Biotoxicity of Water-Soluble UV Photodegradation Products for 10 Typical Gaseous VOCs
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
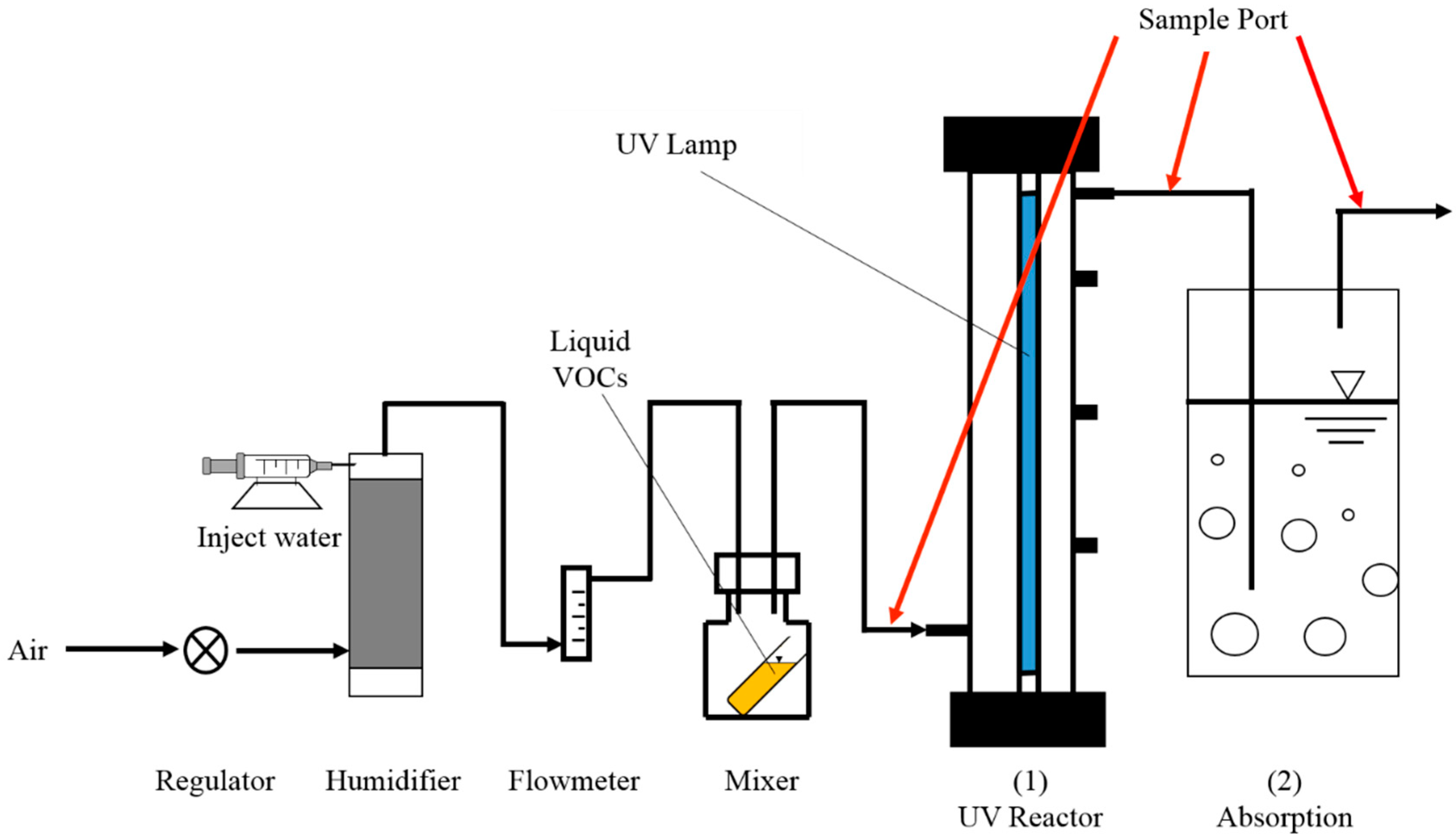
2.2. UV Photodegradation Reactor Design
2.3. Experimental Procedure
2.4. Analytical Methods
3. Results and Discussion
3.1. TOC and TN of Off-Gas Absorption Solutions
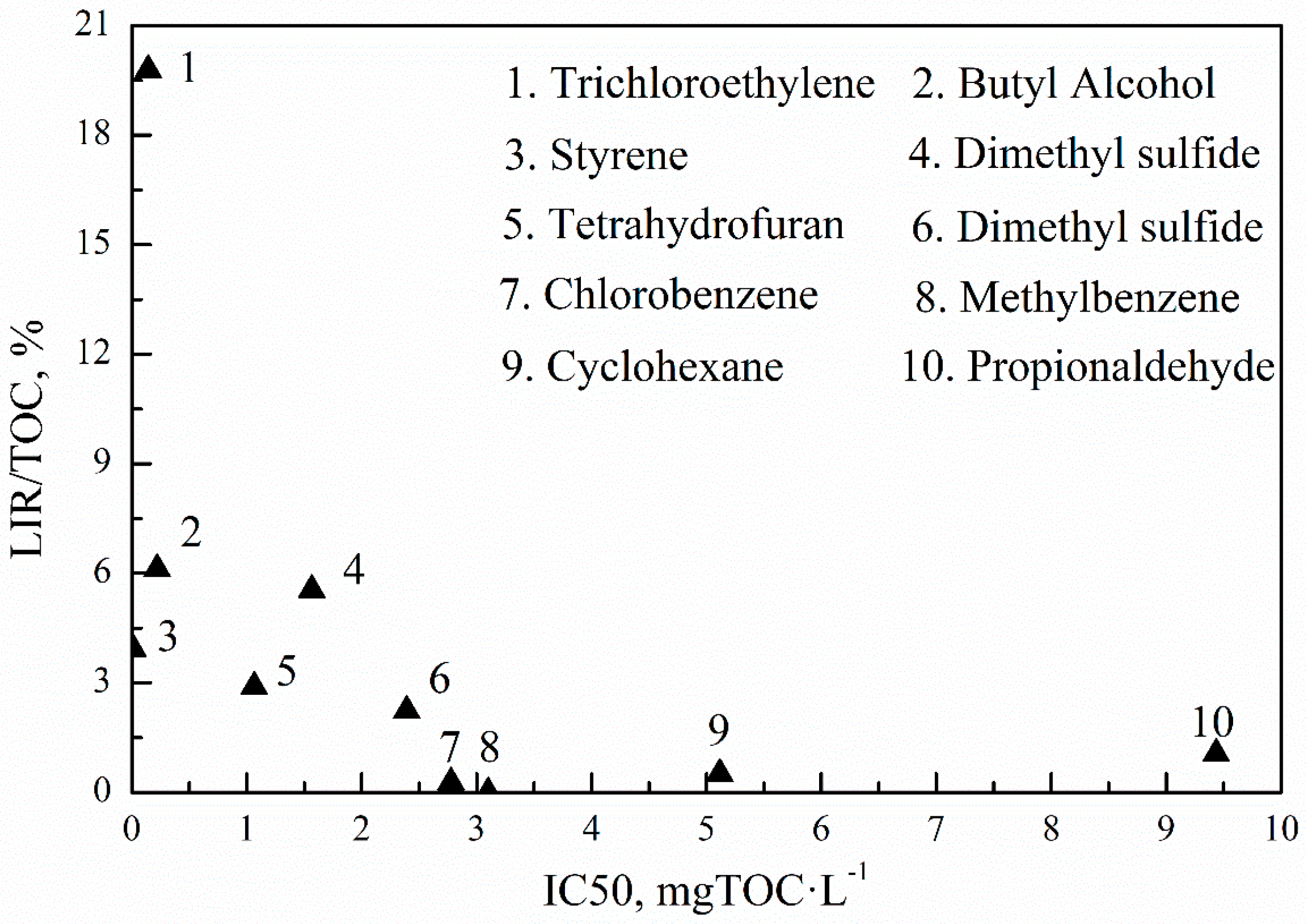
3.2. Acute Toxicity for Luminescent Bacterium
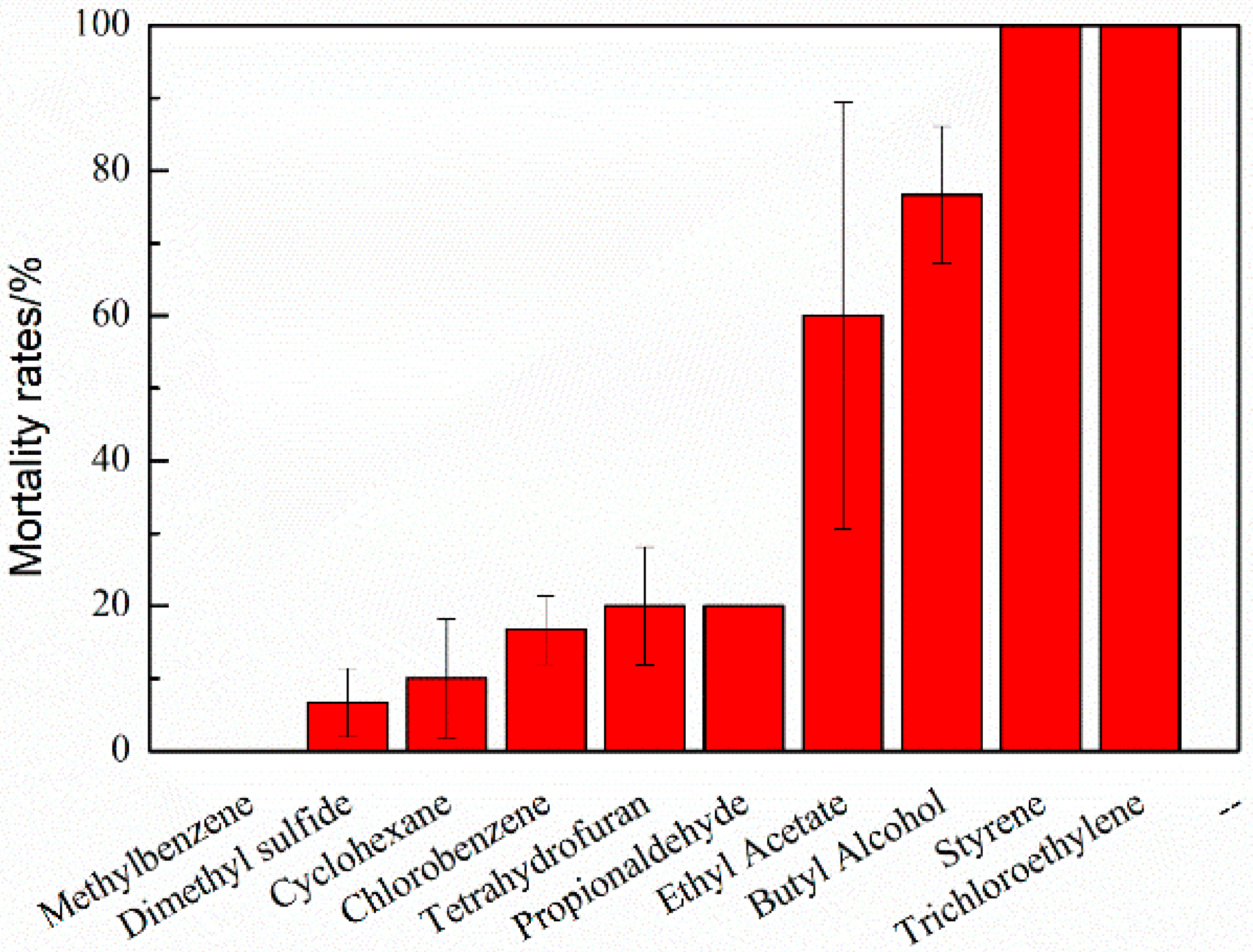
3.3. Acute Toxicity for Daphnia Magna
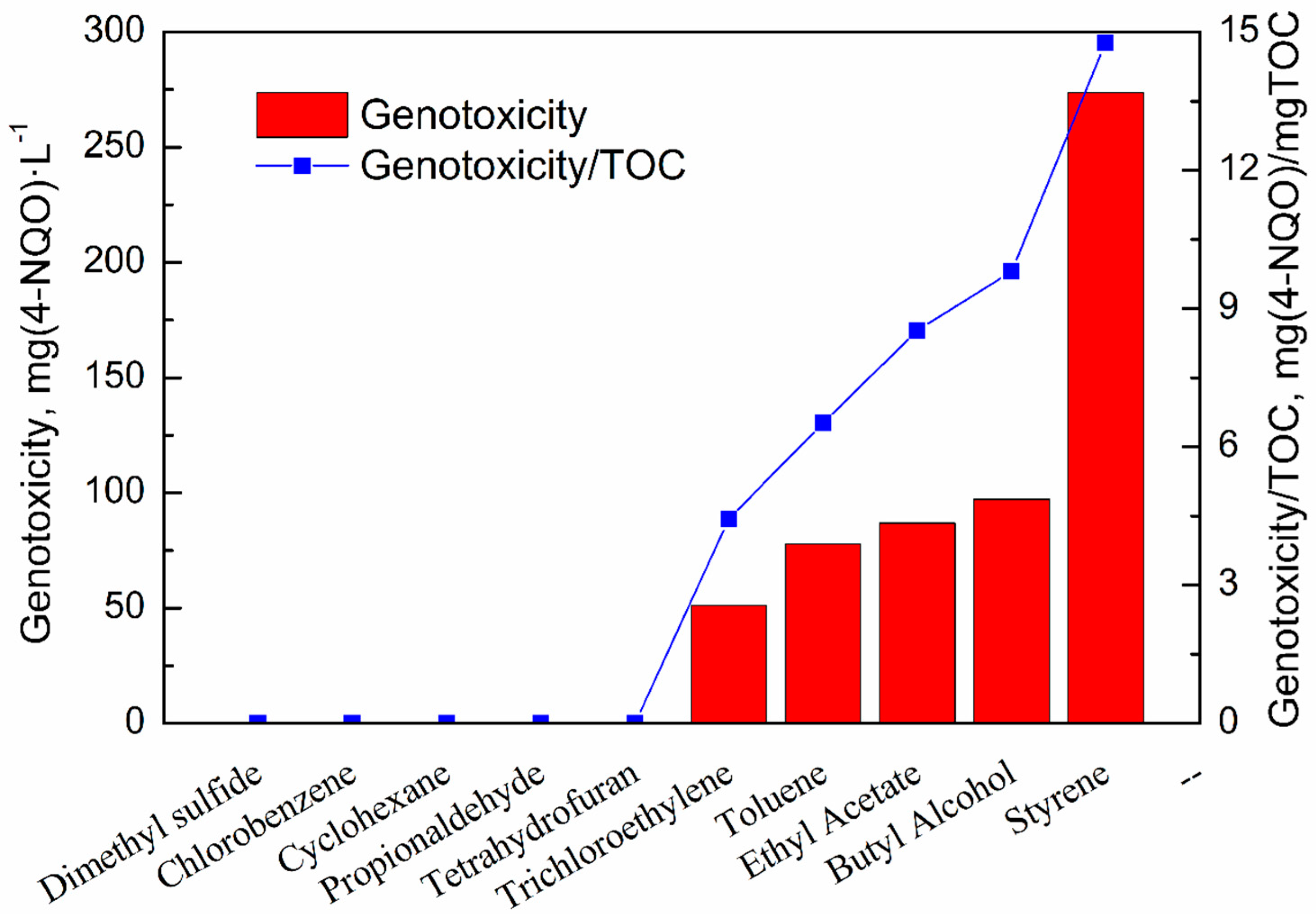
3.4. Genotoxicity
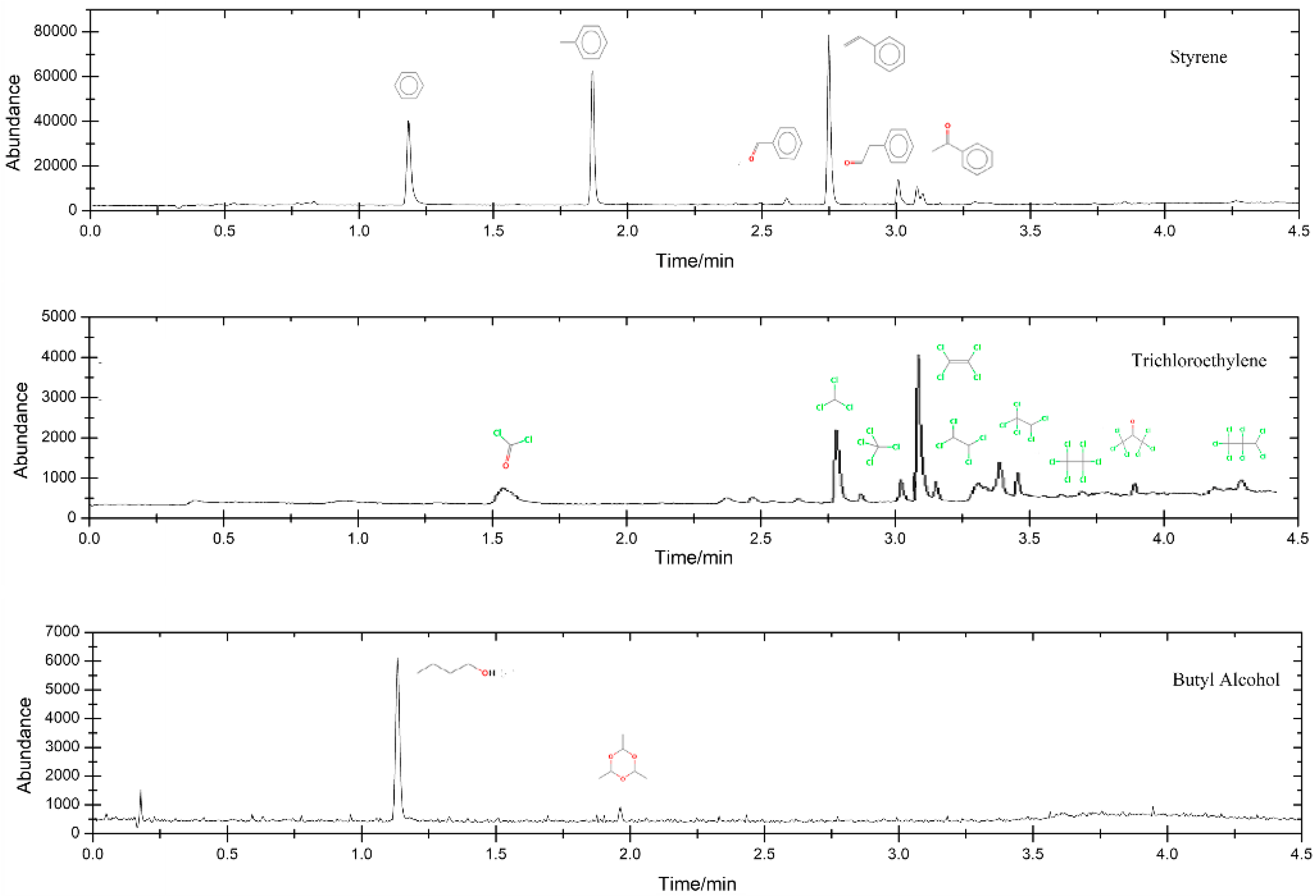
3.5. Intermediate Production
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kamal, M.S.; Hossain, M.; Abdur Razzak, S. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Ojala, S.; Pitkäaho, S.; Laitinen, T.; Niskala Koivikko, N.; Brahmi, R.; Gaálová, J.; Matejova, L.; Kucherov, A.; Päivärinta, S.; Hirschmann, C.; et al. Catalysis in VOC Abatement. Top. Catal. 2011, 54, 1224. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shangguan, W. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Berenjian, A.; Chan, N.; Malmiri, H.J. Volatile Organic Compounds Removal Methods: A Review. Am. J. Biochem. Biotechnol. 2012, 8, 220–229. [Google Scholar]
