Assessment of Microbiological Safety of Water in Public Swimming Pools in Guangzhou, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Swimming Pools Selection and Water Sample Collection
2.2. Detection of Microbiological Pathogens from Water
2.3. Testing P. aeruginosa Susceptibility to Antibiotics
2.4. Multilocus Sequence Typing (MLST) of P. aeruginosa
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Swimming Pools
3.2. Occurrence of Protozoa in Swimming Pool Water
3.3. Occurrence of Bacteria in Swimming Pool Water
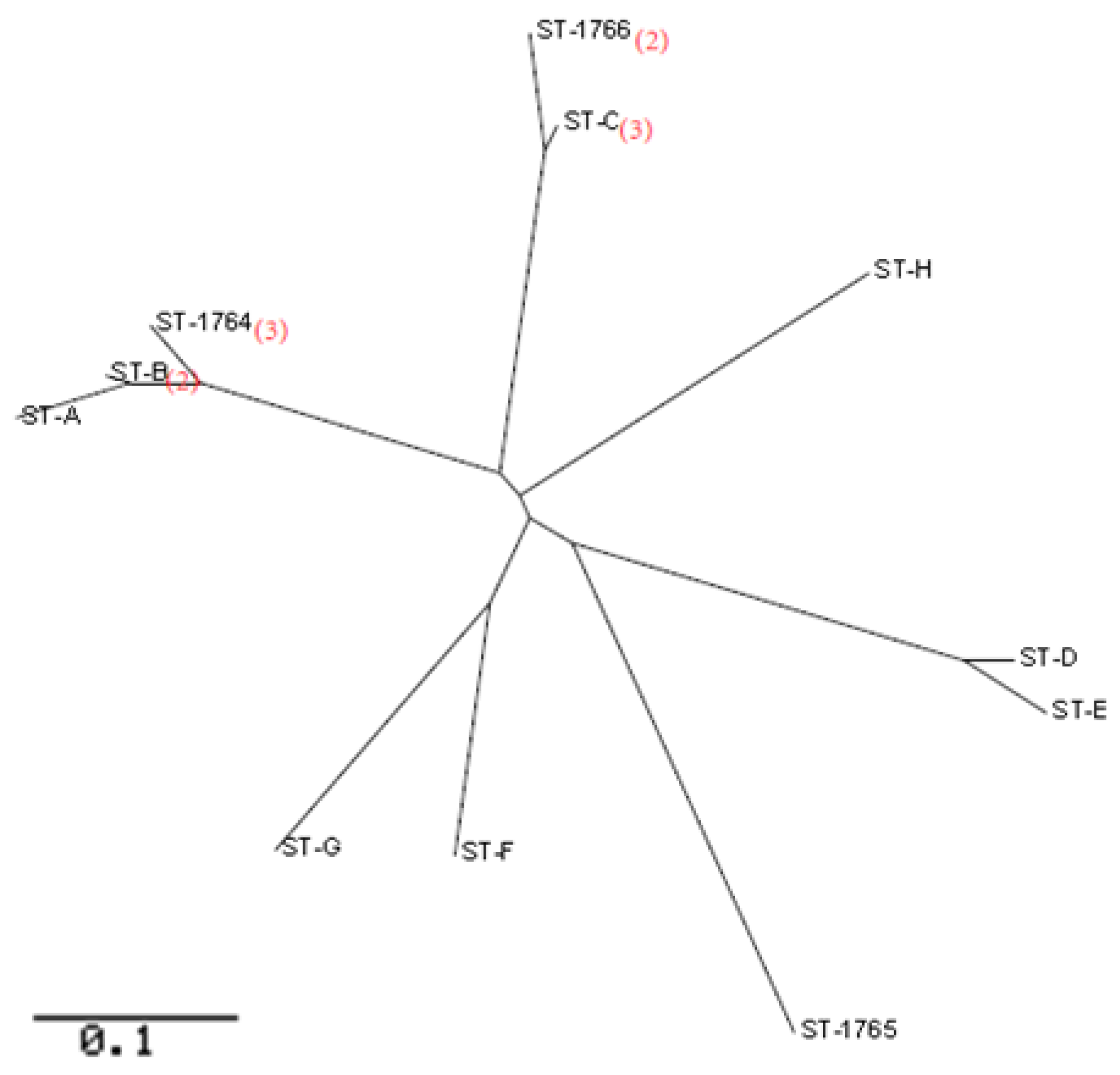
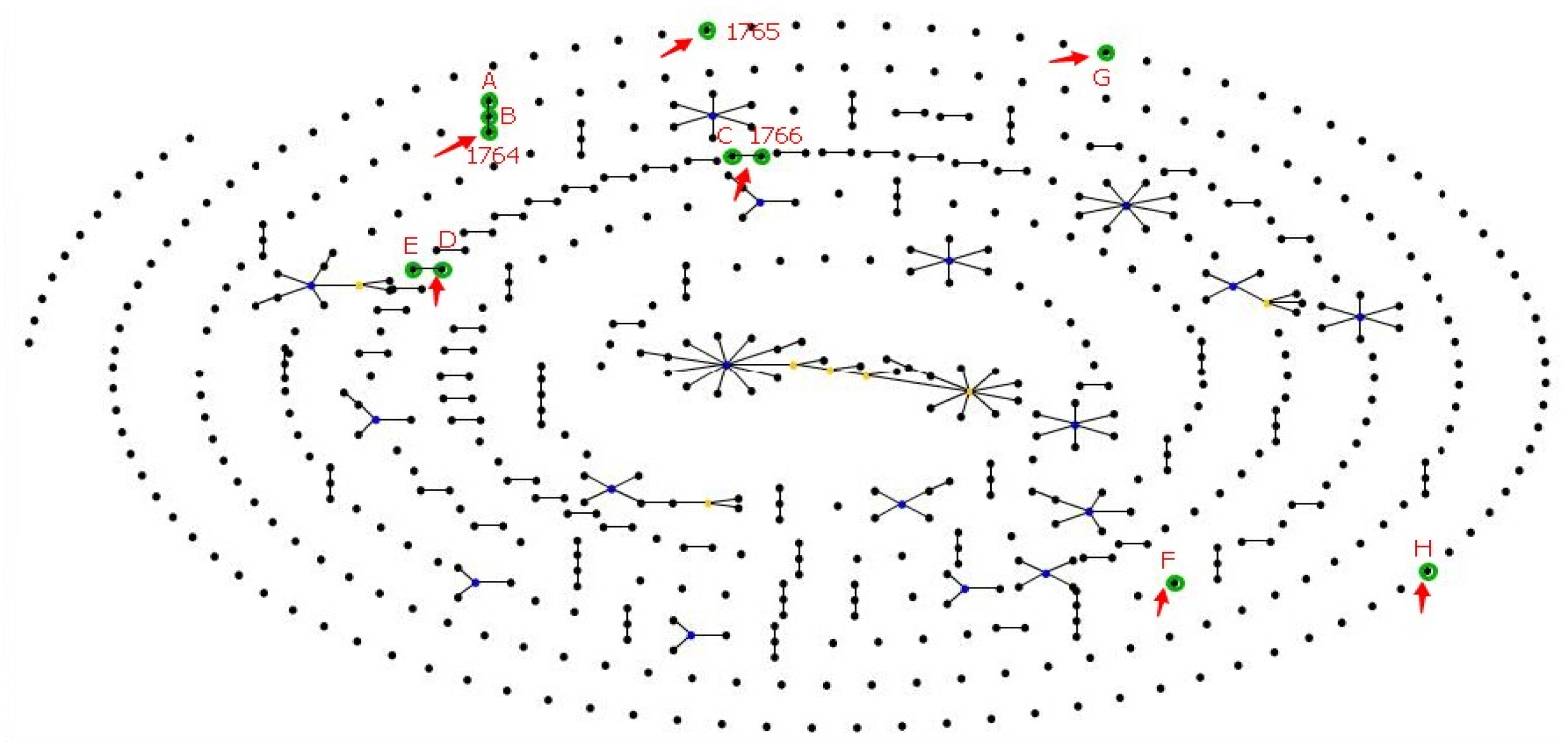
3.4. MLST of P. aeruginosa
3.5. P. aeruginosa Susceptibility to Antibiotics
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leclerc, H.; Schwartzbrod, L.; Dei-Cas, E. Microbial agents associated with waterborne diseases. Crit. Rev. Microbiol. 2002, 28, 371–409. [Google Scholar] [CrossRef] [PubMed]
- Bitton, G. Microbiology of Drinking Water Production and Distribution, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; p. 312. [Google Scholar]
- WHO. Water Sanitation Hygiene. Available online: http://www.who.int/water_sanitation_health/en/ (accessed on 6 June 2018).
