Effect of Time and Mixing in Thermal Pretreatment on Faecal Indicator Bacteria Inactivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sludge Sampling and Characterization
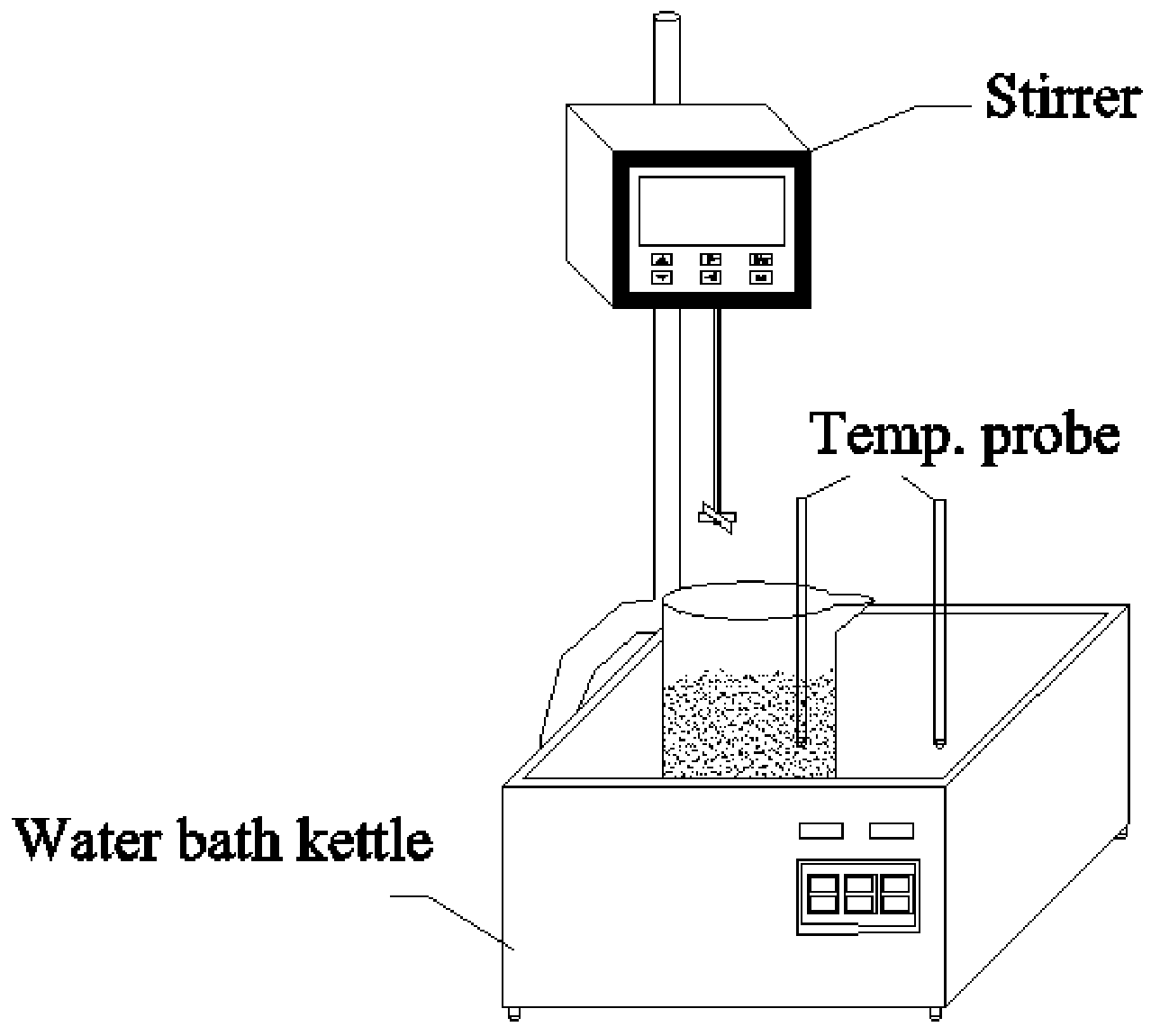
2.2. Thermal Pretreatment
2.3. Anaerobic Digestion Reactors
2.4. Analytical Methods
2.5. Kinetic Analysis
