Geographical Clustering and Environmental Determinants of Schistosomiasis from 2007 to 2012 in Jianghan Plain, China
Abstract
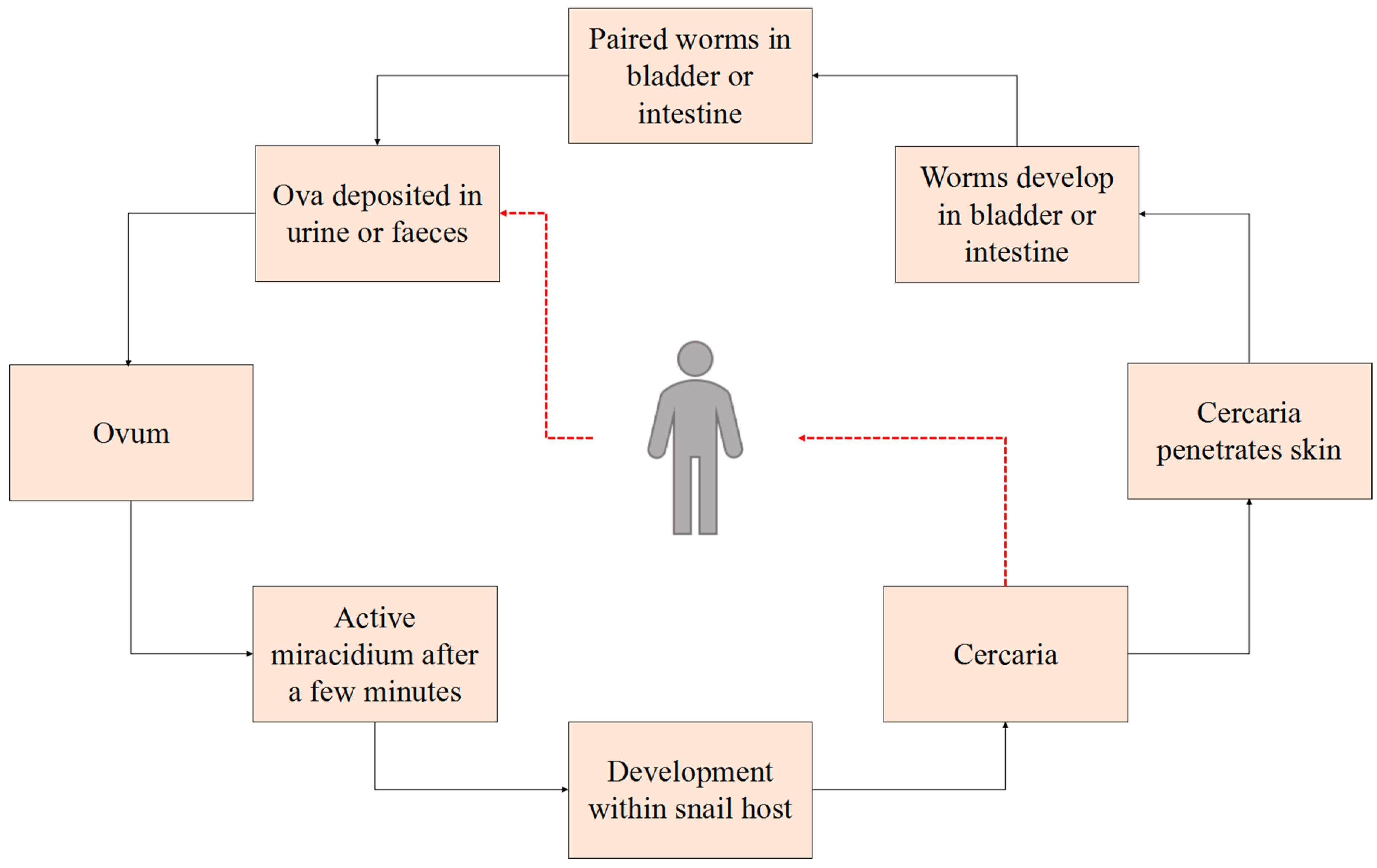
1. Introduction
2. Materials and Methods
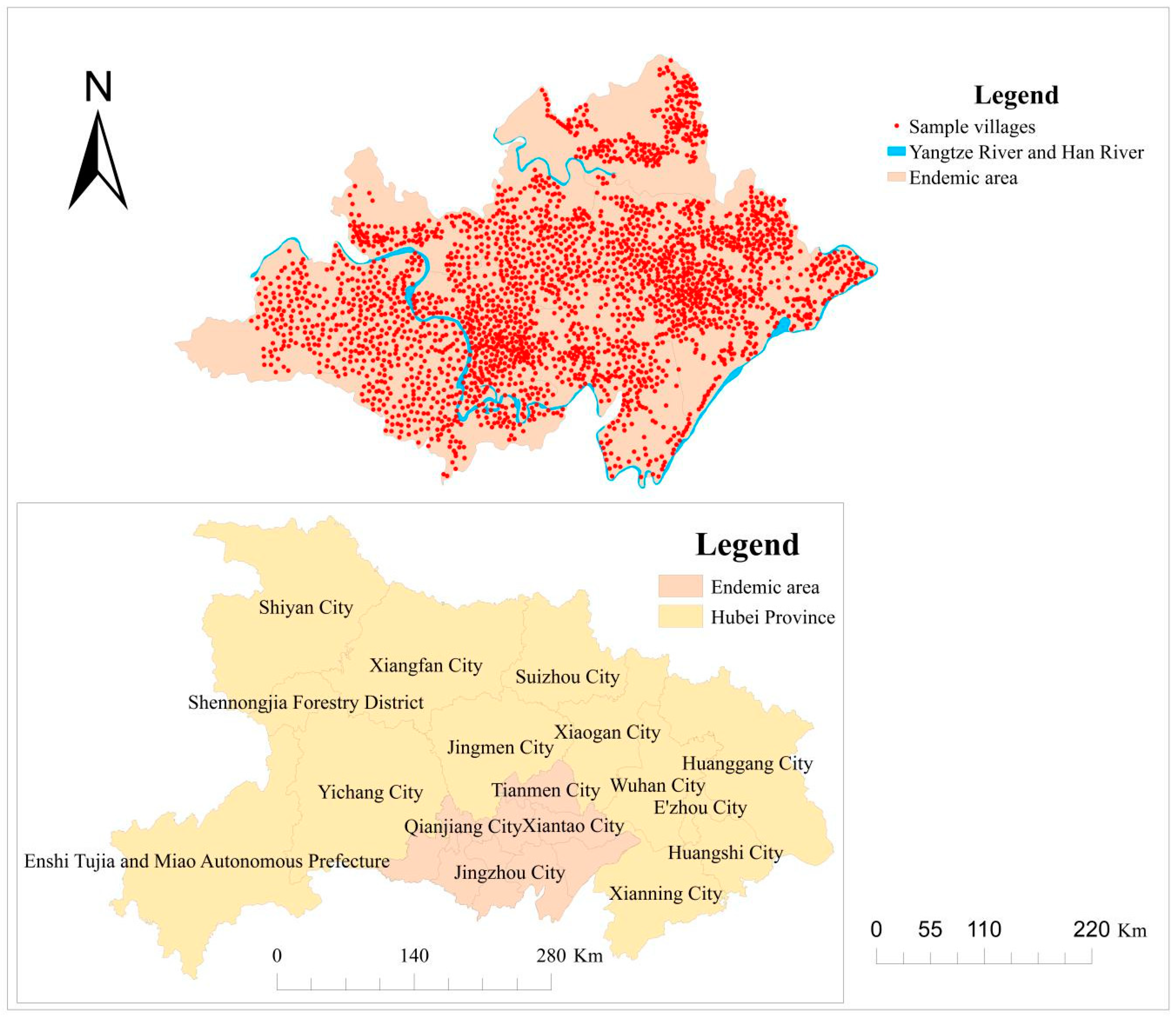
2.1. Study Area
2.2. Parasitological Data
2.3. Environmental Data
2.4. Statistical Analysis
2.4.1. Cluster Analysis
2.4.2. Detection of Geographical Environment Factors on Schistosomiasis
3. Results
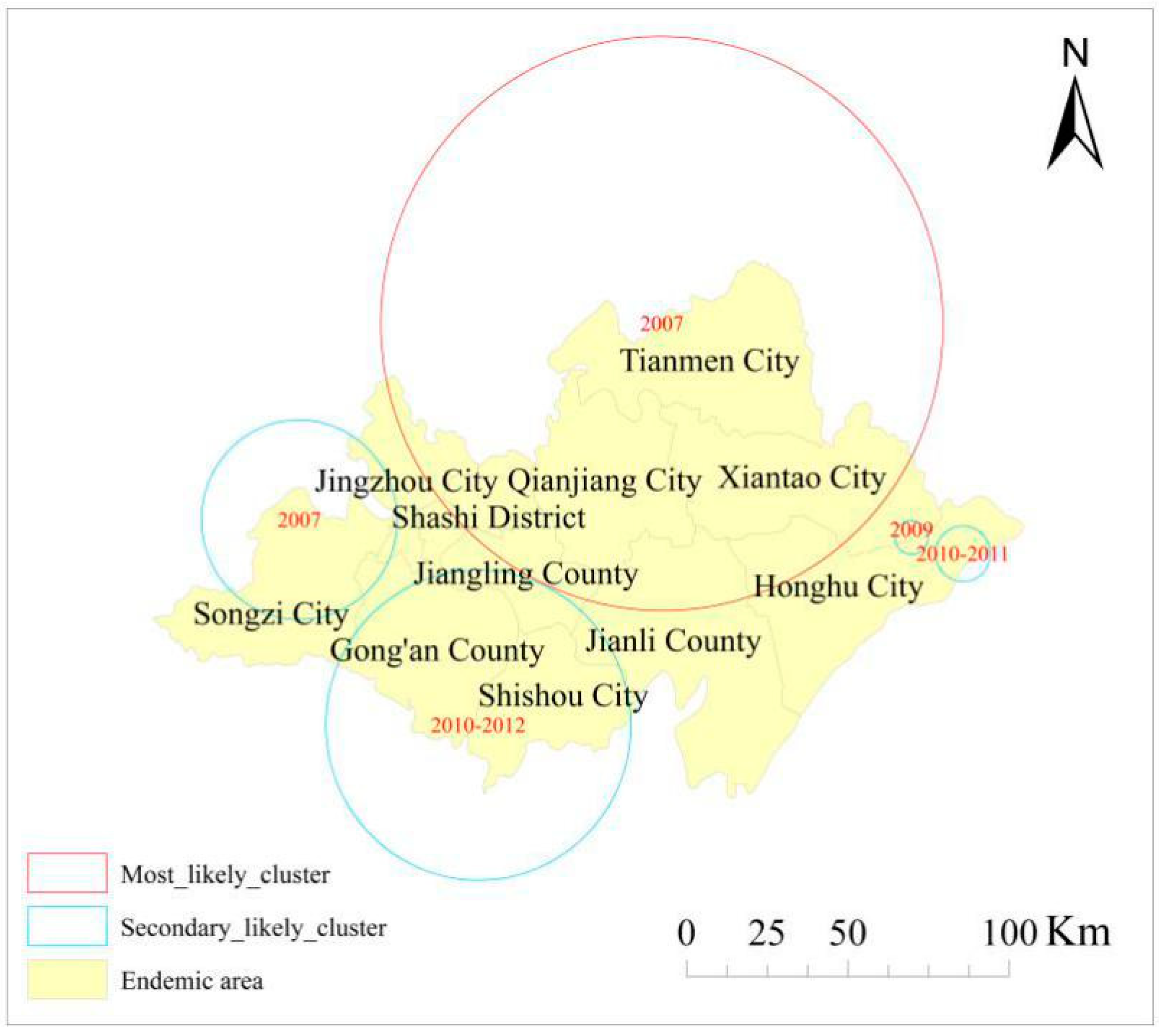
3.1. Spatial Cluster Analysis
3.2. Environmental Factor Analysis
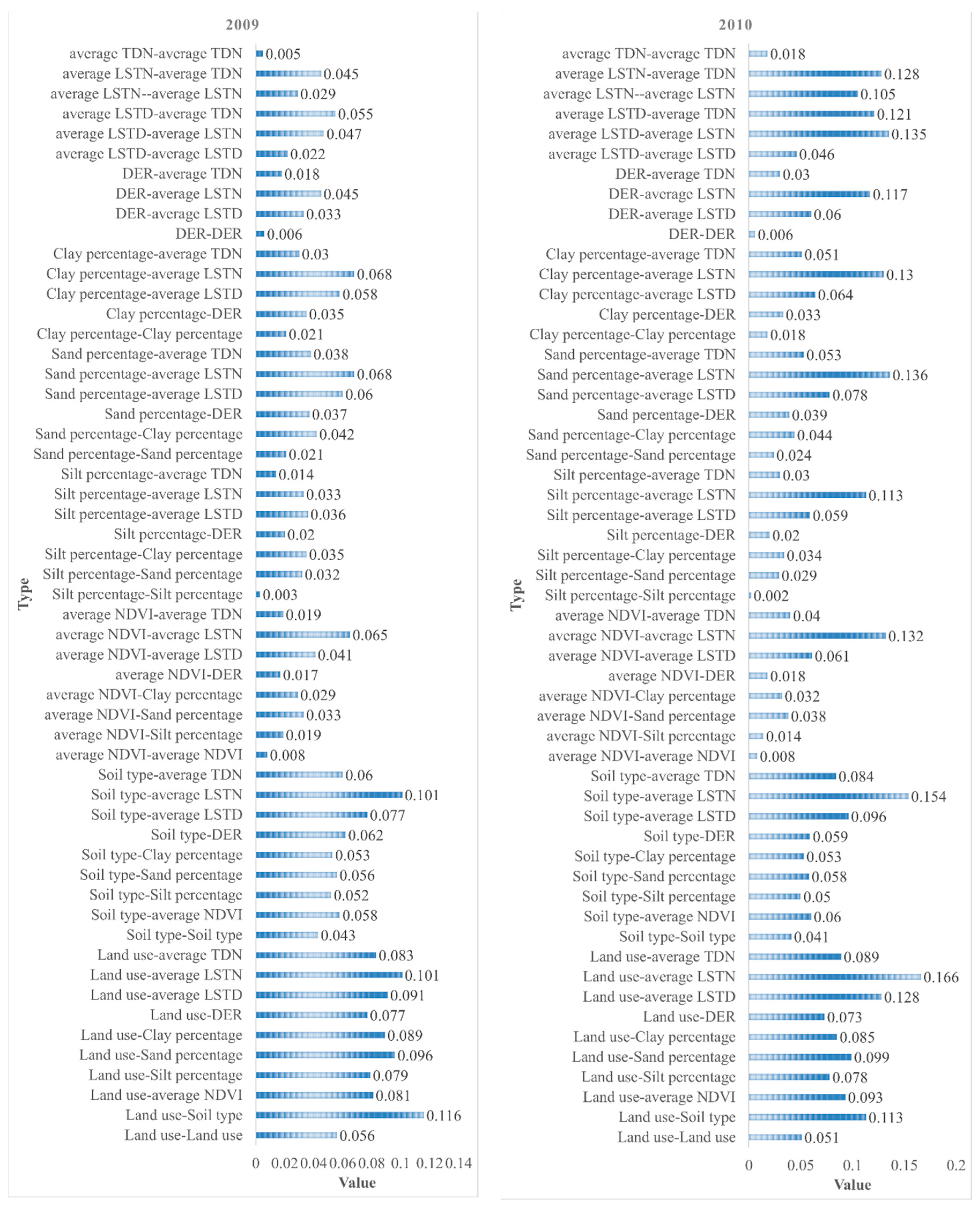
3.2.1. Factor Detector
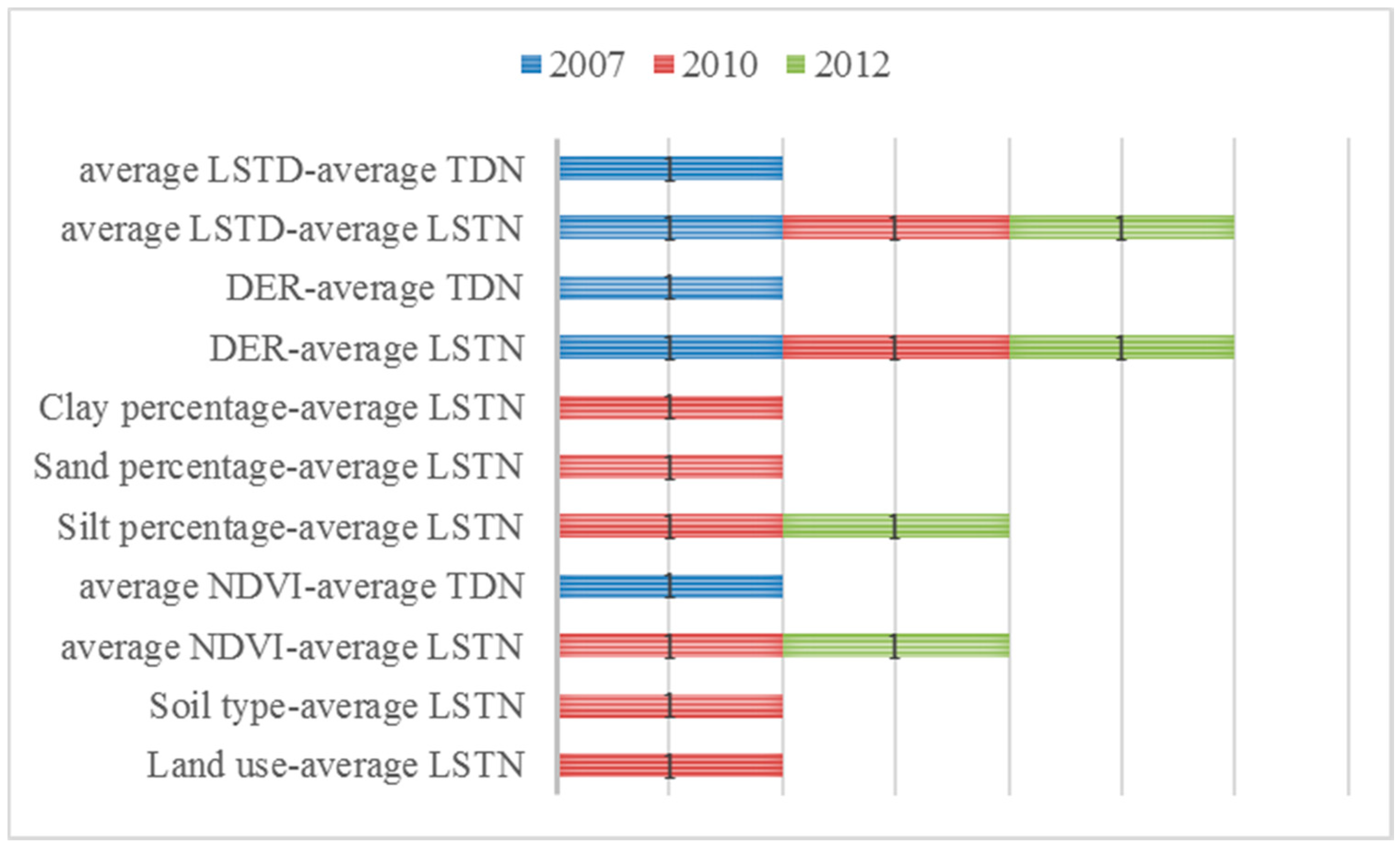
3.2.2. Ecological Detector
3.2.3. Interaction Detector
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Chitsulo, L.; Engels, D.; Montresor, A.; Savioli, L. The global status of schistosomiasis and its control. Acta Trop. 2000, 77, 41–51. [Google Scholar] [CrossRef]
- Thétiot-Laurent, S.A.; Boissier, J.; Robert, A.; Meunier, B. Schistosomiasis Chemotherapy. Angew. Chem. Int. Ed. Engl. 2013, 52, 7936–7956. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Liu, J.B.; Jiang, Y.; Li, G.; Shan, X.W.; Zhang, J.; Cai, S.X.; Huang, X.B. Dynamics of spatiotemporal distribution of schistosomiasis in Hubei Province, China. Acta Trop. 2018, 180, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Jin, J.-N.; Xu, J.; Li, S.-Z.; Zhou, X.-N.; Sun, J.-L.; Li, Z.-J.; Lu, S. Surveillance of schistosomiasis in People’s Republic of China in 2015. Chin. J. Schistosomiasis Control 2017, 29, 273–280. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, J.-L.; Xu, M.-Z.; Quan, J.-Y.; Dang, H.; Lu, S.; Xu, J.; Li, Z.-S.; Zhou, N.