The Influence of Phosphogypsum Addition on Phosphorus Release in Biochemical Treatment of Sewage Sludge
Abstract
1. Introduction
- -
- Determination of the characteristics of phosphogypsum for use as a substrate of bacteria growth;
- -
- Determining phosphogypsum’s influence on the process of phosphorus release from sewage sludge;
- -
- Study the effect of the COD loading and phosphogypsum dosage on the metabolic activity of anaerobic microorganism groups in the process of phosphate release.
2. Materials and Methods
2.1. Sewage Sludge
2.2. Phosphogypsum
2.3. Methods of Investigation
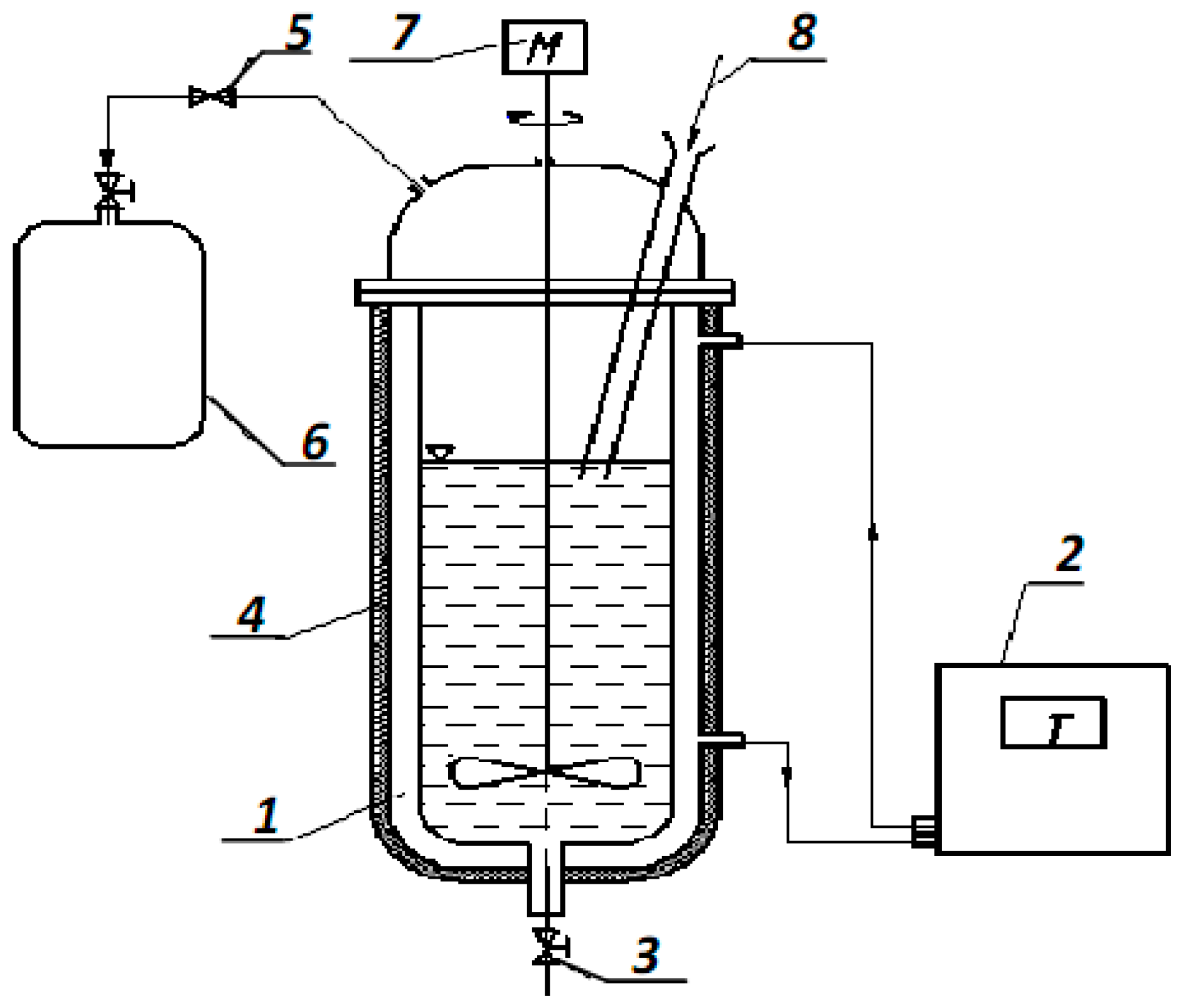