- Jousse, F.; Atteia, O.; Höhener, P.; Cohen, G. Removal of NAPL from columns by oxidation, sparging, surfactant and thermal treatment. Chemosphere 2017, 188, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Parmar, G.R.; Rao, N.N. Emerging Control Technologies for Volatile Organic Compounds. Crit. Rev. Environ. Sci. Technol. 2008, 39, 41–78. [Google Scholar] [CrossRef]
- Debono, O.; Thevenet, F.; Gravejat, P.; Hequet, V.; Raillard, C.; Lecoq, L.; Locoge, N. Toluene photocatalytic oxidation at ppbv levels: Kinetic investigation and carbon balance determination. Appl. Catal. B Environ. 2011, 106, 600–608. [Google Scholar] [CrossRef]
- Wang, J.H.; Ray, M.B. Application of ultraviolet photooxidation to remove organic pollutants in the gas phase. Sep. Purif. Technol. 2000, 19, 11–20. [Google Scholar] [CrossRef]
- Kang, I.; Xi, J.; Hu, H. Effects of different operating conditions on the VOCs removal performance of UV irradiation reactors. J. Odor Indoor Environ. 2017, 16, 72–80. [Google Scholar] [CrossRef]
- Hay, S.O.; Obee, T.; Luo, Z.; Jiang, T.; Meng, Y.; He, J.; Murphy, S.C.; Suib, S. The viability of photocatalysis for air purification. Molecules 2015, 20, 1319–1356. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xi, J.-Y.; Hu, H.