-X. Endemic status of schistosomiasis in People’s Republic of China in 2016. Chin. J. Schistosomiasis Control 2017, 29, 669–677. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; You, S. Application of Geo-informatics to the Study of Schistosomiasis Habitat. Geo Spat. Inf. Sci. 2007, 9, 17–20. (In Chinese) [Google Scholar]
- Lv, S.B.; Lin, D.D. Natural environment and schistosomiasis transmission in Poyang Lake region. Chin. J. Schistosomiasis Control 2014, 26, 561–564. (In Chinese) [Google Scholar]
- Zhang, X.; Yang, X.; Peng, Z. Relationships between the surviving Oncomelania and beaches environmental factors. Acta Ecol. Sin. 1999, 19, 265–269. [Google Scholar]
- Qian, X.; Yang, J.; Chen, L.; Li, L. The study on the microenvironment physical-chemical factor of snail habitat in Anning River Valley. Mod. Prev. Med. 2000, 27, 15–17. (In Chinese) [Google Scholar]
- Maszle, D.R.; Whitehead, P.G.; Johnson, R.C.; Spear, R.C. Hydrological studies of schistosomiasis transport in Sichuan Province, China. Sci. Total Environ. 1998, 216, 193–203. [Google Scholar] [CrossRef]
- Zhong, J.; Zhang, S.; Liu, Z.; Wu, W.; Wu, S.; Zhou, M.; Hu, L. Studies on relationship of snail distribution with plantation and soil property of marshland in Poyang Lake. Chin. J. Schistosomiasis Control 1995, 4, 206–209. (In Chinese) [Google Scholar]
- Minggang, C.; Zheng, F. Schistosomiasis control in China. Parasitol. Int. 1999, 48, 11–19. [Google Scholar] [CrossRef]