3. Results
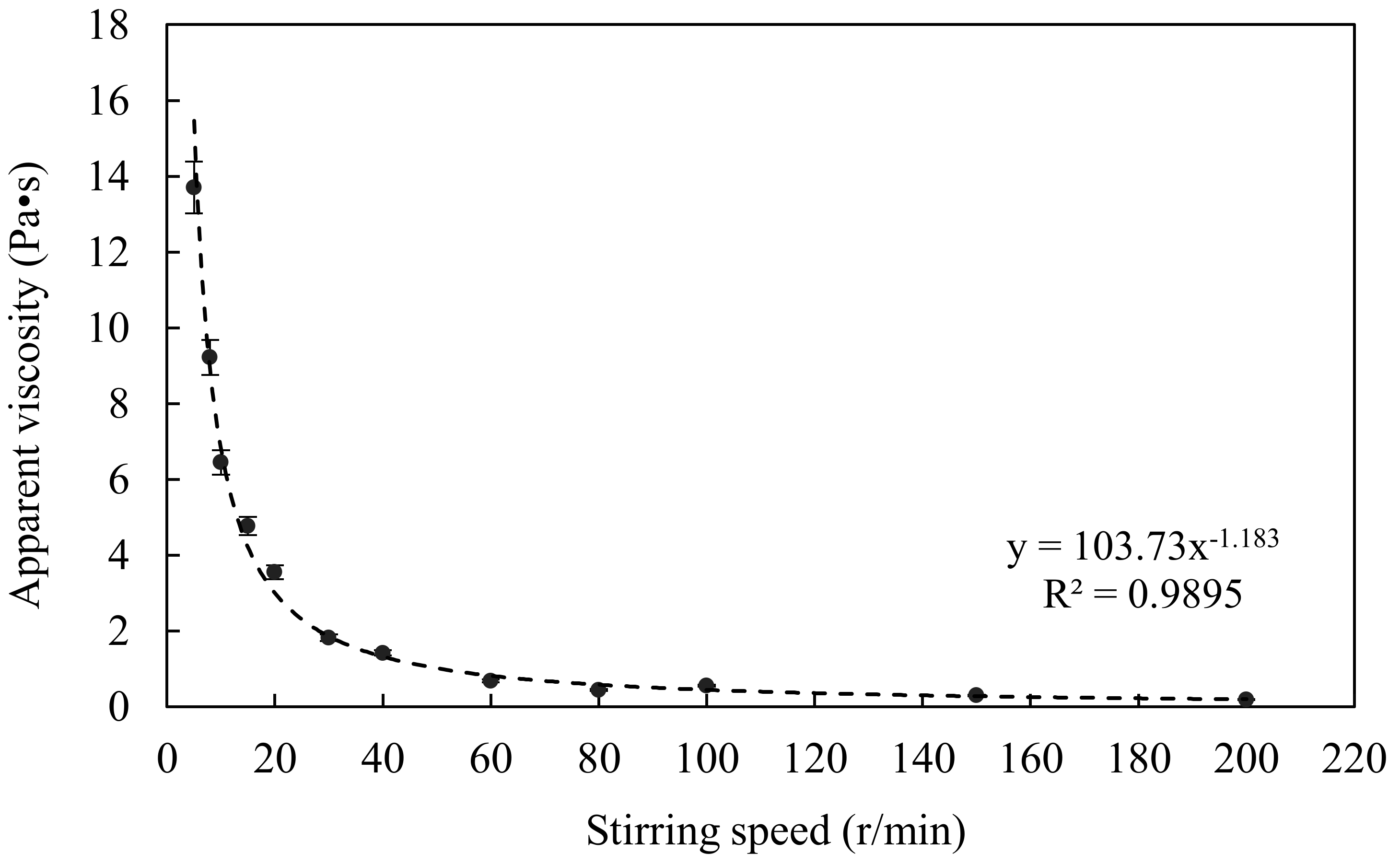
3.1. Velocity Gradient
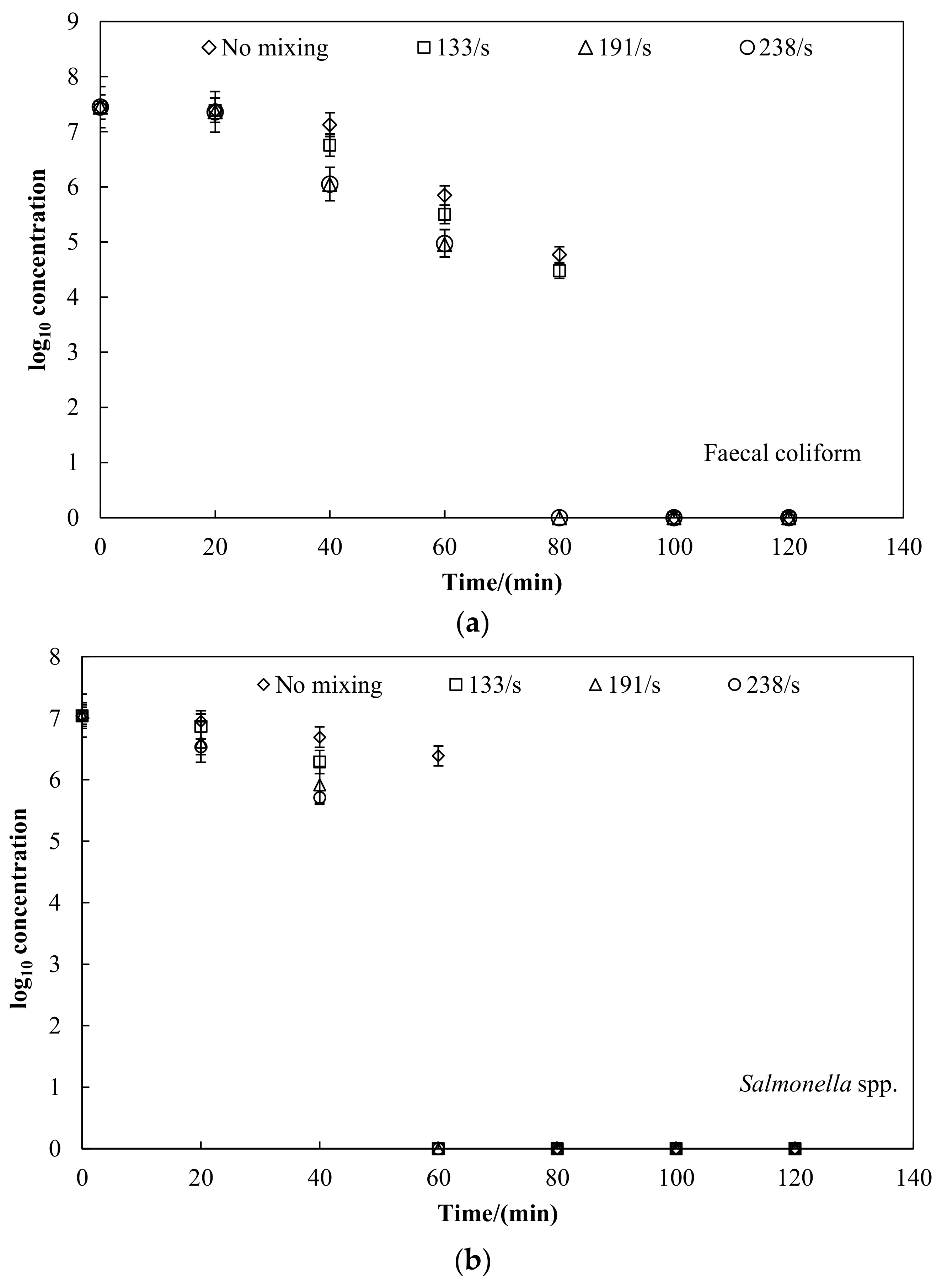
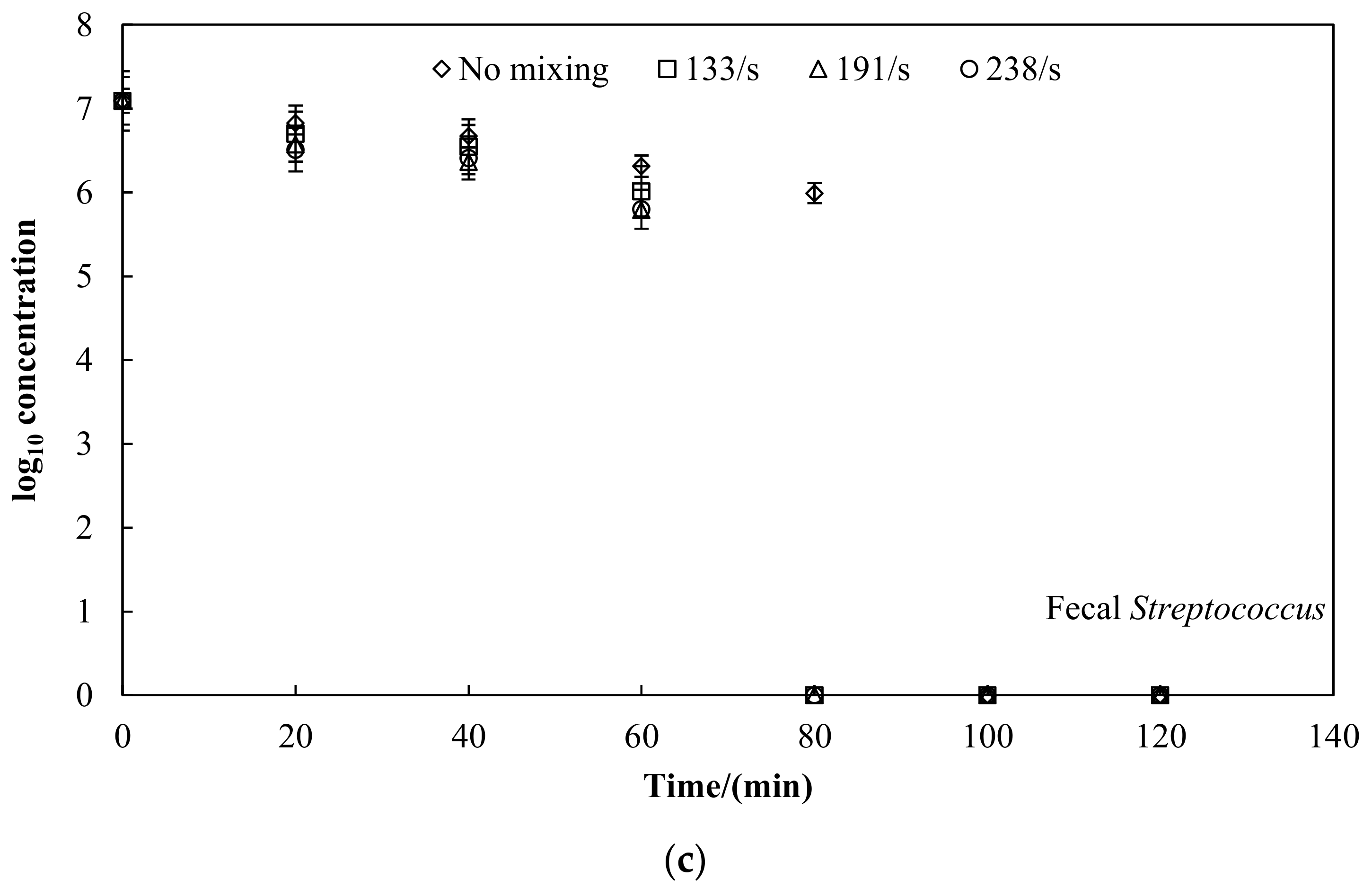
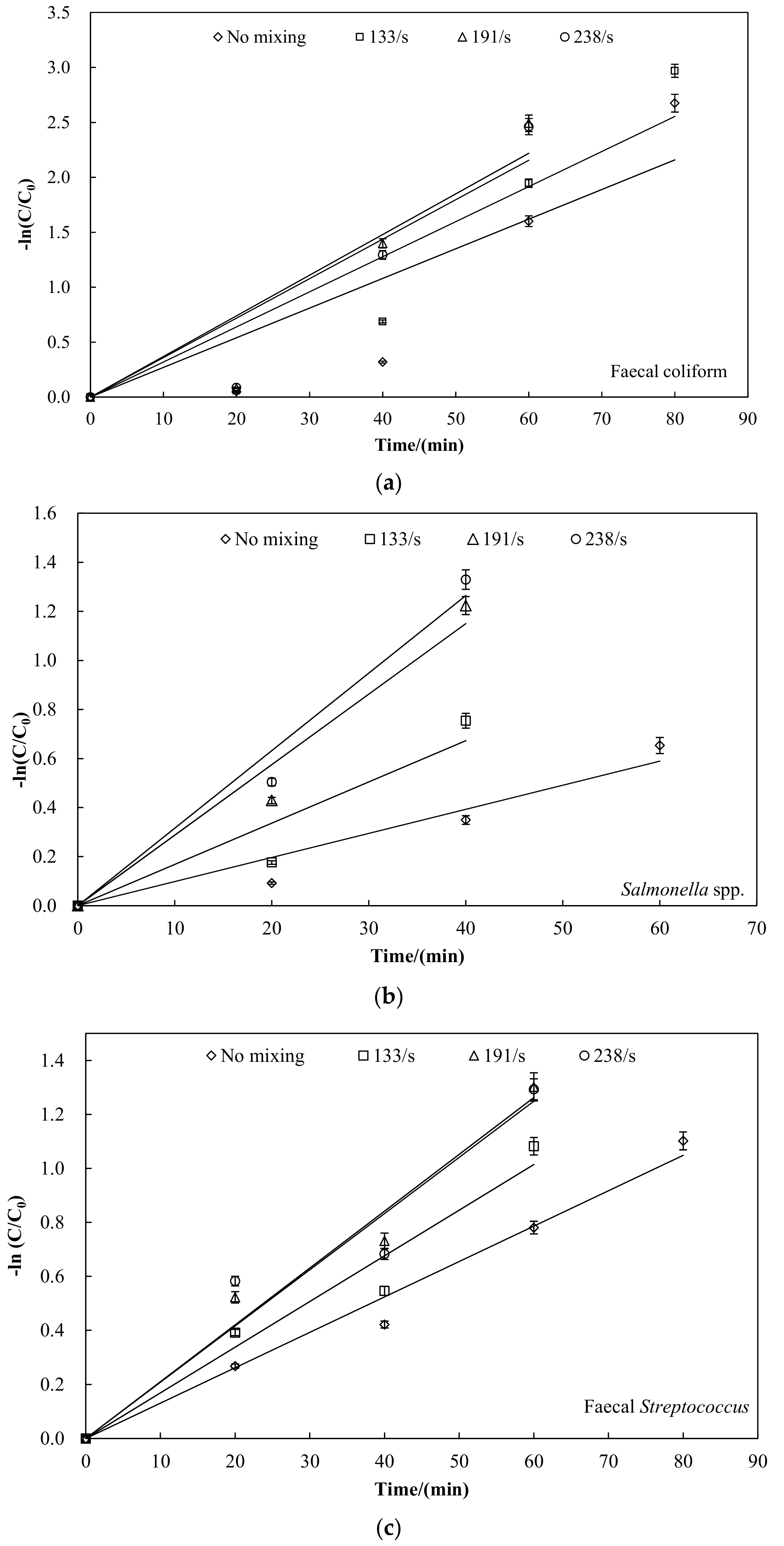
3.2. Faecal Indicator Bacteria Inactivation
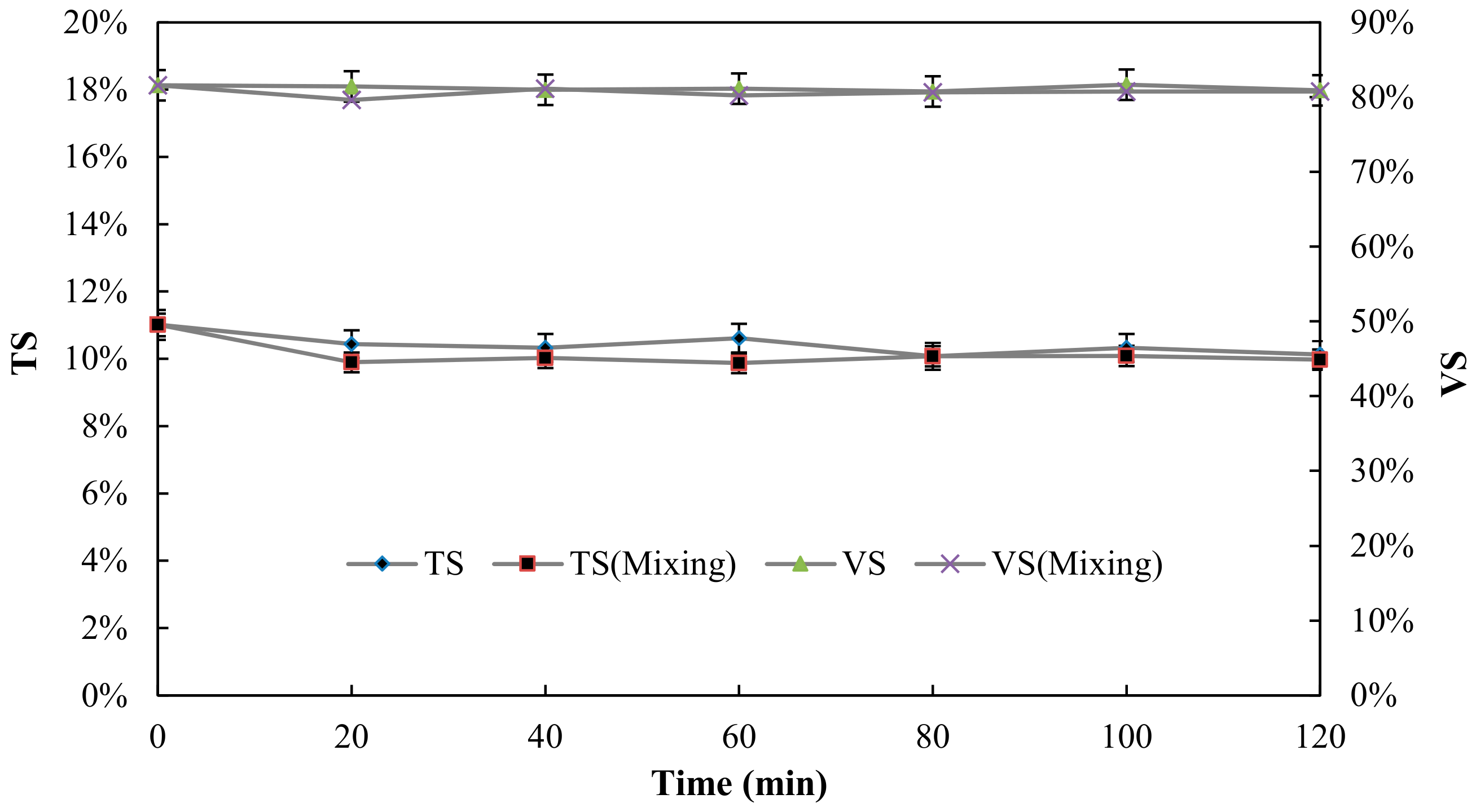
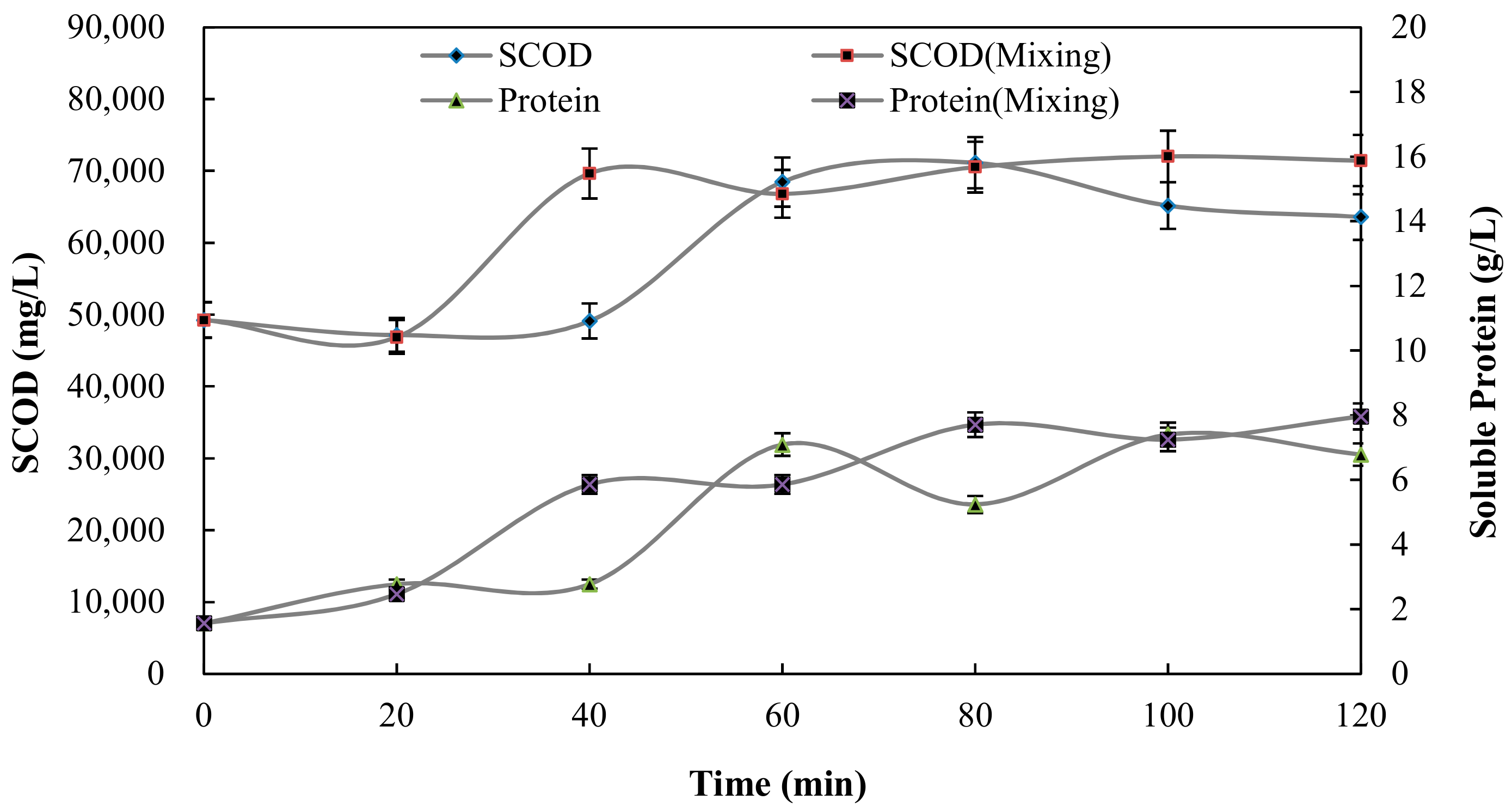
3.3. Sludge Solubilisation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sidhu, J.; Toze, S. Human pathogens and their indicators in biosolids: A literature review. Environ. Int. 2009, 35, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Mackie Jensen, P.K.; Phuc, P.D.; Knudsen, L.G.; Dalsgaard, A.; Konradsen, F. Hygiene versus fertiliser: The use of human excreta in agriculture—A Vietnamese example. Int. J. Hyg. Environ. Health 2008, 211, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, B.; Wang, Y.; Jiang, Q.; Liu, H. Reactor performance and bacterial pathogen removal in response to sludge retention time in a mesophilic anaerobic digester treating sewage sludge. Bioresour. Technol. 2012, 106, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Bond, T.; Templeton, M.R. History and future of domestic biogas plants in the developing world. Energy Sustain. Dev. 2011, 15, 347–354. [Google Scholar] [CrossRef]
- Astals, S.; Venegas, C.; Peces, M.; Jofre, J.; Lucena, F.; Mata-Alvarez, J. Balancing hygienization and anaerobic digestion of raw sewage sludge. Water Res. 2012, 46, 6218–6227. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Biosolids Applied to Land: Advancing Standards and Practices; National Academies Press: Washington, DC, USA, 1988.