-Y.; Yao, Y. Effects of UV pretreatment on microbial community structure and metabolic characteristics in a subsequent biofilter treating gaseous chlorobenzene. Bioresour. Technol. 2009, 100, 5581–5587. [Google Scholar] [CrossRef] [PubMed]
- Cantavenera, M.J.; Catanzaro, I.; Loddo, V.; Palmisano, L.; Sciandrello, G. Photocatalytic degradation of paraquat and genotoxicity of its intermediate products. J. Photochem. Photobiol. A Chem. 2007, 185, 277–282. [Google Scholar] [CrossRef]
- Koh, I.-O.; Thiemann, W. Study of photochemical oxidation of standard chlorinated paraffins and identification of degradation products. J. Photochem. Photobiol. A Chem. 2001, 139, 205–215. [Google Scholar] [CrossRef]
- Koh, L.H.; Kuhn David, C.S.; Mohseni, M.; Allen, D.G. Utilizing ultraviolet photooxidation as a pre-treatment of volatile organic compounds upstream of a biological gas cleaning operation. J. Chem. Technol. Biotechnol. 2004, 79, 619–625. [Google Scholar] [CrossRef]
- Dai, H.; Jing, S.; Wang, H.; Ma, Y.; Li, L.; Song, W.; Kan, H. VOC characteristics and inhalation health risks in newly renovated residences in Shanghai, China. Sci. Total Environ. 2017, 577, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Den, W.; Ravindran, V.; Pirbazari, M. Photooxidation and biotrickling filtration for controlling industrial emissions of trichloroethylene and perchloroethylene. Chem. Eng. Sci. 2006, 61, 7909–7923. [Google Scholar] [CrossRef]
