How Accurate Are the ISAAC Questions for Diagnosis of Allergic Rhinitis in Korean Children?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Case Definitions and Questionnaire
2.3. Allergens
2.4. SPT
2.5. Diagnostic Accuracy
2.6. Statistical Analysis
2.7. Ethics Statement
3. Results
3.1. Demographic Characteristics and Prevalence
3.2. The Accuracy of the Questions
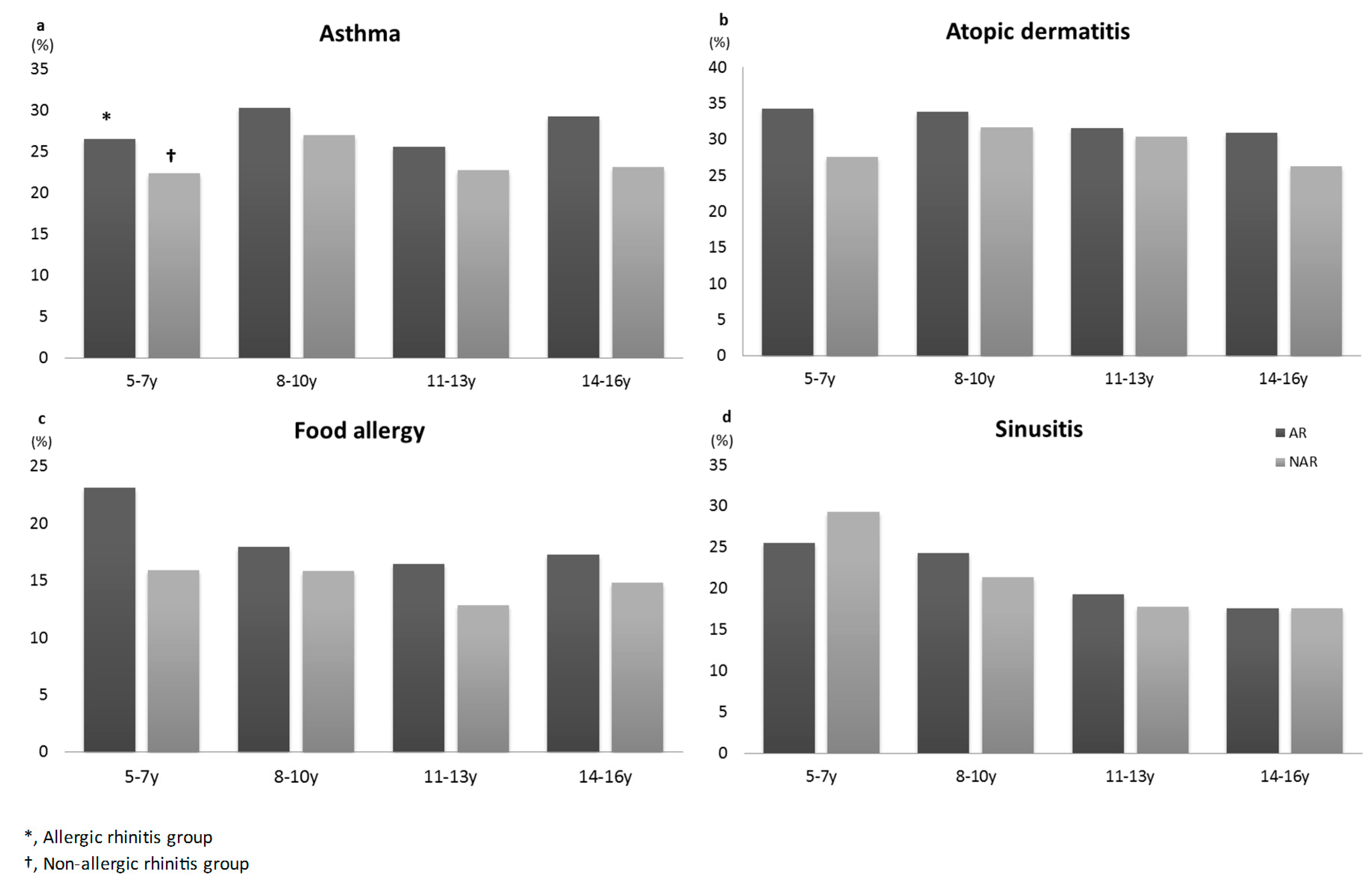
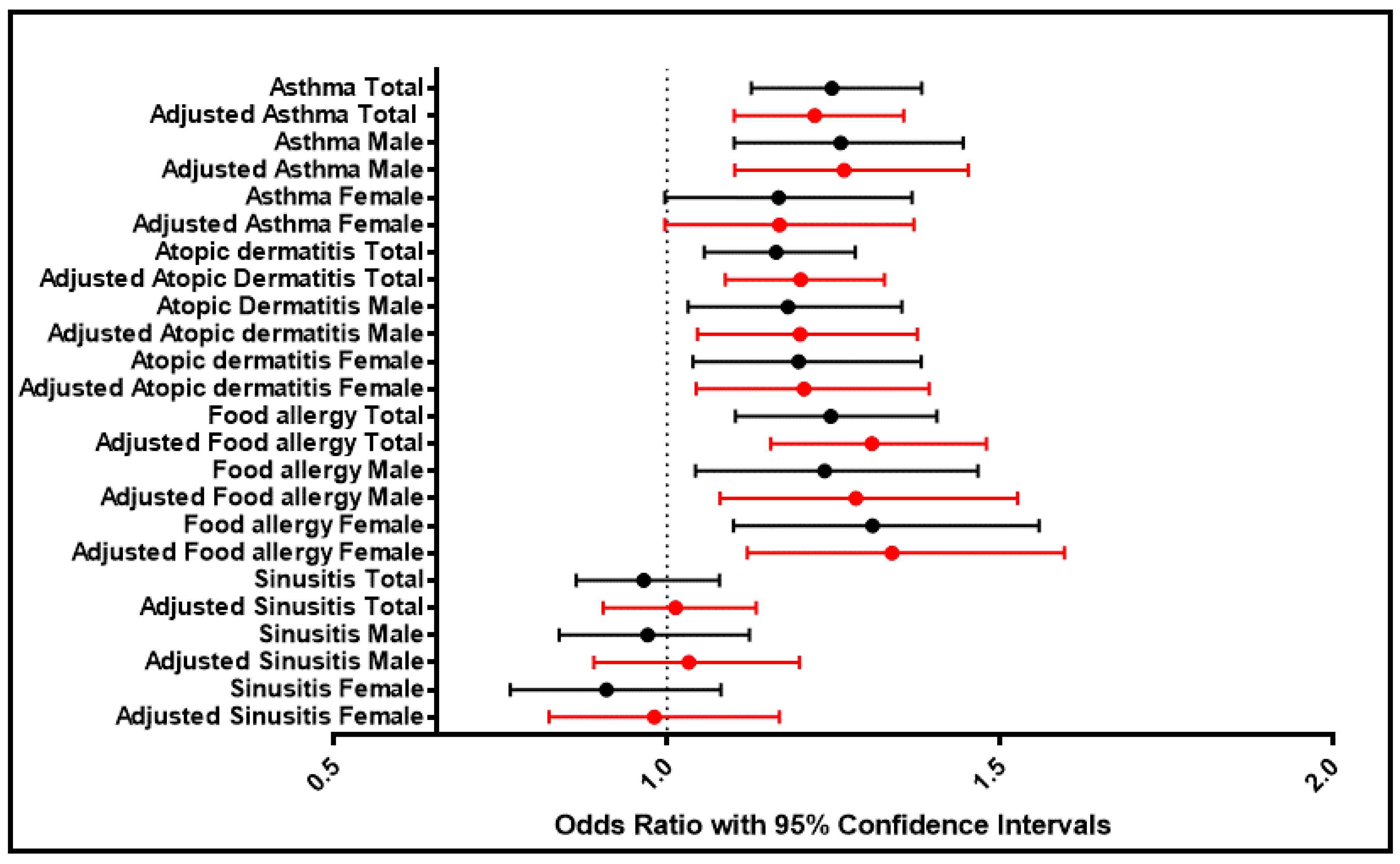
3.3. Comorbidity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; Van Weel, C.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008, 63, 8–160. [Google Scholar] [CrossRef] [PubMed]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.W. International study of asthma and allergies in childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Jee, H.M.; Kim, K.W.; Kim, C.S.; Sohn, M.H.; Shin, D.C.; Kim, K.E. Prevalence of Asthma, Rhinitis and Eczema in Korean Children Using the International Study of Asthma and Allergies in Childhood (ISAAC) Questionnaires. Pediatr. Allergy Respir. Dis. 2009, 19, 165–172. [Google Scholar]
- Kwon, S.E.; Lim, D.H.; Kim, J.H.; Son, B.K.; Park, Y.S.; Jang, H.J.; Kim, B.H.; Kim, G.M.; Yoo, Y.S.; Park, K.W. Prevalence and allergens of allergic rhinitis in children and adolescents in Gwangju. Asthma Respir. Dis. 2015, 3, 54–61. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, Y.S.; Ji Jang, H.; Kim, J.H.; Lim, D.H. Prevalence and allergen of allergic rhinitis in Korean children. Am. J. Rhinol. Allergy 2016, 30, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Ju, Y.S.; Kim, D.S.; Kim, J.Y.; Kwon, H.J.; Kang, D.H.; Lee, S.I.; Cho, S.H. Reliability and validity of the Korean version of ISAAC questionnaire [in Korean]. Korean J. Prev. Med. 1998, 31, 361–371. [Google Scholar]