2.4. Anaerobic Microbiological Degradation Pilot Plant
3. Results and Discussion
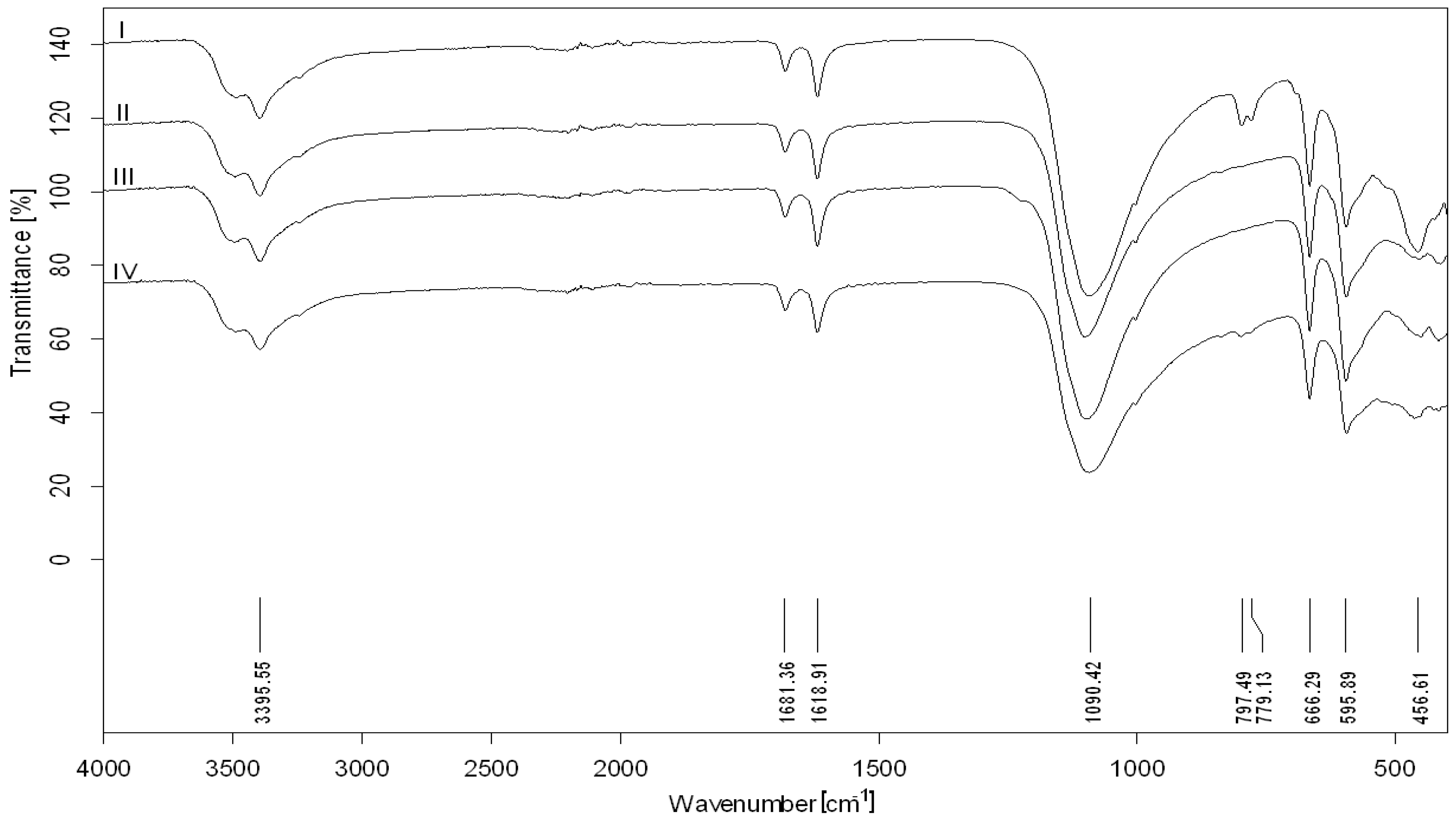
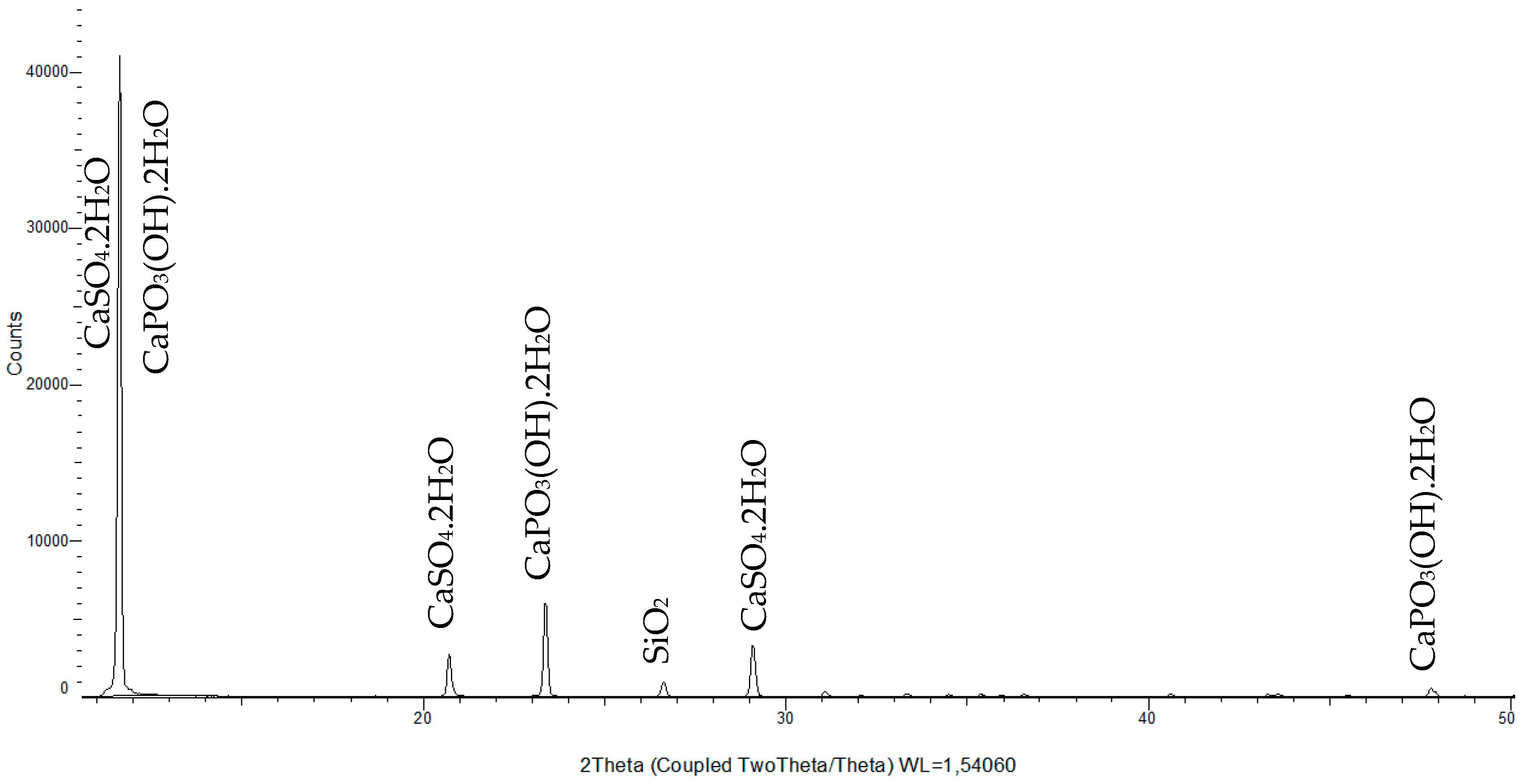
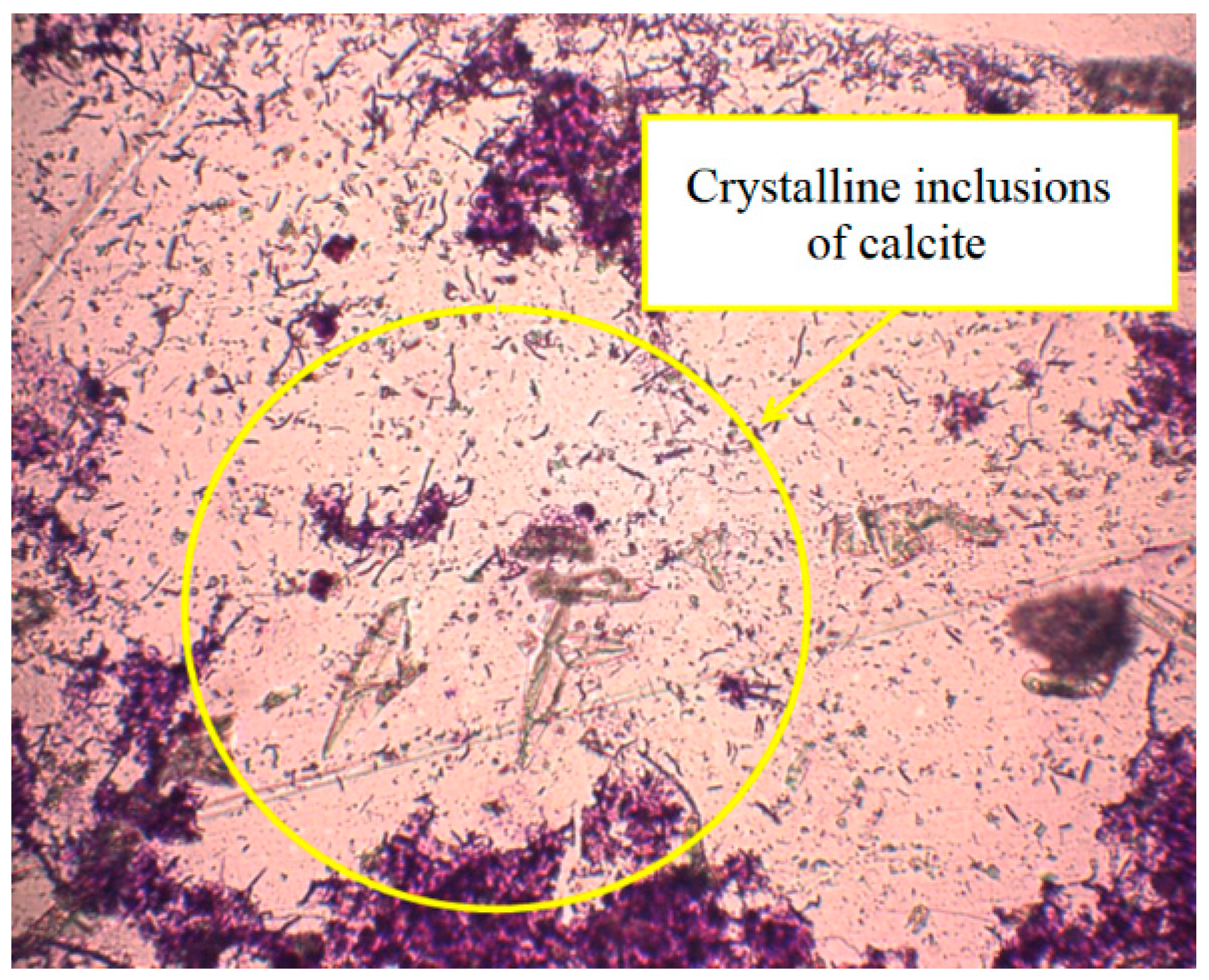
3.1. Phosphogypsum Composition Investigation
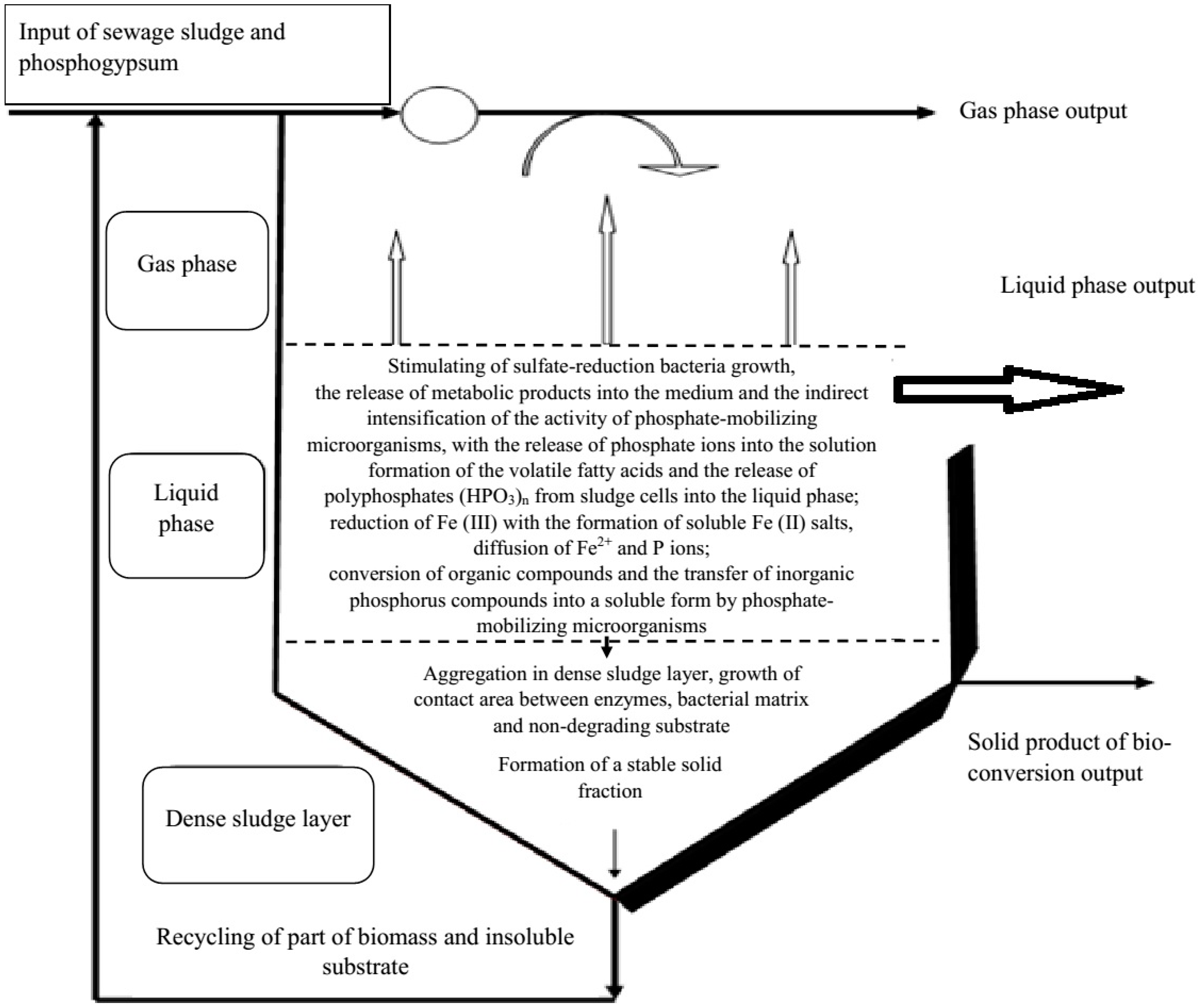