- Alhamlan, F.S.; Al-Qahtani, A.A.; Al-Ahdal, M.N. Recommended advanced techniques for waterborne pathogen detection in developing countries. J. Infect. Dev. Ctries. 2015, 9, 128–135. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Water Supply and Sanitation Assessment 2000 Report. Available online: http://www.who.int/water_sanitation_health/monitoring/jmp2000.pdf (accessed on 30 May 2018).
- Barna, Z.; Kadar, M. The risk of contracting infectious diseases in public swimming pools. A review. Ann. Ist. Super. Sanita 2012, 48, 374–386. [Google Scholar] [CrossRef] [PubMed]
- WHO. Water Recreation and Disease. 2005. Available online: http://www.who.int/water_sanitation_health/bathing/recreadis.pdf (accessed on 28 May 2018).
- Giampaoli, S.; Romano Spica, V. Health and safety in recreational waters. Bull. World Health Organ. 2014, 92, 79. [Google Scholar] [CrossRef] [PubMed]
- Mavridou, A.; Pappa, O.; Papatzitze, O.; Blougoura, A.; Drossos, P. An overview of pool and SPA regulations in Mediterranean countries with a focus on the tourist industry. J. Water Health 2014, 12, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Waldron, L.S.; Ferrari, B.C.; Cheung-Kwok-Sang, C.; Beggs, P.J.; Stephens, N.; Power, M.L. Molecular epidemiology and spatial distribution of a waterborne cryptosporidiosis outbreak in Australia. Appl. Environ. Microbiol. 2011, 77, 7766–7771. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Reacher, M.; Smerdon, W.; Adak, G.K.; Nichols, G.; Chalmers, R.M. Outbreaks of waterborne infectious intestinal disease in England and Wales, 1992–2003. Epidemiol. Infect. 2006, 134, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.A.; van den Akker, B.; Pomati, F.; Roser, D. A risk assessment of Pseudomonas aeruginosa in swimming pools: A review. J. Water Health 2012, 10, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Dallolio, L.; Belletti, M.; Agostini, A.; Teggi, M.; Bertelli, M.; Bergamini, C.; Chetti, L.; Leoni, E. Hygienic Surveillance in Swimming pools: Assessment of the Water Quality in Bologna Facilities in the Period 2010–2012. Microchem. J. 2013, 110, 624–628. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Chen, R.Y.; Li, Y. Investigation of various pathogenic bacteria in indoor swimming pool water. Chin. J. Health Lab. Technol. 2008, 18, 335–337. [Google Scholar]
- Zhang, H.X.; Li, D.; Guo, Q. Monitoring results of water quality of swimming pools in Dalian city during 2009–2010. Occup. Health 2011, 27, 2–3. [Google Scholar]
- Zhou, W.M.; Han, R.P.; Qiu, Y.R. Detection result of water quality of swimming pools in Kunming City from 2009–2011. Occup. Health 2013, 29, 990–991. [Google Scholar]