- Corren, J.; Baroody, F.M.; Pawankar, R. Allergic and nonallergic rhinitis. In Middleton’s Allergy: Principles and Practice, 8th ed.; Adkinson, N.F., Jr., Bochner, B.S., Burks, A.W., Busse, W.W., Holgate, S.T., Lemanske, R.F., O’Hehir, R.E., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2014; pp. 664–685. [Google Scholar]
- Lee, J.E.; Ahn, J.C.; Han, D.H.; Kim, D.Y.; Kim, J.W.; Cho, S.H.; Park, H.W.; Rhee, C.S. Variability of offending allergens of allergic rhinitis according to age: optimization of skin prick test allergens. Allergy Asthma Immunol. Res. 2014, 6, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Yum, H.Y.; Sheen, Y.H.; Park, Y.M.; Lee, Y.J.; Choi, B.S.; Jee, H.M.; Choi, S.H.; Kim, H.H.; Park, Y.; et al. Korean Academy of Pediatric Allergy and Respiratory Disease (KAPARD) Work Group on Rhinitis. Comorbidities and Phenotypes of Rhinitis in Korean Children and Adolescents: A Cross-sectional, Multicenter Study. Allergy Asthma Immunol. Res. 2017, 9, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Braun-Fahrländer, C.; Wüthrich, B.; Gassner, M.; Grize, L.; Sennhauser, F.H.; Varonier, H.S.; Vuille, J.C. Validation of a rhinitis symptom questionnaire (ISAAC core questions) in a population of Swiss school children visiting the school health services. SCARPOL-team. Swiss Study on Childhood Allergy and Respiratory Symptom with respect to Air Pollution and Climate. International Study of Asthma and Allergies in Childhood. Pediatr. Allergy Immunol. 1997, 8, 75–82. [Google Scholar] [PubMed]