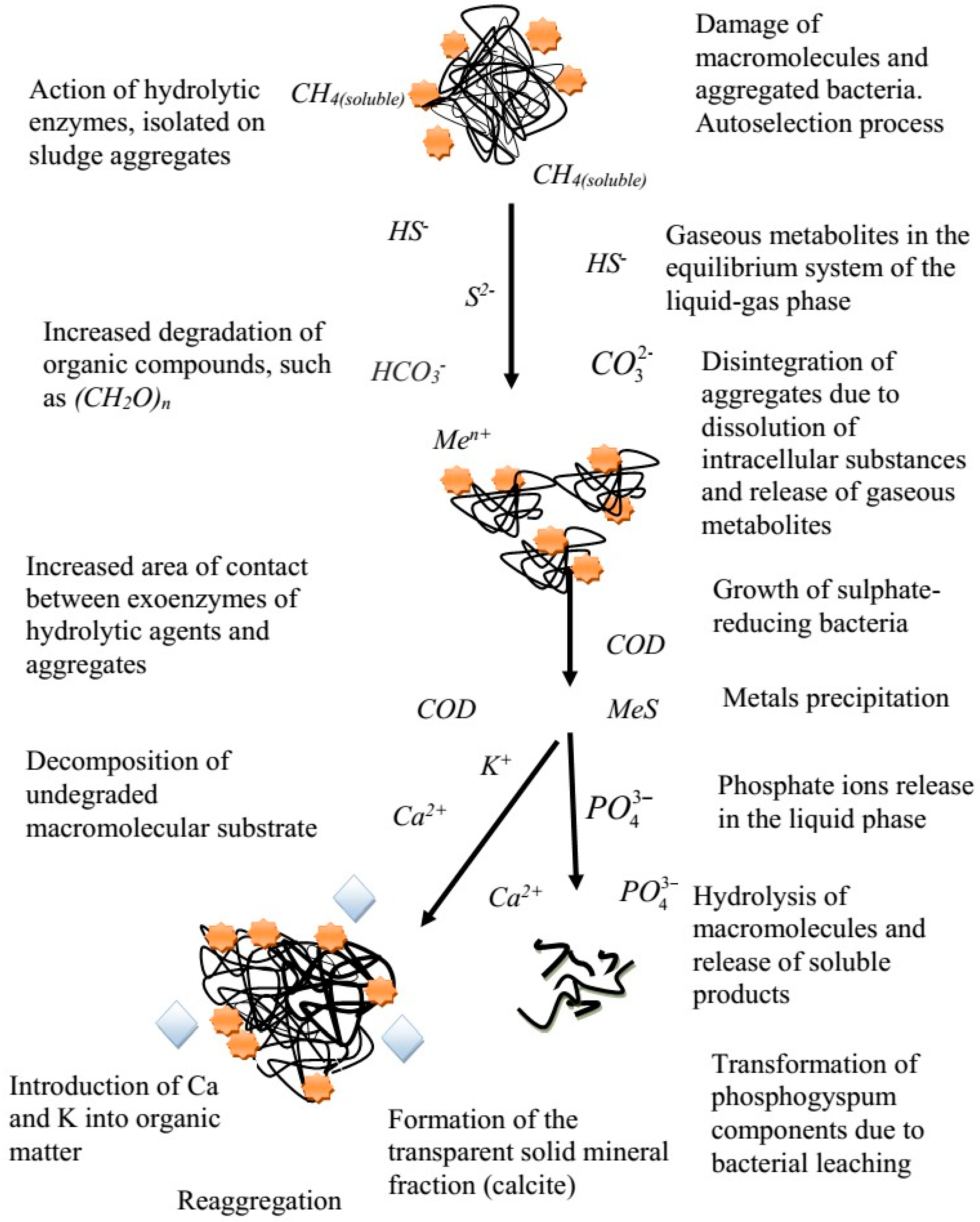
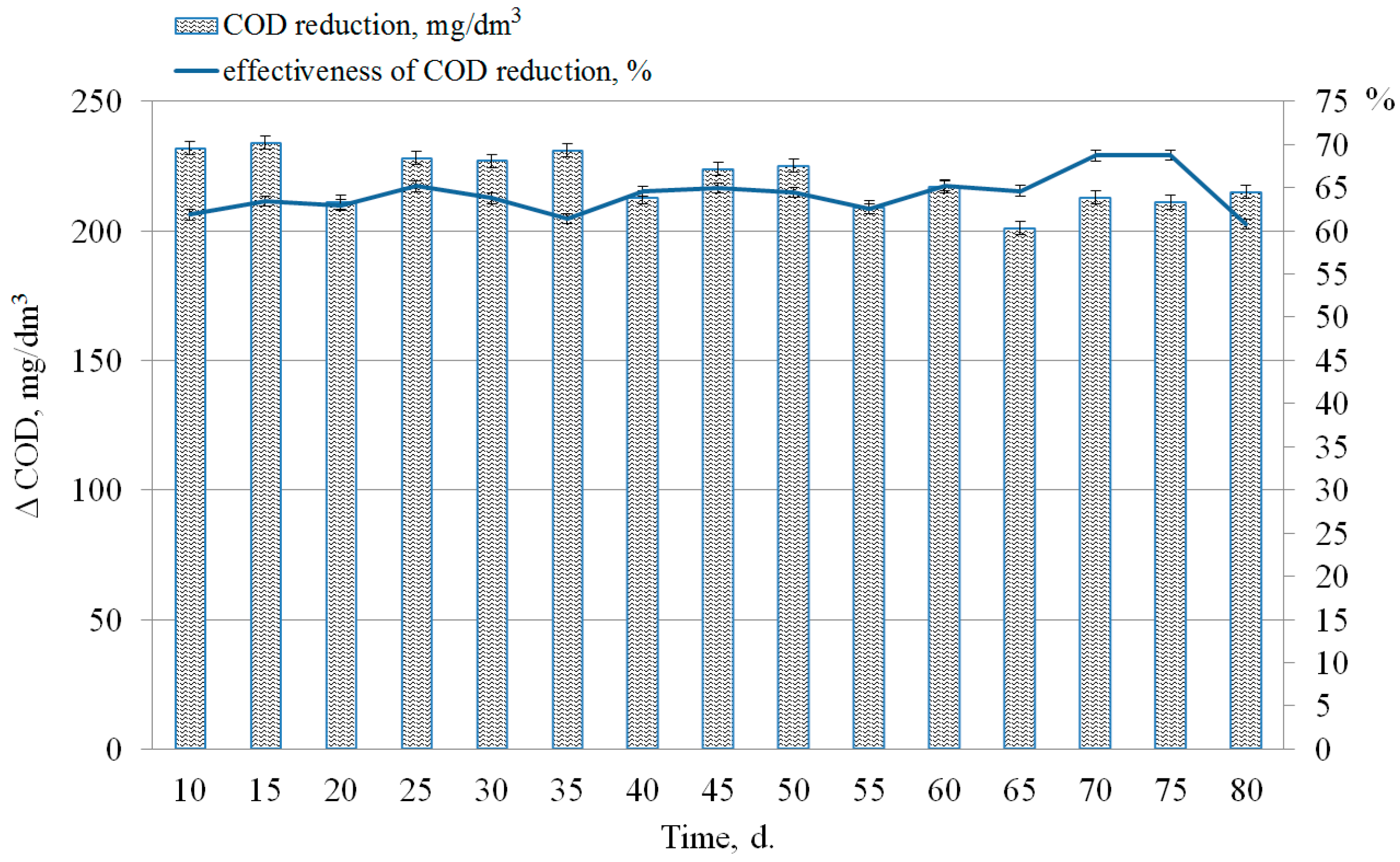
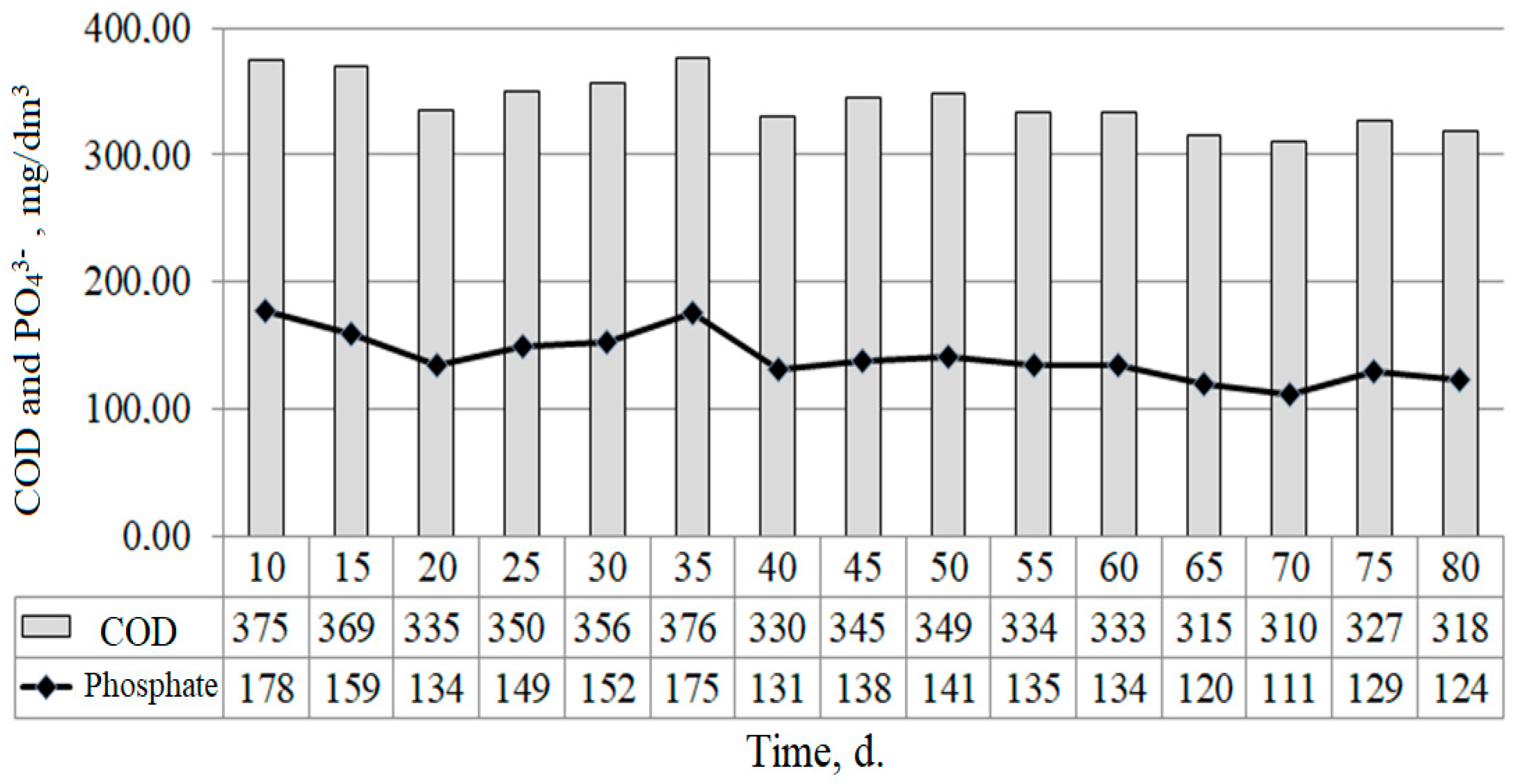
3.2. The Phosphorus Release Process of Sewage Sludge and Phosphogypsum under Sulphate-Reducing Conditions
- -
- Low-cost raw material base that is rich in biogenic elements (calcium, sulphates, phosphorus, etc.);
- -
- Sulphur compounds contained in the waste can be freely used by SRB as a mineral substrate for their growth, which is due to the high sulphate/sulphite ion affinity of microbial cells;
- -
- Technogenic pressure reduction from chemical waste dumps on the environment.
