Exposure to Environmental Contaminants and Lung Function in Adolescents—Is There a Link?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Medical Examination
2.3. Lung Function
2.4. Laboratory Analyses
2.5. Statistical Analyses
3. Results
3.1. Prenatal and Lactational Dioxin Exposure in Relation to Lung Function
3.2. Serum Dioxin, dl-PCBs, and Total TEQ in Relation to Lung Function
3.3. Serum BDEs in Relation to Lung Function
4. Discussion
4.1. Accidents
4.2. Dioxins and PCBs
4.3. Brominated Diphenyl Ethers
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hjelmborg, J.; Korhonen, T.; Holst, K.; Skytthe, A.; Pukkala, E.; Kutschke, J.; Harris, J.R.; Mucci, L.A.; Christensen, K.; Czene, K.; et al. Lung cancer, genetic predisposition and smoking: The nordic twin study of cancer. Thorax 2017, 72, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Loxham, M.; Davies, D.E. Phenotypic and genetic aspects of epithelial barrier function in asthmatic patients. J. Allergy Clin. Immunol. 2017, 139, 1736–1751. [Google Scholar] [CrossRef] [PubMed]
- Sergio, L.; de Paoli, F.; Mencalha, A.L.; da Fonseca, A.S. Chronic obstructive pulmonary disease: From injury to genomic stability. Copd 2017, 14, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Marsh, G.M.P.F.; Riordan, A.S.M.; Keeton, K.A.M.; Benson, S.M.P. Non-occupational exposure to asbestos and risk of pleural mesothelioma: Review and meta-analysis. Occup. Environ. Med. 2017, 74, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Ten Tusscher, G.W.; Koppe, J.G. Perinatal dioxin exposure and later effects—A review. Chemosphere 2004, 54, 1329–1336. [Google Scholar] [CrossRef]
- Leijs, M.M.; Koppe, J.G.; Olie, K.; van Aalderen, W.M.; de Voogt, P.; ten Tusscher, G.W. Effects of dioxins, PCBs, and PBDEs on immunology and hematology in adolescents. Environ. Sci. Technol. 2009, 43, 7946–7951. [Google Scholar] [CrossRef] [PubMed]
- Leijs, M.M.; ten Tusscher, G.W.; Olie, K.; van Teunenbroek, T.; van Aalderen, W.M.; de Voogt, P.; Vulsma, T.; Bartonova, A.; Krayer von Krauss, M.; Mosoiu, C.; et al. Thyroid hormone metabolism and environmental chemical exposure. Environ. Health 2012, 11 (Suppl. 1), S10. [Google Scholar] [CrossRef] [PubMed]
- Leijs, M.M.; Esser, A.; Amann, P.M.; Schettgen, T.; Gube, M.; Merk, H.F.; Kraus, T.; Baron, J.M. Hyperpigmentation and higher incidence of cutaneous malignancies in moderate-high PCB- and dioxin exposed individuals. Environ. Res. 2018, 164, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.W.; Kerger, B.D.; Paustenbach, D.J. Elimination half-lives of selected polychlorinated dibenzodioxins and dibenzofurans in breast-fed human infants. J. Toxicol. Environ. Health A 2006, 69, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Pirkle, J.L.; Wolfe, W.H.; Patterson, D.G.; Needham, L.L.; Michalek, J.E.; Miner, J.C.; Peterson, M.R.; Phillips, D.L. Estimates of the half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in vietnam veterans of operation ranch hand. J. Toxicol. Environ. Health A 1989, 27, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Hagenmaier, H.; Wiesmuller, T.; Golor, G.; Krowke, R.; Helge, H.; Neubert, D. Transfer of various polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDDs and PCDFs) via placenta and through milk in a marmoset monkey. Arch. Toxicol. 