- Wang, C.; Xi, J.; Hu, H.-Y. A novel integrated UV-biofilter system to treat high concentration of gaseous chlorobenzene. Chin. Sci. Bull. 2008, 53, 2712–2716. [Google Scholar] [CrossRef]
- Chen, F.; Pehkonen, S.O.; Ray, M.B. Kinetics and mechanisms of UV-photodegradation of chlorinated organics in the gas phase. Water Res. 2002, 36, 4203–4214. [Google Scholar]
- Menz, J.; Schneider, M.; Kümmerer, K. Toxicity testing with luminescent bacteria—Characterization of an automated method for the combined assessment of acute and chronic effects. Chemosphere 2013, 93, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Venkataraman, C.; Mukherji, S. A review on advantages of implementing luminescence inhibition test (Vibrio fischeri) for acute toxicity prediction of chemicals. Environ. Int. 2006, 32, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, X.; Dzakpasu, M.; Wang, X.C. Evaluation of ecotoxicological effects of benzophenone UV filters: Luminescent bacteria toxicity, genotoxicity and hormonal activity. Ecotoxicol. Environ. Saf. 2017, 142, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Sugihara, K.; Sanoh, S.; Kitamura, S.; Ohta, S. Photodegradation of pharmaceuticals in the aquatic environment by sunlight and UV-A, -B and -C irradiation. J. Toxicol. Sci. 2013, 38, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Di Delupis, G.D.; Macrí, A.; Civitareale, C.; Migliore, L. Antibiotics of zootechnical use: Effects of acute high and low dose contamination on Daphnia magna Straus. Aquat. Toxicol. 1992, 22, 53–59. [Google Scholar] [CrossRef]
- Ikenaka, Y.; Sakamoto, M.; Nagata, T.; Takahashi, H.; Miyabara, Y.; Hanazato, T.; Ishizuka, M.; Isobe, T.; Kim, J.-W.; Chang, K.-H. Effects of polycyclic aromatic hydrocarbons (PAHs) on an aquatic ecosystem: Acute toxicity and community-level toxic impact tests of benzo[a]pyrene using lake zooplankton community. J. Toxicol. Sci. 2013, 38, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Nakamura, S.-I.; Oki, I.; Kato, T.; Shinagawa, H. Evaluation of the new system (umu-test) for the detection of environmental mutagens and carcinogens. Mutat. Res.-Environ. Mutagen. Relat. Subj. 1985, 147, 219–229. [Google Scholar] [CrossRef]