- Xia, C.; Bergquist, R.; Lynn, H.; Hu, F.; Lin, D.; Hao, Y.; Li, S.; Hu, Y.; Zhang, Z. Village-based spatio-temporal cluster analysis of the schistosomiasis risk in the Poyang Lake Region, China. Parasit. Vectors 2017, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Li, R.D.; Huang, D.; Shi, Y.Y.; Xu, X.J.; Wei, F.H.; Wang, H.F. Spatiotemporal heterogeneity in cattle schistosomiasis japonicum infection at village level in Jianghan Plain, China. Resour. Environ. Yangtze Basin 2018, 27, 405–411. (In Chinese) [Google Scholar]
- Qiu, J.; Li, R.D.; Xu, X.J. Characteristics analysis of Oncomelania hupensis’s geographical distribution in Hubei Province. Resour. Environ. Yangtze Basin 2012, 21, 100–104. (In Chinese) [Google Scholar]
- Wang, J.F.; Li, X.H.; Christakos, G.; Liao, Y.L.; Zhang, T.; Xue, G.; Zheng, X.Y. Geographical detectors-based health risk assessment and its application in the neural tube defects study of the Heshun Region, China. Int. J. Geogr. Inf. Sci. 2010, 24, 107–127. [Google Scholar] [CrossRef]
- Wang, J.F.; Hu, Y. Environmental health risk detection with GeogDetector. Environ. Model Softw. 2012, 33, 114–115. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C. Geodetector: Principle and prospective. Acta Geogr. Sin. 2017, 72, 116–134. [Google Scholar] [CrossRef]
- Zhao, F.; Zhu, R.; Zhang, L.J.; Zhang, Z.J.; Li, Y.P.; He, M.Z.; Zhou, Y.B.; Guo, J.G.; Zhao, G.M.; Jiang, Q.W. Application of saTScan in detection of schistosomiasis clusters in marshland and lake areas. Chin. J. Schistosomiasis Control. 2011, 23, 28–31. (In Chinese) [Google Scholar]
- Chen, Y.; Cai, S.; Liu, J.; Xiao, Y.; Li, G.; Shan, X.; Zhang, J. Epidemic and spatial distribution of Schistosomiasis in Hubei province from 2008 to 2012. Chin. J. Epidemiol. 2014, 35, 1366–1370. (In Chinese) [Google Scholar] [CrossRef]
