] [CrossRef]
- Husain, S.; Lame, M.; DeBoer, B. Rat testicular tissue glucose metabolism in the presence of delta-9-tetrahydrocannabinol. Proc. West. Pharmacol. Soc. 1979, 22, 355–358. [Google Scholar] [PubMed]
- Rato, L.; Alves, M.G.; Socorro, S.; Carvalho, R.A.; Cavaco, J.E.; Oliveira, P.F. Metabolic modulation induced by oestradiol and DHT in immature rat Sertoli cells cultured in vitro. Biosci. Rep. 2012, 32, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.S.; Martins, A.D.; Rato, L.; Silva, B.M.; Oliveira, P.F.; Alves, M.G. Melatonin alters the glycolytic profile of Sertoli cells: Implications for male fertility. Mol. Hum. Reprod. 2014, 20, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, S.C.; Jubendradass, R.; Jayakanthan, M.; Rani, S.J.; Mathur, P.P. Bisphenol A impairs insulin signaling and glucose homeostasis and decreases steroidogenesis in rat testis: An in vivo and in silico study. Food Chem. Toxicol. 2012, 50, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- De Liz, O.C.V.; Cattani, D.; Heinz, R.C.; Pierozan, P.; Zanatta, L.; Benedetti, P.E.; Wilhelm, F.D.; Mena, B.S.F.; Pessoa-Pureur, R.; Zamoner, A. Roundup disrupts male reproductive functions by triggering calcium-mediated cell death in rat testis and Sertoli cells. Free Radic. Biol. Med. 2013, 65, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.A.; El-Beshbishy, H.A.; Banjar, Z.M. Mitochondrial dysfunction induced impairment of spermatogenesis in LPS-treated rats: Modulatory role of lycopene. Eur. J. Pharmacol. 2012, 677, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Dias, T.R.; Alves, M.G.; Bernardino, R.L.; Martins, A.D.; Moreira, A.C.; Silva, J.; Barros, A.; Sousa, M.; Silva, B.M.; Oliveira, P.F. Dose-dependent effects of caffeine in human Sertoli cells metabolism and oxidative profile: Relevance for male fertility. Toxicology 2015, 328, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Galardo, M.N.; Riera, M.F.; Pellizzari, E.H.; Chemes, H.E.; Venara, M.C.; Cigorraga, S.B.; Meroni, S.B. Regulation of expression of Sertoli cell glucose transporters 1 and 3 by FSH, IL1 beta, and bFGF at two different time-points in pubertal development. Cell Tissue Res. 2008, 334, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.G.; Martins, A.D.; Vaz, C.V.; Correia, S.; Moreira, P.I.; Oliveira, P.F.; Socorro, S. Metformin and male reproduction: Effects on Sertoli cell metabolism. Br. J. Pharmacol. 2014, 171, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.F.; Murray, F.T.; Drylie, D.D. Interstitial compartment pathology and spermatogenic disruption in testes from impotent diabetic men. Anat. Rec. 1985, 213, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, R.; Abhilash, P.A.; Syam, D.S.; Prathibha, P.; Rejitha, S.; John, F.; Kavitha, S.; Indira, M. Protective effect of ascorbic acid against ethanol-induced reproductive toxicity in male guinea pigs. Br. J. Nutr. 2013, 110, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Anifandis, G.; Amiridis, G.; Dafopoulos, K.; Daponte, A.; Dovolou, E.; Gavriil, E.; Gorgogietas, V.; Kachpani, E.; Mamuris, Z.; Messini, C.I.; et al. The In Vitro Impact of the Herbicide Roundup on Human Sperm Motility and Sperm Mitochondria. Toxics 2017, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Bender, H.S.; Derolf, S.Z.; Misra, H.P. Effects of gossypol on the antioxidant defense system of the rat testis. Arch. Androl. 1988, 21, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Alagbonsi, I.A.; Olayaki, L.A.; Salman, T.M. Melatonin and vitamin C exacerbate Cannabis sativa-induced testicular damage when administered separately but ameliorate it when combined in rats. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Sundarraj, K.; Manickam, V.; Raghunath, A.; Periyasamy, M.; Viswanathan, M.P.; Perumal, E. Repeated exposure to iron oxide nanoparticles causes testicular toxicity in mice. Environ. Toxicol. 2017, 32, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.A.; Lightfoot, D.A.; El-Shemy, H.A. Modulatory role of lipoic acid on lipopolysaccharide-induced oxidative stress in adult rat Sertoli cells in vitro. Chem. Biol. Interact. 2009, 182, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Gan, X.; Liu, K.; Lv, X.; Shen, H.; Dai, G.; Xu, H. Iridoid glycoside from Cornus officinalis ameliorated diabetes mellitus-induced testicular damage in male rats: Involvement of suppression of the AGEs/RAGE/p38 MAPK signaling pathway. J. Ethnopharmacol. 2016, 194, 850–860. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).