1990, 64, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Koppe, J.G.; Pluim, H.J.; Olie, K.; van Wijnen, J. Breast milk, dioxins and the possible effects on the health of newborn infants. Sci. Total Environ. 1991, 106, 33–41. [Google Scholar] [CrossRef]
- Lancz, K.; Murinova, L.; Patayova, H.; Drobna, B.; Wimmerova, S.; Sovcikova, E.; Kovac, J.; Farkasova, D.; Hertz-Picciotto, I.; Jusko, T.A.; et al. Ratio of cord to maternal serum PCB concentrations in relation to their congener-specific physicochemical properties. Int. J. Hyg. Environ. Health 2015, 218, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Van Wijnen, J.; van Bavel, B.; Lindström, G.; Koppe, J.G.; Olie, K. Placental transport of PCDDs and PCDFs in humans. Organohalogen Compd. 1990, 1, 47–50. [Google Scholar]
- Harrad, S.; Hunter, S. Concentrations of polybrominated diphenyl ethers in air and soil on a rural-urban transect across a major UK conurbation. Environ. Sci. Technol. 2006, 40, 4548–4553. [Google Scholar] [CrossRef] [PubMed]
- Gomara, B.; Herrero, L.; Ramos, J.J.; Mateo, J.R.; Fernandez, M.A.; Garcia, J.F.; Gonzalez, M.J. Distribution of polybrominated diphenyl ethers in human umbilical cord serum, paternal serum, maternal serum, placentas, and breast milk from Madrid population, Spain. Environ. Sci. Technol. 2007, 41, 6961–6968. [Google Scholar] [CrossRef] [PubMed]
- Leijs, M.M.; van Teunenbroek, T.; Olie, K.; Koppe, J.G.; ten Tusscher, G.W.; van Aalderen, W.M.; de Voogt, P. Assessment of current serum levels of PCDD/Fs, dl-PCBs and PBDEs in a Dutch cohort with known perinatal PCDD/F exposure. Chemosphere 2008, 73, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Ten Tusscher, G.W.; de Weerdt, J.; Roos, C.M.; Griffioen, R.W.; De Jongh, F.H.; Westra, M.; van der Slikke, J.W.; Oosting, J.; Olie, K.; Koppe, J.G. Decreased lung function associated with perinatal exposure to Dutch background levels of dioxins. Acta Paediatr. 2001, 90, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Pesatori, A.C.; Zocchetti, C.; Guercilena, S.; Consonni, D.; Turrini, D.; Bertazzi, P.A. Dioxin exposure and non-malignant health effects: A mortality study. Occup. Environ. Med. 1998, 55, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Pluim, H.J.; Koppe, J.G.; Olie, K.; Vd Slikke, J.W.; Kok, J.H.; Vulsma, T.; Van Tijn, D.; De Vijlder, J.J. Effects of dioxins on thyroid function in newborn babies. Lancet 1992, 339, 1303. [Google Scholar] [CrossRef]
- Ilsen, A.; Briet, J.M.; Koppe, J.G.; Pluim, H.J.; Oosting, J. Signs of enhanced neuromotor maturation in children due to perinatal load with background levels of dioxins. Follow-up until age 2 years and 7 months. Chemosphere 1996, 33, 1317–1326. [Google Scholar] [CrossRef]