- Chen, Y.Y.; Huang, X.B.; Xiao, Y.; Jiang, Y.; Shan, X.W.; Zhang, J.; Cai, S.X.; Liu, J.B. Spatial analysis of Schistosomiasis in Hubei Province, China: A GIS-Based analysis of Schistosomiasis from 2009 to 2013. PLoS ONE 2015, 10, e0118362. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.X.; Zhang, Z.J.; Zhuang, J.L.; Zhou, Y.B.; Jiang, Q.W. Potential impact of climate changes on spatial distribution of schistosomiasis in China. Sci. Technol. Rev. 2006, 7, 58–60. (In Chinese) [Google Scholar]
- Coleman, M.; Coleman, M.; Mabuza, A.M.; Kok, G.; Coetzee, M.; Durrheim, D.N. Using the SaTScan method to detect local malaria clusters for guiding malaria control programmes. Malar. J. 2009, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Stelling, J.; Yih, W.K.; Galas, M.; Kulldorff, M.; Pichel, M.; Terragno, R.; Tuduri, E.; Espetxe, S.; Binsztein, N.; O’Brien, T.F.; et al. Automated use of WHONET and SaTScan to detect outbreaks of Shigella spp. using antimicrobial resistance phenotypes. Epidemiol. Infect. 2010, 138, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Azage, M.; Kumie, A.; Worku, A.; Bagtzoglou, A.C. Childhood Diarrhea Exhibits Spatiotemporal Variation in Northwest Ethiopia: A SaTScan Spatial Statistical Analysis. PLoS ONE 2015, 10, e0144690. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, L.J.; Zhu, R.; Xu, J.; Li, S.Z.; Guo, J.G.; Xiao, N.; Zhou, X.N. Schistosomiasis situation in People’s Republic of China in 2011. Chin. J. Schistosomiasis Control 2012, 24, 621–626. (In Chinese) [Google Scholar]
- Zhu, H.; Cai, S.X.; Liu, J.B.; Liao, H.Y.; Gong, X.G.; Xu, X.J.; Zhou, Y.; Tu, Z.; Yang, G.B.; Zhu, H.G.; et al. Assessment report on objectives and tasks of schistosomiasis control in Hubei Province (2012). J. Public Health Prev. Med. 2013, 24, 46–50. (In Chinese) [Google Scholar]