- Chen, X.; Atwill, E.R.; Zhong, F.; Wei, Y.; Hou, S.; Li, J.; Xu, C.; Xiao, C.; Yang, Z.; Li, X. Prevalence and risk factors of Cryptosporidium infection in children with clinical diarrhea in Guangzhou, China. J. Bacteriol. Parasitol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Rhodes, E.R.; Villegas, L.F.; Shaw, N.J.; Miller, C.; Villegas, E.N. A modified EPA Method 1623 that uses tangential flow hollow-fiber ultrafiltration and heat dissociation steps to detect waterborne Cryptosporidium and Giardia spp. J. Vis. Exp. 2012, 3791–4177. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; 23th Informational Supplement (Document M100-S23); CLSI: Wayne, PA, USA, 2013. [Google Scholar]
- Causer, L.M.; Handzel, T.; Welch, P.; Carr, M.; Culp, D.; Lucht, R.; Mudahar, K.; Robinson, D.; Neavear, E.; Fenton, S.; et al. An outbreak of Cryptosporidium hominis infection at an Illinois recreational waterpark. Epidemiol. Infect. 2006, 134, 147–156. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, W.R.; Kazmierczak, J.J.; Davis, J.P. An outbreak of cryptosporidiosis associated with a resort swimming pool. Epidemiol. Infect. 1995, 115, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Toriumi, H.; Endo, T.; Yamamoto, N.; Kuroki, T. An outbreak of cryptosporidiosis associated with swimming pools. Kansenshogaku zasshi J. Jpn. Assoc. Infect. Dis. 2008, 82, 14–19. [Google Scholar] [CrossRef]
- Gao, J.Z. Clinical Laboratory Parasitology; People’s Health Publishing House: Beijing, China, 2009. [Google Scholar]
- White, G.C. Handbook of Chlorination and Alternative Disinfectants, 4th ed.; John Wiley & Sons Inc.: New York, NY, USA, 1999. [Google Scholar]
- Baldursson, S.; Karanis, P. Waterborne transmission of protozoan parasites: Review of worldwide outbreaks—An update 2004–2010. Water Res. 2011, 45, 6603–6614. [Google Scholar] [CrossRef] [PubMed]
- Hijnen, W.A.; Beerendonk, E.F.; Medema, G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Korich, D.G.; Mead, J.R.; Madore, M.S.; Sinclair, N.A.; Sterling, C.R. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl. Environ. Microbiol. 1990, 56, 1423–1428. [Google Scholar] [PubMed]
- Hall, V.; Taye, A.; Walsh, B.; Maguire, H.; Dave, J.; Wright, A.; Anderson, C.; Crook, P. A large outbreak of gastrointestinal illness at an open-water swimming event in the River Thames, London. Epidemiol. Infect. 2017, 145, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.A.; Bens, M.S.; Craun, G.F.; Calderon, R.L.; Herwaldt, B.L. Surveillance for waterborne-disease outbreaks—United States, 1995–1996. MMWR CDC Surveill. Summ. Morb. Mortal. Wkly. Rep. CDC Surveill. Summ. 1998, 47, 1–34. [Google Scholar]
- McFeters, G.A.; Kippin, J.S.; LeChevallier, M.W. Injured coliforms in drinking water. Appl. Environ. Microbiol. 1986, 51, 1–5. [Google Scholar] [PubMed]
- Craun, G.F.; Calderon, R.L.; Craun, M.F. Outbreaks associated with recreational water in the United States. Int. J. Environ. Health Res. 2005, 15, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Mena, K.D.; Gerba, C.P. Risk assessment of Pseudomonas aeruginosa in water. Rev. Environ. Contam. Toxicol. 2009, 201, 71–115. [Google Scholar] [PubMed]
- Hajjartabar, M. Poor-quality water in swimming pools associated with a substantial risk of otitis externa due to Pseudomonas Aeruginosa. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2004, 50, 63–67. [Google Scholar] [CrossRef]