- Ten Tusscher, G.W. Later Childhood Effects of Perinatal Exposure to Background Levels of Dioxins in the Netherlands; University of Amsterdam: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Zapletal, A.S.M.; Paul, T. Lung function in children and adolescents. Prog. Respir. Res. 1987, 22, 114–218. [Google Scholar]
- Pluim, H.J.; Koppe, J.G.; Olie, K.; van der Slikke, J.W.; Slot, P.C.; van Boxtel, C.J. Clinical laboratory manifestations of exposure to background levels of dioxins in the perinatal period. Acta Paediatr. 1994, 83, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Rylander, L.; Nilsson-Ehle, P.; Hagmar, L. A simplified precise method for adjusting serum levels of persistent organohalogen pollutants to total serum lipids. Chemosphere 2006, 62, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L.R. Chemicals and children’s environment: What we don’t know about risks. Environ. Health Perspect. 1998, 106 (Suppl. 3), 875–880. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T. Yusho in Japan. Ind. Health 2003, 41, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Rogan, W.J.; Gladen, B.C.; Hung, K.L.; Koong, S.L.; Shih, L.Y.; Taylor, J.S.; Wu, Y.C.; Yang, D.; Ragan, N.B.; Hsu, C.C. Congenital poisoning by polychlorinated biphenyls and their contaminants in Taiwan. Science 1988, 241, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Bertazzi, P.A.; Consonni, D.; Bachetti, S.; Rubagotti, M.; Baccarelli, A.; Zocchetti, C.; Pesatori, A.C. Health effects of dioxin exposure: A 20-year mortality study. Am. J. Epidemiol. 2001, 153, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Budtz-Jorgensen, E.; Barr, D.B.; Needham, L.L.; Weihe, P.; Heinzow, B. Elimination half-lives of polychlorinated biphenyl congeners in children. Environ. Sci. Technol. 2008, 42, 6991–6996. [Google Scholar] [CrossRef] [PubMed]
- Patandin, S.; Dagnelie, P.C.; Mulder, P.G.; Op de Coul, E.; van der Veen, J.E.; Weisglas-Kuperus, N.; Sauer, P.J. Dietary exposure to polychlorinated biphenyls and dioxins from infancy until adulthood: A comparison between breast-feeding, toddler, and long-term exposure. Environ. Health Perspect. 1999, 107, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.; Strom, M.; Olsen, S.F.; Dahl, R.; Hoffmann, H.J.; Granstrom, C.; Rytter, D.; Bech, B.H.; Linneberg, A.; Maslova, E.; et al. Prenatal exposure to persistent organic pollutants and offspring allergic sensitization and lung function at 20 years of age. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2016, 46, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Link, B.; Gabrio, T.; Zollner, I.; Piechotowski, I.; Kouros, B. Sentinel health department project in Baden-Wuerttemberg (Germany)—A useful tool for monitoring children’s health and environment. Int. J. Hyg. Environ. Health 2007, 210, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Gascon, M.; Sunyer, J.; Martinez, D.; Guerra, S.; Lavi, I.; Torrent, M.; Vrijheid, M. Persistent organic pollutants and children’s respiratory health: The role of cytokines and inflammatory biomarkers. Environ. Int. 2014, 69, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Stolevik, S.B.; Nygaard, U.C.; Namork, E.; Haugen, M.; Meltzer, H.M.; Alexander, J.; Knutsen, H.K.; Aaberge, I.; Vainio, K.; van Loveren, H.; et al. Prenatal exposure to polychlorinated biphenyls and dioxins from the maternal diet may be associated with immunosuppressive effects that persist into early childhood. Food Chem. Toxicol. 2013, 51, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Gascon, M.; Morales, E.; Sunyer, J.; Vrijheid, M. Effects of persistent organic pollutants on the developing respiratory and immune systems: A systematic review. Environ. Int. 2013, 52, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.H.; Kuo, Y.C.; Tsai, M.H.; Ho, C.C.; Tsai, H.T.; Hsu, C.Y.; Chen, Y.C.; Lin, P. Interleukin-24 as a target cytokine of environmental aryl hydrocarbon receptor agonist exposure in the lung. Toxicol. Appl. Pharmacol. 2017, 324, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zuurbier, M.; Leijs, M.; Schoeters, G.; ten Tusscher, G.; Koppe, J.G. Children’s exposure to polybrominated diphenyl ethers. Acta Paediatr. 2006, 95, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Holden, A.; Smith, S.C.; Gephart, R.; Petreas, M.; Park, J.S. PBDE levels in breast milk are decreasing in California. Chemosphere 2016, 150, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Dodson, R.E.; Perovich, L.J.; Covaci, A.; Van den Eede, N.; Ionas, A.C.; Dirtu, A.C.; Brody, J.G.; Rudel, R.A. After the PBDE phase-out: A broad suite of flame retardants in repeat house dust samples from California. Environ. Sci. Technol. 2012, 46, 13056–13066. [Google Scholar] [CrossRef] [PubMed]
- Harrad, S.; Hazrati, S.; Ibarra, C. Concentrations of polychlorinated biphenyls in indoor air and polybrominated diphenyl ethers in indoor air and dust in Birmingham, United Kingdom: Implications for human exposure. Environ. Sci. Technol. 2006, 40, 4633–4638. [Google Scholar] [CrossRef] [PubMed]
- Roze, E.; Meijer, L.; Bakker, A.; Van Braeckel, K.N.; Sauer, P.J.; Bos, A.F. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ. Health Perspect. 2009, 117, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Viberg, H. Neonatal ontogeny and neurotoxic effect of decabrominated diphenyl ether (PBDE 209) on levels of synaptophysin and tau. Int. J. Dev. Neurosci. 2009, 27, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Viberg, H. Exposure to polybrominated diphenyl ethers 203 and 206 during the neonatal brain growth spurt affects proteins important for normal neurodevelopment in mice. Toxicol. Sci. 2009, 109, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Talsness, C.E. Overview of toxicological aspects of polybrominated diphenyl ethers: A flame-retardant additive in several consumer products. Environ. Res. 2008, 108, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Talsness, C.E.; Kuriyama, S.N.; Sterner-Kock, A.; Schnitker, P.; Grande, S.W.; Shakibaei, M.; Andrade, A.; Grote, K.; Chahoud, I. In utero and lactational exposures to low doses of polybrominated diphenyl ether-47 alter the reproductive system and thyroid gland of female rat offspring. Environ. Health Perspect. 