- Oda, Y.; Nakayama, S.; Harada, K.H.; Koizumi, A. Negative results of umu genotoxicity test of fluorotelomer alcohols and perfluorinated alkyl acids. Environ. Health Prev. Med. 2007, 12, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Best, G.; Mourato, D.; Singh, M.; Firman, M.; Basu, S. Application of immersed ultrafiltration membranes for colour & TOC removal. Membr. Technol. 1999, 1999, 5–12. [Google Scholar]
- Yuan, S.; Chen, J.; Lin, Z.; Li, W.; Sheng, G.; Yu, H. Nitrate formation from atmospheric nitrogen and oxygen photocatalysed by nano-sized titanium dioxide. Nat. Commun. 2013, 4, 2249. [Google Scholar] [CrossRef] [PubMed]
- Ishii, R.; Yoshikawa, K. Microbial bioassay of acute toxicity by the pH inhibition method and comparison of IC50 (pHI) with LD50 for rats and mice. J. Ferment. Bioeng. 1993, 76, 361–366. [Google Scholar] [CrossRef]
- Grisolia, C.; Barros Barata de Oliveira, A.; Bonfim, H.; Klautau, N. Genotoxicity evaluation of domestic sewage in a municipal wastewater treatment plant. Genet. Mol. Biol. 2005, 28, 334–338. [Google Scholar] [CrossRef]
| VOCs | Molecular Formula | MW/(g·mol−1) | BP/°C |
|---|---|---|---|
| Propionaldehyde | C3H6O | 58.08 | 48.0 |
| Trichloroethylene | C2HCl3 | 131.39 | 87.1 |
| Chlorobenzene | C6H5Cl | 112.56 | 131.0 |
| Dimethyl sulfide | (CH3)2S | 62.13 | 37.5 |
| Tetrahydrofuran | (CH2)4O | 72.11 | 66.0 |
| Methylbenzene | C6H5CH3 | 92.14 | 111.0 |