- Hutcheson, C.; Cira, R.; Gaines, S.L.; Jones, K.R.; Howard, W.; Hornsby, D.; Redmond, M.; Rustin, C.; Hlavsa, M.C.; Murphy, J.L.; et al. Microbes in Pool Filter Backwash as Evidence of the Need for Improved Swimmer Hygiene—Metro-Atlanta, Georgia, 2012. MMWR-Morbid. Mortal. Wkly. Rep. 2013, 62, 385–388. [Google Scholar]
- Reali, D.; Rosati, S. Antibiotic susceptibility and serotyping of Pseudomonas aeruginosa strains isolated from surface waters, thermomineral waters and clinical specimens. Zent. Hyg. Umweltmed. 1994, 196, 75–80. [Google Scholar]
- WHO. Microbial hazards. In Guidelines for Safe Recreational Water Environments. Swimming Pools and Similar Environments; WHO Press: Geneva, Switzerland, 2006. [Google Scholar]
- Nichols, G. Infection risks from water in natural and man-made environments. Euro Surveill. Bull. Eur. Mal. Transm. Eur. Commun. Dis. Bull. 2006, 11, 76–78. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Economou, V.; Sakkas, H.; Gousia, P.; Giannakopoulos, X.; Dontorou, C.; Filioussis, G.; Gessouli, H.; Karanis, P.; Leveidiotou, S. Microbiological quality of indoor and outdoor swimming pools in Greece: Investigation of the antibiotic resistance of the bacterial isolates. Int. J. Hyg. Environ. Health 2008, 211, 385–397. [Google Scholar] [CrossRef] [PubMed]
| Locus and Functions | Primers Sequence (5′—3′) | Size (bp) | ||
|---|---|---|---|---|
| Forward | Reverse | |||
| acsA | Amplification | ACCTGGTGTACGCCTCGCTGAC | GACATAGATGCCCTGCCCCTTGAT | 842 |
| Sequencing | GCCACACCTACATCGTCTAT | GTGGACAACCTCGGCAACCT | 390 | |
| aroE | Amplification | TGGGGCTATGACTGGAAACC | TAACCCGGTTTTGTGATTCCTACA | 825 |
| Sequencing | ATGTCACCGTGCCGTTCAAG | TGAAGGCAGTCGGTTCCTTG | 495 | |
| guaA | Amplification | CGGCCTCGACGTGTGGATGA | GAACGCCTGGCTGGTCTTGTGGTA | 940 |
| Sequencing | AGGTCGGTTCCTCCAAGGTC | TCAAGTCGCACCACAACGTC | 372 | |
| mutL | Amplification | CCAGATCGCCGCCGGTGAGGTG | CAGGGTGCCATAGAGGAAGTC | 940 |
| Sequencing | AGAAGACCGAGTTCGACCAT | ATGACTTCCTCTATGGCACC | 441 | |
| nuoD | Amplification | ACCGCCACCCGTACTG | TCTCGCCCATCTTGACCA | 1042 |
| Sequencing | ACGGCGAGAACGAGGACTAC | TTCACCTTCACCGACCGCCA | 366 | |
| ppsA | Amplification | GGTCGCTCGGTCAAGGTAGTGG | GGGTTCTCTTCTTCCGGCTCGTAG | 989 |
| Sequencing | GGTGACGACGGCAAGCTGTA | TCCTGTGCCGAAGGCGATAC | 369 | |
| trpE | Amplification | GCGGCCCAGGGTCGTGAG | CCCGGCGCTTGTTGATGGTT | 811 |
| Sequencing | TTCAACTTCGGCGACTTCCA | GGTGTCCATGTTGCCGTTCC | 441 | |
| Residual Levels of Free Chlorine (mg/L) | % (Positive/Total) Occurrence of P. aeruginosa | p | OR (95% CI) |
|---|---|---|---|
| <0.3 | 66.7 (8/12) | - | - |
| 0.3 to 0.5 | 77.8 (7/9) | 1.00 | 1.00 (0.21, 4.71) |
| >0.5 | 66.7 (12/18) | 0.55 | 1.75 (0.28, 11.15) |
| Total | 69.2 (27/39) | - | - |
| acsA | aroE | guaA | mutL | nuoD | ppsA | trpE | Group | STs | SLV | DLV | Freq | Pool Type | District |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 81 | 11 | 112 | 5 | 73 | 20 | 139 | 1 | ST-A | 1 | 1 | 1 | Residential | Haizhu |