- Huang, X.; Zhang, X.; Zhu, H.; Xiao, Y.; Liu, J.; Su, Z.; Chen, Y. Endemic situation of schistosomiasis in Hubei Province, 2008. Chin. J. Schistosomiasis Control 2009, 21, 486–490. (In Chinese) [Google Scholar]
- Hong, X.C.; Xu, X.J.; Chen, X.; Li, Y.S.; Yu, C.H.; Yuan, Y.; Chen, Y.Y.; Li, R.D.; Qiu, J.; Liu, Z.C.; et al. Assessing the Effect of an Integrated Control Strategy for Schistosomiasis Japonica Emphasizing Bovines in a Marshland Area of Hubei Province, China: A Cluster Randomized Trial. PLoS Negl. Trop. Dis. 2013, 7, e2122. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Gray, D.J.; Li, Y.; Feng, Z.; Williams, G.M.; Stewart, D.; Rey-Ladino, J.; Ross, A.G. Schistosomiasis in the People’s Republic of China: The era of the Three Gorges Dam. Clin. Microbiol. Rev. 2010, 23, 442–466. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.M.; Xiang, S.; Yang, K.; Wu, X.H.; Zhou, X.N. Three Gorges Dam and Its Impact on the Potential Transmission of Schistosomiasis in Regions along the Yangtze River. Ecohealth 2008, 5, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Lu, J.Y.; Wei, G.Y.; Yao, S.M. Impact of environment changes on Oncomelania spread. J. Yangtze River Sci. Res. Inst. 2007, 24, 16–19. (In Chinese) [Google Scholar]
- Hu, Y.; Xiong, C.; Zhang, Z.; Luo, C.; Cohen, T.; Gao, J.; Zhang, L.; Jiang, Q. Changing Patterns of Spatial Clustering of Schistosomiasis in Southwest China between 1999–2001 and 2007–2008: Assessing Progress toward Eradication after the World Bank Loan Project. Int. J. Environ. Res. Public Health 2014, 11, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Xu, B.; Liang, S. Remote