2008, 116, 308–314. [Google Scholar] [CrossRef] [PubMed]
| N = 33 (18 Girls, 15 Boys) | Median | Mean | Range |
|---|---|---|---|
| Age (years) | 14.3 | 15.0 | 14.0–18.7 |
| Prenatal PCDD/F exposure I-TEQ (pg/g lipid) | 29.8 | 32.6 | 9.05–88.8 |
| Lactational PCDD/F exposure (I-TEQ (ng)) | 45.9 | 66.9 | 4.34–279 |
| Serum PCDD/F (WHO 2005) TEQ (pg/g lipid) | 1.6 | 2.2 | 0.4–6.1 |
| Serum dl-PCBs (WHO 2005) TEQ (pg/g lipid) | 1.8 | 2.2 | 0.04–7.8 |
| Serum sum-BDEs (ng/g lipid) | 9.9 | 14 | 4.9–73.6 |
| Spirometry | Diffusion Measurements | Body Box | |||
|---|---|---|---|---|---|
| VC MAX | β: 0.49 * | TLCO SB | β: 0.11 | VC MAX | β: 0.479 * |
| FEV 1 | β: 0.63 * | VA | β: 0.392 | TLC | β: 0.261 |
| FEV1/VC MAX | β: 0.11 | VIN | β: 0.459 * | RV | β: −0.2 |
| PEF | β: 0.43 | RV%TLC | β: −0.45 | ||
| FEF 50 | β: 0.36 | FRC | β: −0.16 | ||
| Spirometry | Diffusion Measurements | Body Box | |||
|---|---|---|---|---|---|
| VC MAX | (ρ): 0.27 | TLCO SB | (ρ): 0.211 | VC MAX | (ρ): 0.282 |
| FVC | (ρ): 0.208 | VA | (ρ): 0.212 | TLC | (ρ): 0.084 |
| FEV 1 | (ρ): −0.539 * | VIN | (ρ): 0.243 | RV | (ρ): −0.272 |
| FEV1/VC MAX | (ρ): −0.575 * | RV%TLC | (ρ): −0.341 | ||
| PEF | (ρ): −0.171 | FRC | (ρ): −0.169 | ||
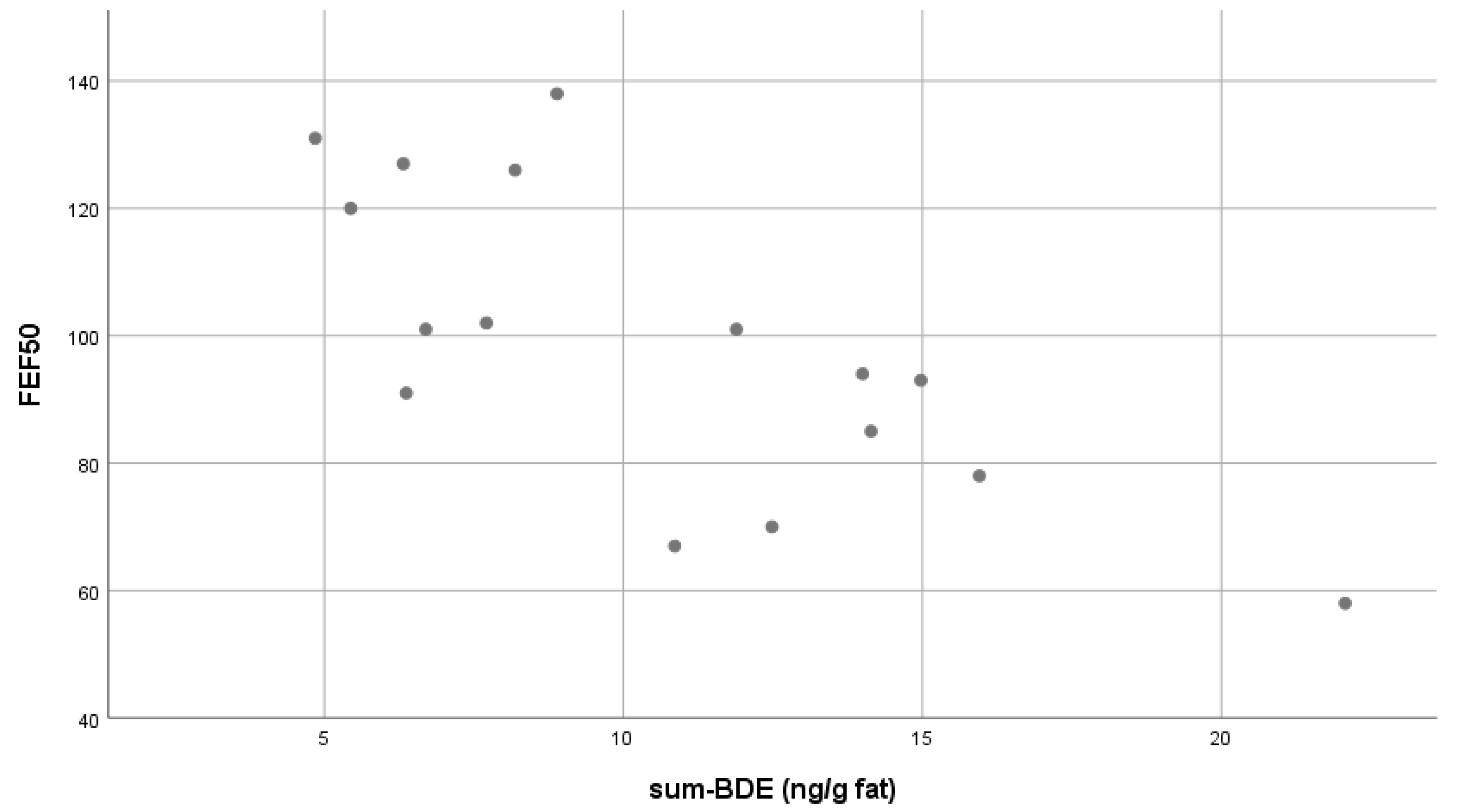
| FEF 50 | (ρ): −0.699 ** | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leijs, M.M.; Koppe, J.G.; Olie, K.; De Voogt, P.; Van Aalderen, W.M.C.; Ten Tusscher, G.W. Exposure to Environmental Contaminants and Lung Function in Adolescents—Is There a Link? Int. J. Environ. Res. Public Health 2018, 15, 1352. https://doi.org/10.3390/ijerph15071352
Leijs MM, Koppe JG, Olie K, De Voogt P, Van Aalderen WMC, Ten Tusscher GW. Exposure to Environmental Contaminants and Lung Function in Adolescents—Is There a Link? International Journal of Environmental Research and Public Health. 2018; 15(7):1352. https://doi.org/10.3390/ijerph15071352
Chicago/Turabian StyleLeijs, Marike M., Janna G. Koppe, Kees Olie, Pim De Voogt, Wim M. C. Van Aalderen, and Gavin W. Ten Tusscher. 2018. "Exposure to Environmental Contaminants and Lung Function in Adolescents—Is There a Link?" International Journal of Environmental Research and Public Health 15, no. 7: 1352. https://doi.org/10.3390/ijerph15071352
APA StyleLeijs, M. M., Koppe, J. G., Olie, K., De Voogt, P., Van Aalderen, W. M. C., & Ten Tusscher, G. W. (2018). Exposure to Environmental Contaminants and Lung Function in Adolescents—Is There a Link? International Journal of Environmental Research and Public Health, 15(7), 1352. https://doi.org/10.3390/ijerph15071352