| 81 | 11 | 112 | 5 | 13 | 20 | 139 | 1 | ST-B | 2 | 0 | 2 | Residential | Baiyun |
| 81 | 11 | 57 | 5 | 13 | 20 | 139 | 1 | ST-1764 | 1 | 1 | 3 | Residential | Baiyun |
| 5 | 3 | 95 | 5 | 93 | 6 | 47 | 2 | ST-1766 | 1 | 0 | 2 | Residential; Water park | Baiyun; Haizhu |
| 5 | 3 | 95 | 5 | 13 | 6 | 47 | 2 | ST-C | 1 | 0 | 3 | Residential; Water park * | Baiyun; Haizhu |
| 70 | 5 | 72 | 2 | 3 | 20 | 26 | 3 | ST-D | 1 | 0 | 1 | School | Haizhu |
| 70 | 5 | 72 | 2 | 3 | 4 | 26 | 3 | ST-E | 1 | 0 | 1 | Water park | Baiyun |
| 5 | 5 | 57 | 13 | 13 | 74 | 3 | Singleton | ST-F | - | - | 1 | Water park | Haizhu |
| 83 | 5 | 9 | 3 | 13 | 10 | 3 | Singleton | ST-G | - | - | 1 | Residential | Baiyun |
| 32 | 13 | 24 | 13 | 13 | 6 | 25 | Singleton | ST-H | - | - | 1 | Water park | Baiyun |
| 39 | 6 | 4 | 14 | 61 | 15 | 2 | Singleton | ST-1765 | - | - | 1 | Residential | Baiyun |
| Isolates ID | PENICILLINS | Β-LACTAM | Cephems | Carbapenems | Aminoglycosides | Fluoroquinolones | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Piperacillin | Ticarcillin-Clavulanic Acid | Tazobactam | Ceftazidime | Cefepime | Imipenem | Gentamicin | Tobramycin | Amikacin | Ciprofloxacin | Levofloxacin | |
| 2 | R * | S | S | S | S | S | S | S | S | S | S |
| 16 | IR ** | S | S | S | S | S | IR | S | S | S | S |
| 17 | S *** | S | S | S | S | R | R | S | S | S | S |
| 32 | IR | S | S | S | S | S | IR | S | S | S | S |
| 33 | S | S | S | S | S | R | S | S | S | S | S |
| 37 | R | S | S | S | S | S | S | S | S | S | S |
| All other isolates | S | S | S | S | S | S | S | S | S | S | S |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Li, J.; Hou, S.; Xu, C.; Zhang, H.; Atwill, E.R.; Li, X.; Yang, Z.; Chen, S. Assessment of Microbiological Safety of Water in Public Swimming Pools in Guangzhou, China. Int. J. Environ. Res. Public Health 2018, 15, 1416. https://doi.org/10.3390/ijerph15071416
Wei X, Li J, Hou S, Xu C, Zhang H, Atwill ER, Li X, Yang Z, Chen S. Assessment of Microbiological Safety of Water in Public Swimming Pools in Guangzhou, China. International Journal of Environmental Research and Public Health. 2018; 15(7):1416. https://doi.org/10.3390/ijerph15071416
Chicago/Turabian StyleWei, Xiaohong, Juntao Li, Shuiping Hou, Conghui Xu, Hao Zhang, Edward Robert Atwill, Xunde Li, Zhicong Yang, and Shouyi Chen. 2018. "Assessment of Microbiological Safety of Water in Public Swimming Pools in Guangzhou, China" International Journal of Environmental Research and Public Health 15, no. 7: 1416. https://doi.org/10.3390/ijerph15071416
APA StyleWei, X., Li, J., Hou, S., Xu, C., Zhang, H., Atwill, E. R., Li, X., Yang, Z., & Chen, S. (2018). Assessment of Microbiological Safety of Water in Public Swimming Pools in Guangzhou, China. International Journal of Environmental Research and Public Health, 15(7), 1416. https://doi.org/10.3390/ijerph15071416