sensing and geographic information systems in the spatial temporal dynamics modeling of infectious diseases. Sci. China C Life Sci. 2006, 49, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Gong, P.; Biging, G.; Liang, S.; Seto, E.; Spear, R. Snail Density Prediction for Schistosomiasis Control Using Ikonos and ASTER Images. Photogramm. Eng. Remote Sens. 2004, 70, 1285–1294. [Google Scholar] [CrossRef]
- Fang, Z.; Huang, L.; Zhang, X.; Sun, Q.; Jiang, J. The relationship between patterns of spatial distribution of Oncomelania and layout of Oncomelania-controlling forest in purple shallow hilly areas of Sichuan. J. Sichuan For. Sci. Technol. 2009, 30, 40–44. (In Chinese) [Google Scholar]
- Chang, B.R.; Li, R.D.; Xu, X.J.; Qiu, J.; Yi, F.J.; Luo, K.S. Impact of land use on Oncomelania’s distribution in Jianghan Plain. Resour. Environ. Yangtze Basin 2014, 23, 380–384. (In Chinese) [Google Scholar]
- Tong, L.; Xu, X.; Fu, Y.; Wei, F.H. Impact of environmental factors on snail distribution using geographical detector model. Prog. Geogr. 2014, 33, 625–635. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, X.; Yang, G.; Sun, L.; Hong, Q.; Yang, K.; Wang, R.; Hua, Z. The potential impact of global warming on the spread of schistosomiasis. Chin. J. Epidemiol. 2002, 2, 8–11. (In Chinese) [Google Scholar]
- Xu, Z.; Wang, Y.; He, Z.; Cai, S.; Tu, Z.; Liu, J.; Zhu, H. Assessment on the outcomes of integrated schistosomiasis control measures implemented from 2009 to2013 in Gongan County, Hubei Province. J. Trop. Dis. Parasitol. 2015, 13, 26–29. (In Chinese) [Google Scholar]