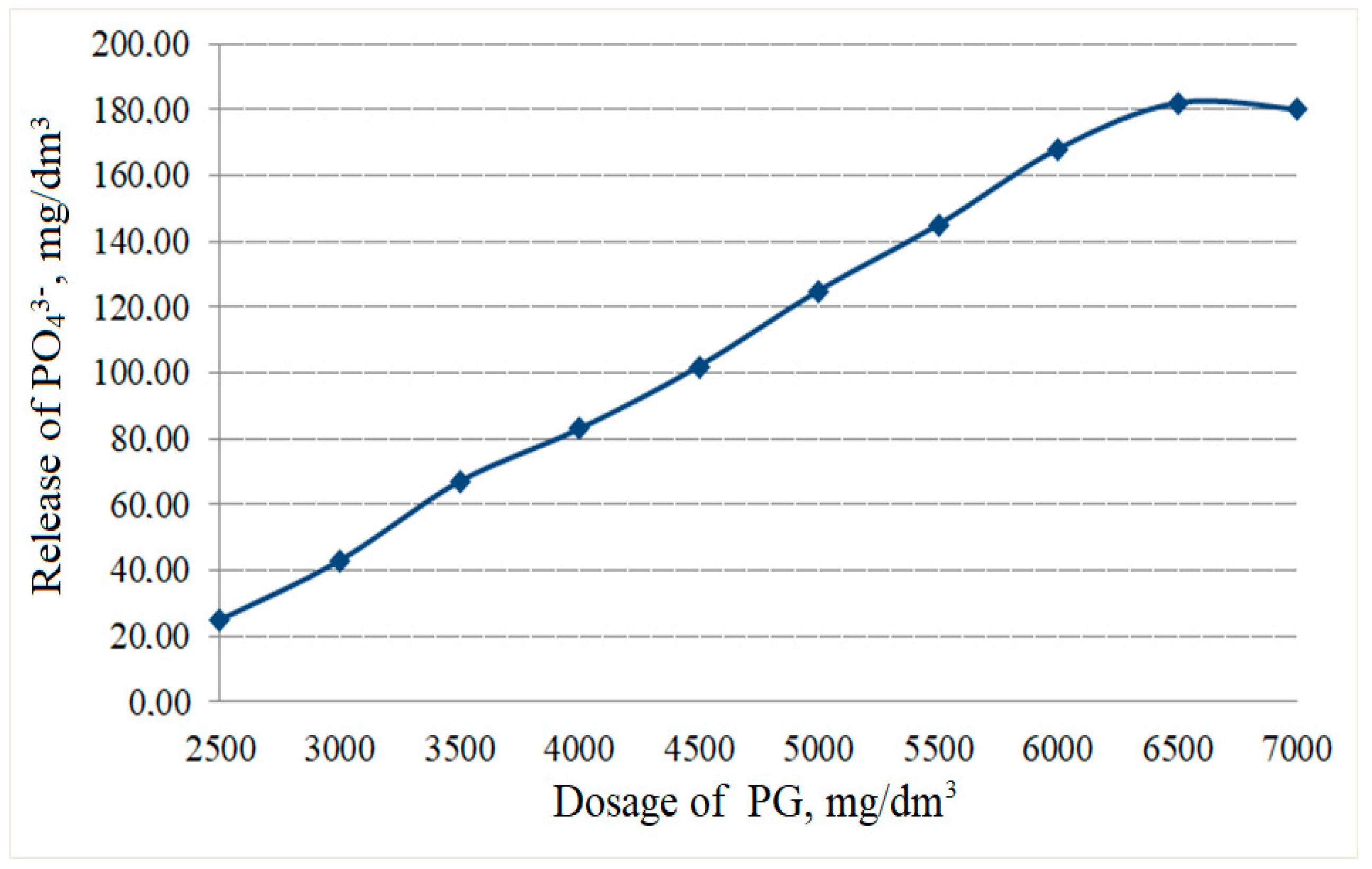
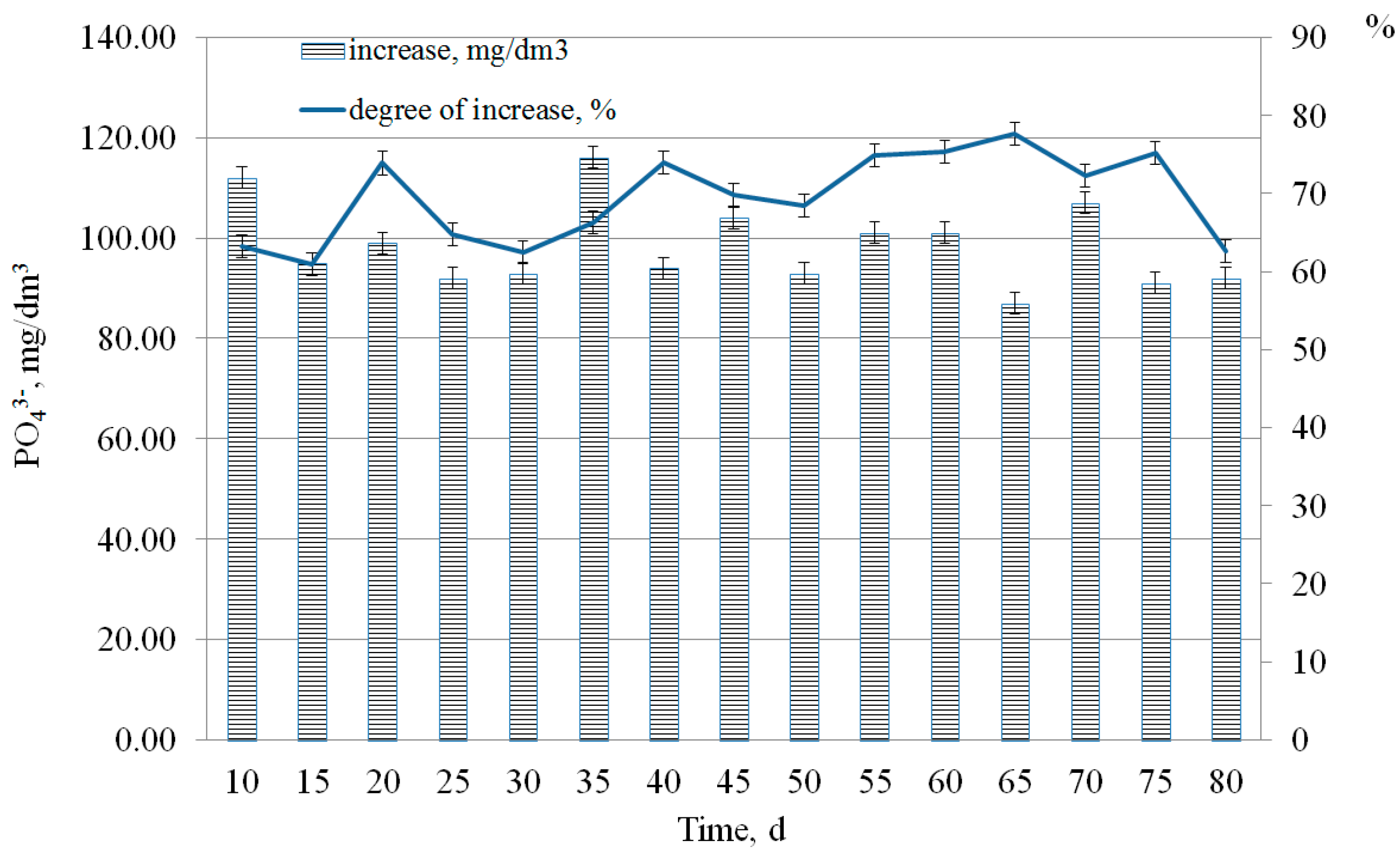
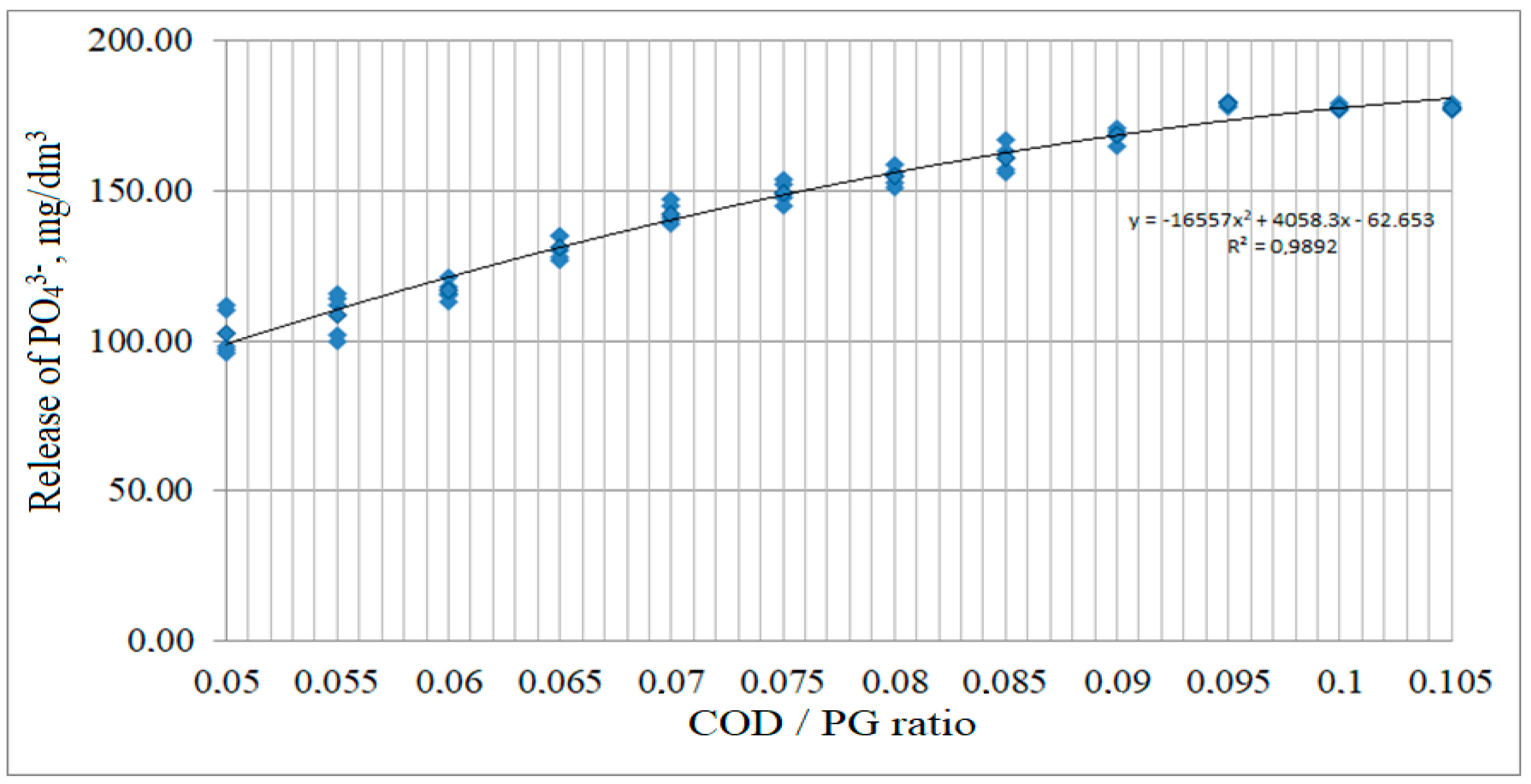
3.3. Effect of the COD Loading and the Phosphogypsum Dosage on the Process of Phosphate Release
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Muryanto, S.; Bayuseno, A.P. Wastewater Treatment for a Sustainable Future: Overview of Phosphorus Recovery. Appl. Mech. Mater. 2012, 110–116, 2043–2048. [Google Scholar] [CrossRef]
- Zamora, P.; Georgieva, T.; Salcedo, I.; Elzinga, N.; Kuntke, P.; Buisman, C. Long-term operation of a pilot-scale reactor for phosphorus recovery as struvite from source-separated urine. J. Chem. Technol. Biotechnol. 2017, 92, 1035–1045. [Google Scholar] [CrossRef]
- Tulaydan, Y.; Malovanyy, M.; Kochubei, V.; Sakalova, H. Treatment of high-strength wastewater from ammonium and phosphate ions with the obtaining of struvite. Chem. Chem. Technol. 2017, 11, 463–468. [Google Scholar] [CrossRef]
- Phosphorus Recycling. Information from Site Osaka University Graduate School of Engineering. Available online: http://www.bio.eng.osaka-u.ac.jp/be/eng/precycling.html (accessed on 12 March 2018).