- Ibáñez, M.D.; Valero, A.L.; Montoro, J.; Jauregui, I.; Ferrer, M.; Dávila, I.; Bartra, J.; del Cuvillo, A.; Mullol, J.; Sastre, J. Analysis of comorbidities and therapeutic approach for allergic rhinitis in a pediatric population in Spain. Pediatr. Allergy Immunol. 2013, 24, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Brough, H.A.; Simpson, A.; Makinson, K.; Hankinson, J.; Brown, S.; Douiri, A.; Belgrave, D.C.; Penagos, M.; Stephens, A.C.; McLean, W.I.; et al. Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J. Allergy Clin. Immunol. 2014, 134, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Westman, M.; Stjärne, P.; Asarnoj, A.; Kull, I.; van Hage, M.; Wickman, M.; Toskala, E. Natural course and comorbidities of allergic and nonallergic rhinitis in children. J. Allergy Clin. Immunol. 2012, 129, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Leynaert, B.; Liard, R.; Bousquet, J.; Pin, I.; Soussan, D.; Neukirch, F. Lessons from the French part of the European community respiratory health survey (ECRHS). Allergy Clin. Immunol. Int. 1999, 11, 218–225. [Google Scholar] [CrossRef]
- Chawes, B.L.K.; Bønnelykke, K.; Kreiner-Møller, E.; Bisgaard, H. Children with allergic and nonallergic rhinitis have a similar risk of asthma. J. Allergy Clin. Immunol. 2010, 126, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Eckel, S.P.; Kim, J.H.; Gilliland, F.D. Exhaled NO: Determinants and Clinical Application in Children with Allergic Airway Disease. Allergy Asthma Immunol. Res. 2016, 8, 12–21. [Google Scholar] [CrossRef] [PubMed]
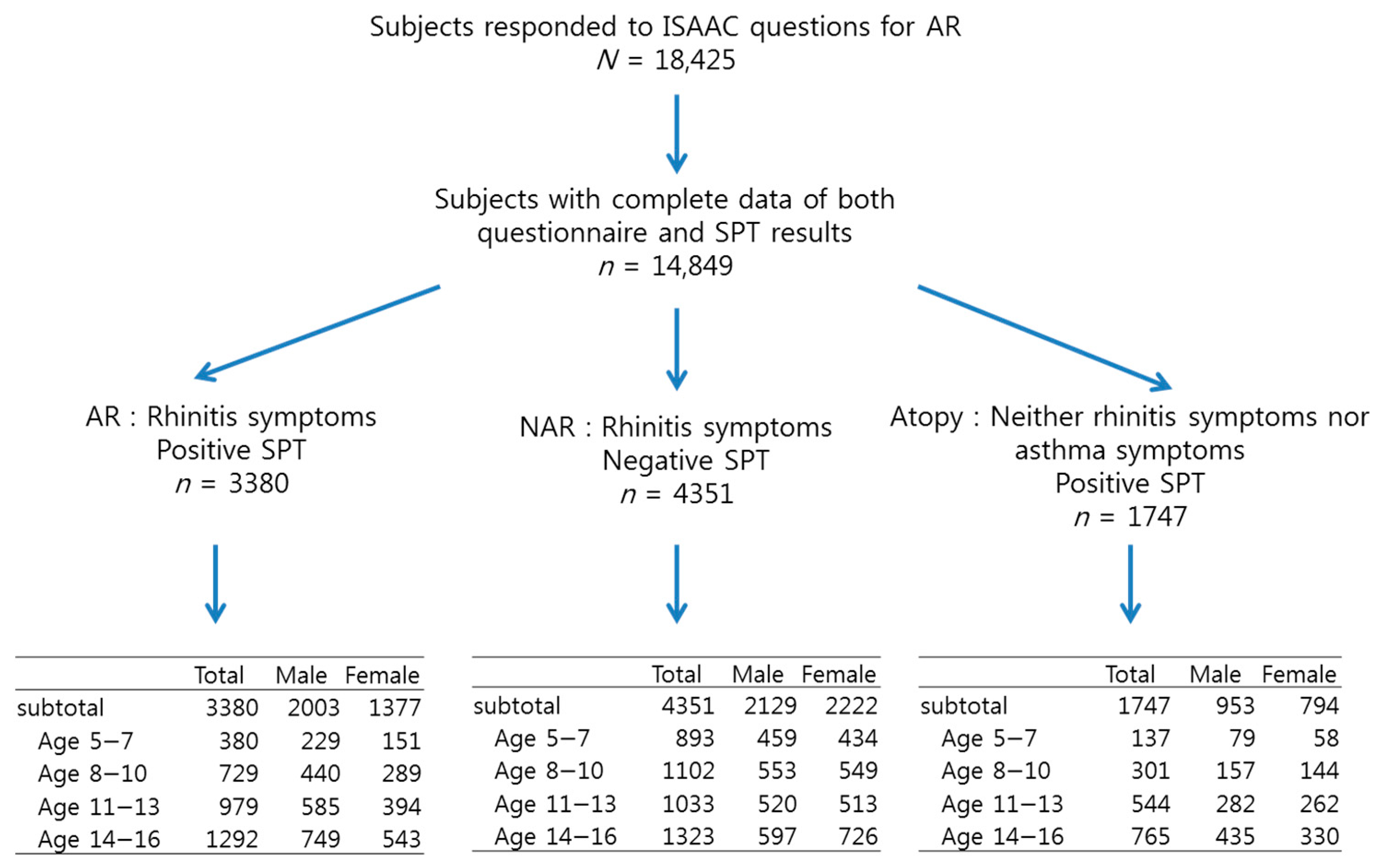
| Rhinitis Questions | Rhinitis by Questions | AR by Questionnaire and Skin Prick Test * | NAR † | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (%) (n = 18,425) | Male (%) (n = 9292) | Female (%) (n = 9133) | Total (%) (n = 3380) | Male (%) (n = 2003) | Female (%) (n = 1377) | Total (%) (n = 4351) | Male (%) (n = 2129) | Female (%) (n = 2222) | |