- Hu, Y.; Xia, C.; Li, S.; Ward, M.P.; Luo, C.; Gao, F.; Wang, Q.; Zhang, S.; Zhang, Z. Assessing environmental factors associated with regional schistosomiasis prevalence in Anhui Province, Peoples’ Republic of China using a geographical detector method. Infect. Dis. Poverty 2017, 6, 87. [Google Scholar] [CrossRef] [PubMed]
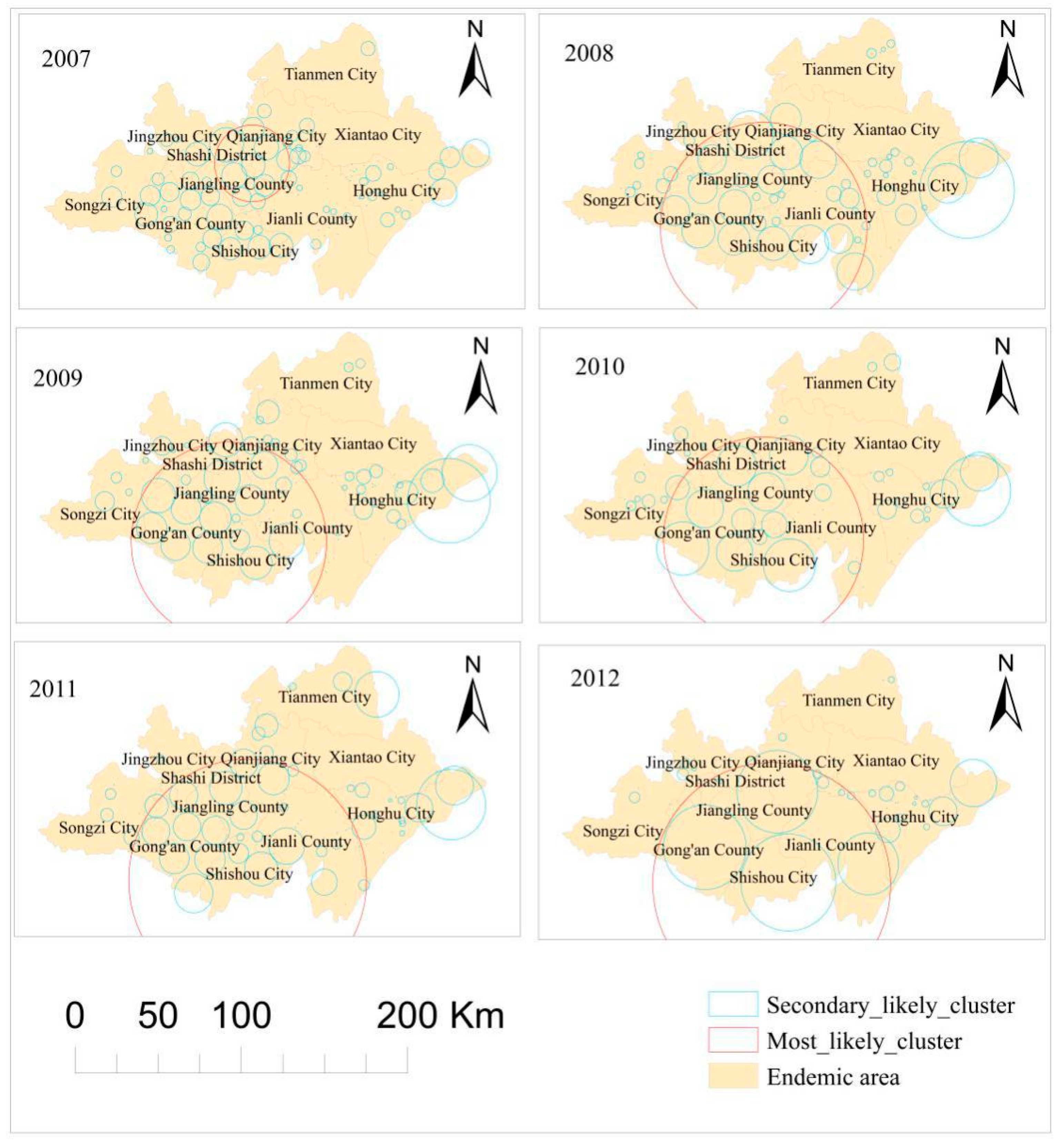


| Year | Number of Clusters | Most Likely Cluster | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of Villages | Latitude | Longitude | Radius (km) | LLR | RR | p-Value | ||
| 2007 | 109 | 259 | 30.201 | 112.575 | 22.760 | 10,609.116 | 2.448 | 0 |
| 2008 | 74 | 1135 | 29.834 | 112.450 | 62.666 | 12,127.637 | 2.118 | 0 |
| 2009 | 88 | 996 | 29.840 | 112.424 | 59.289 | 8431.589 | 1.979 | 0 |
| 2010 | 70 | 1106 | 29.846 | 112.494 | 60.754 | 10,308.459 | 2.261 | 0 |
| 2011 | 68 | 1147 | 29.697 | 112.539 | 72.155 | 8138.424 | 2.112 | 0 |
| 2012 | 42 | 1147 | 29.697 | 112.539 | 72.155 | 7303.115 | 2.174 | 0 |
| Cluster | Number of Clusters | Number of Villages | Latitude | Longitude | Radius (km) | Time | LLR | p-Value |
|---|---|---|---|---|---|---|---|---|
| Most likely cluster | 1 | 1014 | 30.755 | 112.970 | 87.938 | 2007 | 1404.203 | 0 |
| Secondary likely clusters | 2 | 116 | 30.290 | 111.741 | 30.583 | 2007 | 439.265 | 0 |
| 3 | 500 | 29.689 | 112.274 | 47.596 | 2010–2012 | 395.753 | 0 | |
| 4 | 23 | 30.052 | 113.885 | 8.545 | 2010–2011 | 153.546 | 0 | |
| 5 | 4 | 30.110 | 113.723 | 5.146 | 2009 | 82.678 | 0 |
| Year | Land Use (%) | Soil Type (%) | Average NDVI (%) | Silt (%) | Sand (%) | Clay (%) | DER (%) | Average LSTD (%) | Average LSTN (%) | Average TDN (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2007 | 3.37 | 1.93 * | 0.71 | 0.95 | 1.00 | 1.04 | 0.08 * | 0.34 * | 5.48 | 5.71 |