- Chan, C.; Guisasola, A.; Baeza, J.A. Enhanced Biological Phosphorus Removal at low Sludge Retention Time in view of its integration in A-stage systems. Water Res. 2017, 118, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-S.; Pang, J.-W.; Guo, W.-Q.; Yang, X.-Y.; Wu, Z.-Y.; Ren, N.-Q.; Zhao, Z.-Q. Biological phosphorus removal in an extended ASM2 model: Roles of extracellular polymeric substances and kinetic modeling. Bioresour. Technol. 2017, 232, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Oehmen, A.; Lemos, P.C.; Carvalho, G.; Yuan, Z.; Keller, J.; Blackall, L.L.; Reis, M.A.M. Advances in enhanced biological phosphorus removal: From micro to macro scale. Water Res. 2007, 41, 2271–2300. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Pratt, S.; Batstone, D.J. Phosphorus recovery from wastewater through microbial processes. Curr. Opin. Biotechnol. 2012, 23, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Plyatsuk, L.D.; Chernish, E. Intensification of the anaerobic microbiological degradation of sewage sludge under bio-sulfidogenic conditions. Environ. Prot. Eng. 2013, 39, 101–118. [Google Scholar] [CrossRef]
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; López-Delgado, A. Environmental impact and management of phosphogypsum (Review). J. Environ. Manag. 2009, 90, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Plyatsuk, L.D.; Trunova, I.O. Ecological Problems of Chemical Technology, Development of Advanced Technologies and Equipments for Chemical Industry: A Status Report; Sumy State University: Sumy, Ukraine, 2009; pp. 24–25. [Google Scholar]
- Balintova, M.; Demcak, S.; Holub, M.; Hurakova, M. Study of Precipitates from Mine Water after Defrosting and Oxidation. Solid State Phenom. 2016, 244, 234–239. [Google Scholar] [CrossRef]
- Farmer, V.C. Infrared Spectra of Minerals; Mineralogical Society: Great Britain & Ireland, 1974; Volume 4, ISBN 9780903056533. [Google Scholar] [CrossRef]
- Raynaud, S.; Champion, E.; Bernache-Assollant, D.; Thomas, P. Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials 2002, 23, 1065–1072. [Google Scholar] [CrossRef]
- Junakova, N.; Balintova, M. Nutrient Leaching from Reservoir Bottom Sediments. Chem. Eng. Trans. 2013, 35, 1141–1146. [Google Scholar]
- Serra, J.; González, P.; Liste, S.; Serra, C.; Chiussi, S.; León, B.; Pérez-Amor, M.; Ylänen, H.O.; Hupa, M. FTIR and XPS studies of bioactive silica based glasses. J. Non-Cryst. Solids 2003, 332, 20–27. [Google Scholar] [CrossRef]
- Chernysh, Y.Y.; Plyatsuk, L.D. Phosphogypsum utilization in the dephosphotation treatment of wastewater and sewage sludge. In Proceedings of the II International Scientific-Practical Conference “Water Supply and Wastewater Disposal Designing, Construction, Operation and Monitoring”, Lviv, Ukraine, 18–20 October 2017; Orachewska, D., Bobush, O., Eds.; Lviv Polytechnic National University Publisher: Lviv, Ukraine, 2017. [Google Scholar]


| Total Solids | Volatile Solids | Total Suspended Solids | Volatile Suspended Solids | COD | PO43− |
|---|---|---|---|---|---|
| 2100–2130 | 1831–1867 | 2038–2040 | 1800–1960 | 300–376 | 32–67 |
| CaO | SO3 | A12O3 | Fe2O3 |
| 30–42 | 44–52 | 0.3–5.0 | 0.2–2.0 |
| SiO2 | F | P2O5 | H2O |
| 0.3–1.0 | 0.1–1.0 | 1–4 | 25–40 |
| CaO | SO3 | Al2O3 | Fe2O3 | SiO2 | P2O5 |
|---|---|---|---|---|---|
| 28–36 | 52–61 | 0.1–0.2 | 0.1–0.3 | 1.2–19.9 * | 0.3–1.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernysh, Y.; Balintova, M.; Plyatsuk, L.; Holub, M.; Demcak, S. The Influence of Phosphogypsum Addition on Phosphorus Release in Biochemical Treatment of Sewage Sludge. Int. J. Environ. Res. Public Health 2018, 15, 1269. https://doi.org/10.3390/ijerph15061269
Chernysh Y, Balintova M, Plyatsuk L, Holub M, Demcak S. The Influence of Phosphogypsum Addition on Phosphorus Release in Biochemical Treatment of Sewage Sludge. International Journal of Environmental Research and Public Health. 2018; 15(6):1269. https://doi.org/10.3390/ijerph15061269
Chicago/Turabian StyleChernysh, Yelizaveta, Magdalena Balintova, Leonid Plyatsuk, Marian Holub, and Stefan Demcak. 2018. "The Influence of Phosphogypsum Addition on Phosphorus Release in Biochemical Treatment of Sewage Sludge" International Journal of Environmental Research and Public Health 15, no. 6: 1269. https://doi.org/10.3390/ijerph15061269
APA StyleChernysh, Y., Balintova, M., Plyatsuk, L., Holub, M., & Demcak, S. (2018). The Influence of Phosphogypsum Addition on Phosphorus Release in Biochemical Treatment of Sewage Sludge. International Journal of Environmental Research and Public Health, 15(6), 1269. https://doi.org/10.3390/ijerph15061269