| Symptom, ever ‡ | |||||||||
| Subtotal | 47.6 | 50.6 | 44.4 | 21.0 | 24.6 | 17.2 | 26.5 | 25.8 | 27.2 |
| Age 5−7 | 50.1 | 52.0 | 48.0 | 15.4 | 18.2 | 12.4 | 34.9 | 34.7 | 35.2 |
| Age 8−10 | 49.5 | 54.4 | 44.6 | 19.6 | 23.8 | 15.4 | 29.6 | 29.9 | 29.3 |
| Age 11−13 | 45.4 | 50.1 | 40.7 | 22.6 | 26.5 | 18.6 | 23.0 | 23.5 | 22.6 |
| Age 14−16 | 46.6 | 47.7 | 45.6 | 23.0 | 26.6 | 19.4 | 23.4 | 20.9 | 26.0 |
| Symptom, current ∫ | |||||||||
| Subtotal | 43.6 | 46.7 | 40.4 | 19.5 | 23.0 | 16.0 | 23.9 | 23.4 | 24.5 |
| Age 5−7 | 47.1 | 49.0 | 45.0 | 14.4 | 16.9 | 11.7 | 32.7 | 32.2 | 33.2 |
| Age 8−10 | 45.4 | 50.3 | 40.5 | 18.3 | 22.5 | 14.1 | 26.9 | 27.3 | 25.6 |
| Age 11−13 | 41.5 | 46.1 | 36.7 | 21.3 | 25.1 | 17.3 | 20.7 | 21.2 | 20.2 |
| Age 14−16 | 42.1 | 43.3 | 40.9 | 21.2 | 24.3 | 18.0 | 20.7 | 18.6 | 22.7 |
| Diagnosis, ever || | |||||||||
| Subtotal | 29.4 | 32.4 | 26.2 | 14.5 | 17.5 | 11.4 | 14.7 | 14.9 | 14.5 |
| Age 5−7 | 30.3 | 31.3 | 29.1 | 10.1 | 11.9 | 8.1 | 20.5 | 20.5 | 20.5 |
| Age 8−10 | 32.3 | 37.3 | 27.2 | 14.5 | 18.1 | 11.0 | 17.8 | 19.5 | 16.1 |
| Age 11−13 | 28.7 | 33.0 | 24.4 | 15.7 | 19.5 | 11.8 | 13.0 | 13.2 | 12.7 |
| Age 14−16 | 27.2 | 29.0 | 25.5 | 15.5 | 18.1 | 12.8 | 11.5 | 10.7 | 12.4 |
| Treatment, current ¶ | |||||||||
| Subtotal | 22.2 | 25.0 | 19.3 | 11.0 | 13.5 | 8.6 | 10.8 | 11.1 | 10.5 |
| Age 5−7 | 25.7 | 27.2 | 24.0 | 8.7 | 10.4 | 6.8 | 17.5 | 17.6 | 17.3 |
| Age 8−10 | 25.1 | 29.3 | 21.0 | 12.0 | 14.6 | 9.4 | 12.8 | 14.0 | 11.6 |
| Age 11−13 | 21.7 | 26.1 | 17.3 | 12.0 | 15.4 | 8.5 | 9.6 | 10.3 | 8.9 |
| Age 14−16 | 18.3 | 19.7 | 17.0 | 10.7 | 12.6 | 8.8 | 7.6 | 7.0 | 8.1 |
| Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Youden’s Index (%) * | Accuracy (%) || | |