| 2008 | 4.69 | 2.46 | 0.25 * | 0.15 * | 1.38 | 1.08 | 0.51 | 0.63 | 1.61 | 1.59 |
| 2009 | 5.5 | 4.26 | 0.76 | 0.26 * | 2.15 | 2.08 | 0.58 | 2.15 | 2.86 | 0.46 * |
| 2010 | 5.13 | 4.06 | 0.81 | 0.25 * | 2.44 | 1.84 | 0.58 | 4.56 | 10.53 | 1.81 |
| 2011 | 4.05 | 3.09 | 0.34 * | 0.16 * | 2.64 | 1.81 | 0.34 | 2.98 | 2.66 | 3.83 |
| 2012 | 4.28 | 2.67 | 0.43 | 0.57 * | 2.19 | 2.10 | 0.06 * | 0.76 | 6.50 | 4.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Y.; Li, R.; Qiu, J.; Xu, X.; Huang, D.; Qu, Y. Geographical Clustering and Environmental Determinants of Schistosomiasis from 2007 to 2012 in Jianghan Plain, China. Int. J. Environ. Res. Public Health 2018, 15, 1481. https://doi.org/10.3390/ijerph15071481
Niu Y, Li R, Qiu J, Xu X, Huang D, Qu Y. Geographical Clustering and Environmental Determinants of Schistosomiasis from 2007 to 2012 in Jianghan Plain, China. International Journal of Environmental Research and Public Health. 2018; 15(7):1481. https://doi.org/10.3390/ijerph15071481
Chicago/Turabian StyleNiu, Yingnan, Rendong Li, Juan Qiu, Xingjian Xu, Duan Huang, and Yubing Qu. 2018. "Geographical Clustering and Environmental Determinants of Schistosomiasis from 2007 to 2012 in Jianghan Plain, China" International Journal of Environmental Research and Public Health 15, no. 7: 1481. https://doi.org/10.3390/ijerph15071481
APA StyleNiu, Y., Li, R., Qiu, J., Xu, X., Huang, D., & Qu, Y. (2018). Geographical Clustering and Environmental Determinants of Schistosomiasis from 2007 to 2012 in Jianghan Plain, China. International Journal of Environmental Research and Public Health, 15(7), 1481. https://doi.org/10.3390/ijerph15071481