- Baudez, J.C.; Slatter, P.; Eshtiaghi, N. The impact of temperature on the rheological behaviour of anaerobic digested sludge. Chem. Eng. J. 2013, 215–216, 182–187. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, Z.; Wen, Z. Enhancing anaerobic digestibility and phosphorus recovery of dairy manure through microwave-based thermochemical pretreatment. Water Res. 2009, 43, 3493–3502. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, C.; León-Cofreces, C.; García-Encina, P.A. Different pretreatments for increasing the anaerobic biodegradability in swine manure. Bioresour. Technol. 2008, 99, 8710–8714. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Ahn, J.-H. Effect of microwave pretreatment in presence of NaOH on mesophilic anaerobic digestion of thickened waste activated sludge. Bioresour. Technol. 2013, 131, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M.; Bundy, D.A.; Selkirk, M.E.; Smith, D.F.; Anderson, R.M. Immunological modulation and evasion by helminth parasites in human populations. Nature 1993, 365, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Manafi, M. New developments in chromogenic and fluorogenic culture media. Int. J. Food Microbiol. 2000, 60, 205–218. [Google Scholar] [CrossRef]
- Rambach, A. New plate medium for facilitated differentiation of Salmonella spp. from Proteus spp. and other enteric bacteria. Appl. Environ. Microbiol. 1990, 56, 301–303. [Google Scholar] [PubMed]
- Suresh, P.; Rehg, J.E. Comparative evaluation of several techniques for purification of Cryptosporidium parvum oocysts from rat feces. J. Clin. Microbiol. 1996, 34, 38–40. [Google Scholar] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998.
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Mohammed, T.J.; Shakir, E. Effect of settling time, velocity gradient, and camp number on turbidity removal for oilfield produced water. Egypt. J. Pet. 2018, 27, 31–36. [Google Scholar] [CrossRef]
- Popat, S.C.; Yates, M.V.; Deshusses, M.A. Kinetics of inactivation of indicator pathogens during thermophilic anaerobic digestion. Water Res. 2010, 44, 5965–5972. [Google Scholar] [CrossRef] [PubMed]
- Aitken, M.D.; Sobsey, M.D.; Blauth, K.E.; Shehee, M.; Crunk, P.L.; Walters, G.W. Inactivation of Ascaris suum and poliovirus in biosolids under thermophilic anaerobic digestion conditions. Environ. Sci. Technol. 2005, 39, 5804–5809. [Google Scholar] [CrossRef] [PubMed]
- Spillmann, S.K.; Traub, F.; Schwyzer, M.; Wyler, R. Inactivation of animal viruses during sewage sludge treatment. Appl. Environ. Microbiol. 1987, 53, 2077–2081. [Google Scholar] [PubMed]
- Kaparaju, P.; Buendia, I.; Ellegaard, L.; Angelidakia, I. Effects of mixing on methane production during thermophilic anaerobic digestion of manure: Lab-scale and pilot-scale studies. Bioresour. Technol. 2008, 99, 4919–4928. [Google Scholar] [CrossRef] [PubMed]
- Karim, K.; Thomasklasson, K.; Hoffmann, R.; Drescher, S.; Depaoli, D.; Aldahhan, M. Anaerobic digestion of animal waste: Effect of mixing. Bioresour. Technol. 2005, 96, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Uma Rani, R.; Adish Kumar, S.; Kaliappan, S.; Yeom, I.-T.; Rajesh Banu, J. Low temperature thermo-chemical pretreatment of dairy waste activated sludge for anaerobic digestion process. Bioresour. Technol. 2012, 103, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Güelfo, L.A.; Álvarez-Gallego, C.; Sales Márquez, D.; Romero García, L.I. The effect of different pretreatments on biomethanation kinetics of industrial Organic Fraction of Municipal Solid Wastes (OFMSW). Chem. Eng. J. 2011, 171, 411–417. [Google Scholar]