|---|---|---|---|---|---|---|
| Symptom, ever † | ||||||
| Subtotal | 57.5 | 58.4 | 44.2 | 70.6 | 15.9 | 58.1 |
| Age 5−7 | 63.2 | 53.8 | 30.6 | 82.0 | 17.0 | 56.1 |
| Age 8−10 | 60.4 | 56.2 | 39.8 | 74.8 | 16.6 | 57.6 |
| Age 11−13 | 56.4 | 61.6 | 49.5 | 67.9 | 18.0 | 59.5 |
| Age 14−16 | 55.4 | 60.0 | 49.5 | 65.5 | 15.4 | 58.1 |
| Symptom, current ** | ||||||
| Subtotal | 53.5 | 62.4 | 44.9 | 70.1 | 15.9 | 59.2 |
| Age 5−7 | 59.2 | 56.8 | 30.6 | 81.2 | 16.0 | 57.4 |
| Age 8−10 | 56.4 | 60.2 | 40.4 | 74.3 | 16.6 | 59.0 |
| Age 11−13 | 53.1 | 65.5 | 50.7 | 67.6 | 18.6 | 60.5 |
| Age 14−16 | 51.0 | 64.7 | 50.6 | 65.1 | 15.7 | 59.0 |
| Diagnosis, ever ‡ | ||||||
| Subtotal | 39.8 | 76.9 | 49.6 | 69.1 | 16.7 | 63.4 |
| Age 5−7 | 41.4 | 72.9 | 32.9 | 79.5 | 14.3 | 65.2 |
| Age 8−10 | 44.9 | 73.7 | 44.9 | 73.6 | 18.5 | 64.3 |
| Age 11−13 | 39.2 | 78.3 | 54.7 | 65.8 | 17.5 | 62.7 |
| Age 14−16 | 37.3 | 80.3 | 57.3 | 64.3 | 17.6 | 62.4 |
| Treatment, current ∫ | ||||||
| Subtotal | 30.3 | 83.0 | 50.4 | 67.6 | 13.3 | 63.8 |
| Age 5−7 | 35.7 | 76.9 | 33.2 | 78.8 | 12.6 | 66.9 |
| Age 8−10 | 37.1 | 81.1 | 48.4 | 72.9 | 18.2 | 66.8 |
| Age 11−13 | 29.9 | 83.9 | 55.5 | 64.2 | 13.9 | 62.3 |
| Age 14−16 | 25.8 | 87.1 | 58.6 | 62.3 | 12.8 | 61.6 |
| OR * | aOR † | p-Value ** | p-Value ‡ | 95% Confidence Interval ∫ | 95% Confidence Interval || | ||||
|---|---|---|---|---|---|---|---|---|---|
| Asthma | Total | 1.248 | 1.222 | 0.000 | 0.000 | 1.127 | 1.383 | 1.101 | 1.356 |
| Male | 1.261 | 1.266 | 0.001 | 0.000 | 1.101 | 1.445 | 1.102 | 1.453 | |
| Female | 1.168 | 1.169 | 0.054 | 0.055 | 0.997 | 1.368 | 0.997 | 1.371 | |
| Atopic dermatitis | Total | 1.164 | 1.201 | 0.002 | 0.000 | 1.056 | 1.283 | 1.088 | 1.327 |