| Butyl Alcohol | CH3(CH2)3OH | 74.12 | 117.3 |
| Cyclohexane | C6H12 | 84.62 | 80.7 |
| Styrene | C6H5CH=CH2 | 104.14 | 145.2 |
| Ethyl Acetate | CH3COOCH2CH3 | 88.11 | 77.2 |
| VOCs | TOC/(mg·L−1) | TN/(mg·L−1) | MC/mg | CO2 Increase/(ppm) | VOC Decrease/(ppm) |
|---|---|---|---|---|---|
| Propionaldehyde | 4.18 | 0.37 | 16.9 | 97 | 12 |
| Trichloroethylene | 4.31 | 0 | 22.5 | 41 | 0 |
| Chlorobenzene | 4.96 | 0 | 33.8 | 56 | 7 |
| Dimethyl sulfide | 5.79 | 0.60 | 11.3 | 39 | 8 |
| Tetrahydrofuran | 7.87 | 0.26 | 11.3 | 95 | 8 |
| Methylbenzene | 9.13 | 0.15 | 39.4 | 128 | 6 |
| Butyl Alcohol | 9.92 | 0 | 22.5 | 45 | 18 |
| Cyclohexane | 16.55 | 0 | 33.8 | 81 | 42 |
| Styrene | 18.53 | 0.50 | 45.0 | 41 | 8 |
| Ethyl Acetate | 19.60 | 0 | 22.5 | 72 | 27 |
| VOCs | 1 | 1/2 | 1/3 | 1/5 | 1/10 | 1/100 |
|---|---|---|---|---|---|---|
| Propionaldehyde | 94.28 | 95.79 | 84.36 | 37.15 | 0 | 0 |
| Trichloroethylene | 96.48 | 95.29 | 74.19 | 41.58 | 2.54 | 0 |
| Chlorobenzene | 98.17 | 97.73 | 83.74 | 39.68 | 4.44 | 0 |
| Dimethyl sulfide | 100 | 99.88 | 87.39 | 51.14 | 8.46 | 0 |
| Tetrahydrofuran | 97.52 | 96.29 | 92.69 | 61.39 | 22.77 | 0 |
| Methylbenzene | 99.28 | 99.17 | 94.73 | 49.84 | 32.13 | 0 |
| Butyl Alcohol | 94.36 | 93.89 | 90.66 | 80.37 | 43.82 | 0 |