- Braguglia, C.M.; Gianico, A.; Mininni, G. Comparison between ozone and ultrasound disintegration on sludge anaerobic digestion. J. Environ. Manag. 2012, 95, S139–S143. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Li, Y.; Fei, X.; Wang, S.; Yuan, H. Enhancement of thermophilic anaerobic digestion of thickened waste activated sludge by combined microwave and alkaline pretreatment. J. Environ. Sci. 2011, 23, 1257–1265. [Google Scholar]
- Dimock, R.; Morgenroth, E. The influence of particle size on microbial hydrolysis of protein particles in activated sludge. Water Res. 2006, 40, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Dhar, B.R.; Youssef, E.; Nakhla, G.; Ray, M.B. Pretreatment of municipal waste activated sludge for volatile sulfur compounds control in anaerobic digestion. Bioresour. Technol. 2011, 102, 3776–3782. [Google Scholar] [CrossRef] [PubMed]
| Level | Faecal Indicator Bacteria | US-EPA/625/R92 | Mexico-NOM-004-SEMARNAT |
|---|---|---|---|
| A | faecal coliforms | <103 CFU/g TS | <103 CFU/g TS |
| Salmonella spp. | <3 CFU/g TS | <3 CFU/g TS | |
| faecal Streptococcus | <103 CFU/g TS | -- | |
| helminth eggs | <1 egg/g TS | <1 egg/g TS | |
| B | faecal indicator bacteria | <2 × 106 CFU/g TS | <103 CFU/g TS |
| faecal coliforms | <3 CFU/g TS | <3 CFU/g TS | |
| Salmonella spp. | <106 CFU/g TS | -- | |
| faecal Streptococcus | <1 egg/g TS | <10 egg/g TS | |
| C | faecal indicator bacteria | -- | <2 × 103 CFU/g TS |
| faecal coliforms | -- | <300 CFU/g TS | |
| Salmonella spp. | -- | -- | |
| faecal Streptococcus | -- | <35 egg/g TS |
| Analytical Parameters | Faecal Sludge |
|---|---|
| Total nitrogen (TN) (g L−1) | 0.209 ± 0.018 |
| Total organic carbon (TOC) (g L−1) | 5.02 ± 0.27 |
| Total solid (TS) | 11 ± 0.7% |
| Volatile solids (VS) | 81.6 ± 1.6% |
| pH | 6.9 ± 0.25 |
| Total chemical oxygen demand (TCOD) (mg L−1) | 101,300 ± 100 |
| Soluble chemical oxygen demand (SCOD) (mg L−1) | 49,250 ± 60 |
| Protein (g L−1) | 24.1 ± 2.3 |
| Soluble protein (g L−1) | 1.56 ± 0.12 |
| Faecal coliform (CFU g−1 (TS)) | (2.79 ± 0.04) × 107 |
| Salmonella spp. (CFU g−1 (TS)) | (1.10 ± 0.02) × 107 |
| Faecal Streptococcus (CFU g−1 (TS)) | (1.24 ± 0.02) × 107 |
| Helminth eggs (Egg g−1 (TS)) | 0 |
| Faecal Indicator Bacteria | Mixing Speed/(r·min−1) | First-Order Kinetics | |
|---|---|---|---|
| k | R2 | ||
| faecal coliform | 0 | 0.027 | 0.803 |
| 40 | 0.031 | 0.871 | |
| 60 | 0.037 | 0.878 | |
| 80 | 0.035 | 0.873 | |
| Salmonella spp. | 0 | 0.009 | 0.894 |
| 40 | 0.016 | 0.905 | |
| 60 | 0.029 | 0.965 | |
| 80 | 0.030 | 0.977 | |
| faecal Streptococcus | 0 | 0.013 | 0.932 |
| 40 | 0.016 | 0.959 | |
| 60 | 0.021 | 0.972 | |
| 80 | 0.020 | 0.968 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, F.; Dong, H.; Shang, B.; Zhang, W. Effect of Time and Mixing in Thermal Pretreatment on Faecal Indicator Bacteria Inactivation. Int. J. Environ. Res. Public Health 2018, 15, 1225. https://doi.org/10.3390/ijerph15061225
Yin F, Dong H, Shang B, Zhang W. Effect of Time and Mixing in Thermal Pretreatment on Faecal Indicator Bacteria Inactivation. International Journal of Environmental Research and Public Health. 2018; 15(6):1225. https://doi.org/10.3390/ijerph15061225
Chicago/Turabian StyleYin, Fubin, Hongmin Dong, Bin Shang, and Wanqin Zhang. 2018. "Effect of Time and Mixing in Thermal Pretreatment on Faecal Indicator Bacteria Inactivation" International Journal of Environmental Research and Public Health 15, no. 6: 1225. https://doi.org/10.3390/ijerph15061225
APA StyleYin, F., Dong, H., Shang, B., & Zhang, W. (2018). Effect of Time and Mixing in Thermal Pretreatment on Faecal Indicator Bacteria Inactivation. International Journal of Environmental Research and Public Health, 15(6), 1225. https://doi.org/10.3390/ijerph15061225