| Male | 1.182 | 1.200 | 0.016 | 0.009 | 1.032 | 1.353 | 1.046 | 1.376 | |
| Female | 1.198 | 1.206 | 0.013 | 0.010 | 1.039 | 1.382 | 1.044 | 1.394 | |
| Food allergy | Total | 1.246 | 1.308 | 0.000 | 0.000 | 1.103 | 1.406 | 1.156 | 1.480 |
| Male | 1.237 | 1.284 | 0.015 | 0.005 | 1.043 | 1.467 | 1.080 | 1.527 | |
| Female | 1.309 | 1.338 | 0.003 | 0.001 | 1.100 | 1.559 | 1.121 | 1.597 | |
| Sinusitis | Total | 0.965 | 1.013 | 0.533 | 0.829 | 0.864 | 1.079 | 0.904 | 1.134 |
| Male | 0.971 | 1.033 | 0.691 | 0.672 | 0.838 | 1.124 | 0.890 | 1.199 | |
| Female | 0.909 | 0.981 | 0.281 | 0.831 | 0.765 | 1.081 | 0.823 | 1.169 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.H.; Lim, D.H.; Samra, M.; Kim, E.H.; Kim, J.H. How Accurate Are the ISAAC Questions for Diagnosis of Allergic Rhinitis in Korean Children? Int. J. Environ. Res. Public Health 2018, 15, 1527. https://doi.org/10.3390/ijerph15071527
Kim DH, Lim DH, Samra M, Kim EH, Kim JH. How Accurate Are the ISAAC Questions for Diagnosis of Allergic Rhinitis in Korean Children? International Journal of Environmental Research and Public Health. 2018; 15(7):1527. https://doi.org/10.3390/ijerph15071527
Chicago/Turabian StyleKim, Dong Hyun, Dae Hyun Lim, Mona Samra, Eun Hye Kim, and Jeong Hee Kim. 2018. "How Accurate Are the ISAAC Questions for Diagnosis of Allergic Rhinitis in Korean Children?" International Journal of Environmental Research and Public Health 15, no. 7: 1527. https://doi.org/10.3390/ijerph15071527
APA StyleKim, D. H., Lim, D. H., Samra, M., Kim, E. H., & Kim, J. H. (2018). How Accurate Are the ISAAC Questions for Diagnosis of Allergic Rhinitis in Korean Children? International Journal of Environmental Research and Public Health, 15(7), 1527. https://doi.org/10.3390/ijerph15071527