| Cyclohexane | 99.93 | 96.85 | 94.57 | 90.31 | 60.69 | 0.01 |
| Styrene | 99.87 | 96.49 | 97.49 | 92.22 | 72.69 | 0 |
| Ethyl Acetate | 100 | 100 | 99.44 | 100 | 85.28 | 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Kang, I.-S.; Wu, Q.; Xi, J.; Hu, H. Biotoxicity of Water-Soluble UV Photodegradation Products for 10 Typical Gaseous VOCs. Int. J. Environ. Res. Public Health 2018, 15, 1520. https://doi.org/10.3390/ijerph15071520
Sun Z, Kang I-S, Wu Q, Xi J, Hu H. Biotoxicity of Water-Soluble UV Photodegradation Products for 10 Typical Gaseous VOCs. International Journal of Environmental Research and Public Health. 2018; 15(7):1520. https://doi.org/10.3390/ijerph15071520
Chicago/Turabian StyleSun, Zhuqiu, In-Sun Kang, Qianyuan Wu, Jinying Xi, and Hongying Hu. 2018. "Biotoxicity of Water-Soluble UV Photodegradation Products for 10 Typical Gaseous VOCs" International Journal of Environmental Research and Public Health 15, no. 7: 1520. https://doi.org/10.3390/ijerph15071520
APA StyleSun, Z., Kang, I.-S., Wu, Q., Xi, J., & Hu, H. (2018). Biotoxicity of Water-Soluble UV Photodegradation Products for 10 Typical Gaseous VOCs. International Journal of Environmental Research and Public Health, 15(7), 1520. https://doi.org/10.3390/ijerph15071520





