Lung Cancer Risk and Low (≤50 μg/L) Drinking Water Arsenic Levels for US Counties (2009–2013)—A Negative Association
Abstract
1. Introduction
2. Methods and Materials
2.1. Lung Cancer Case Counts
2.2. Groundwater Median Arsenic Concentrations
2.3. Dependency of County Population on Groundwater as Source of Drinking Water
2.4. Smoking Prevalence Rates
2.5. Radon Levels
2.6. Demographic Variables
2.7. Statistical Methods
3. Results
3.1. Data Characteristics
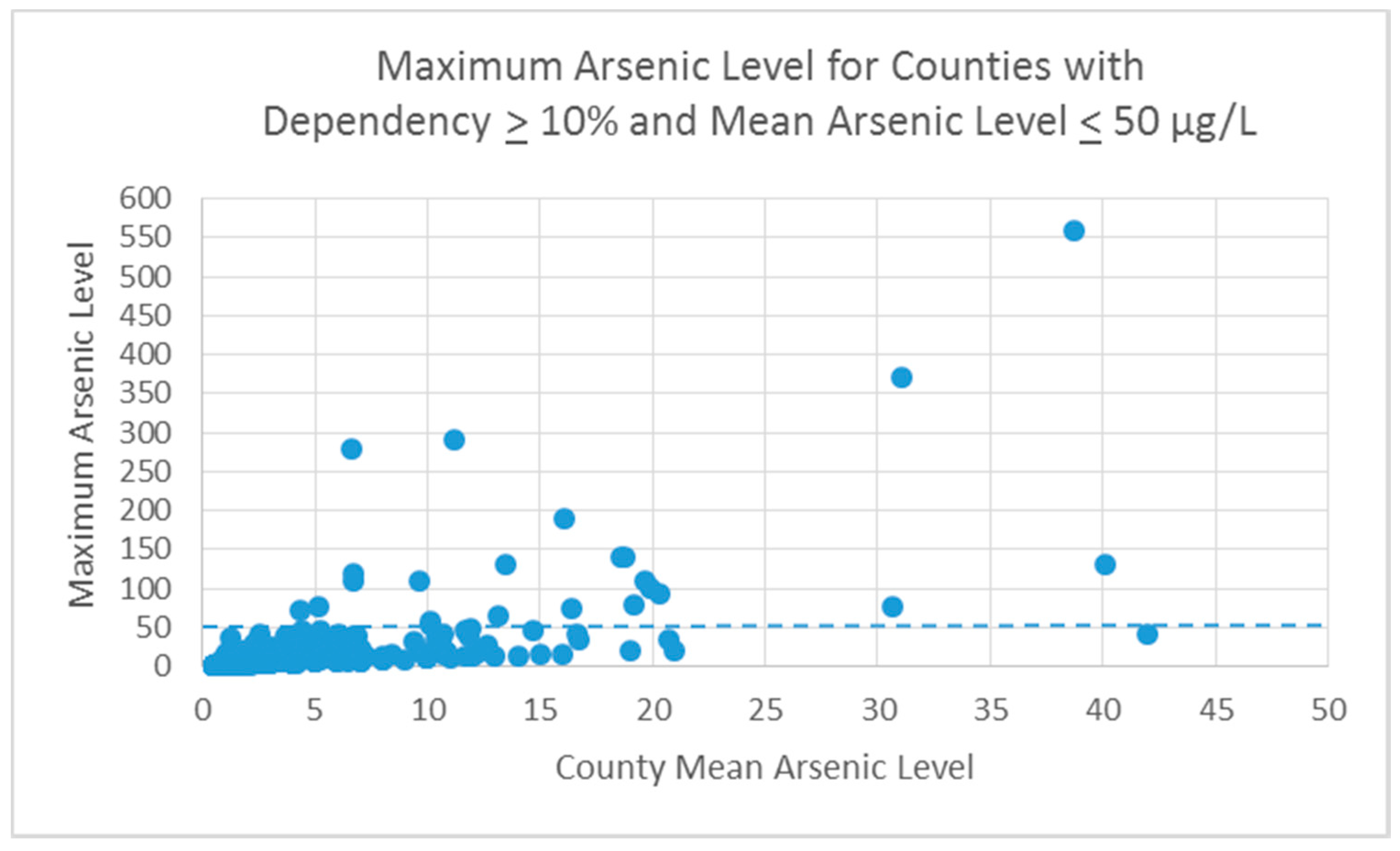
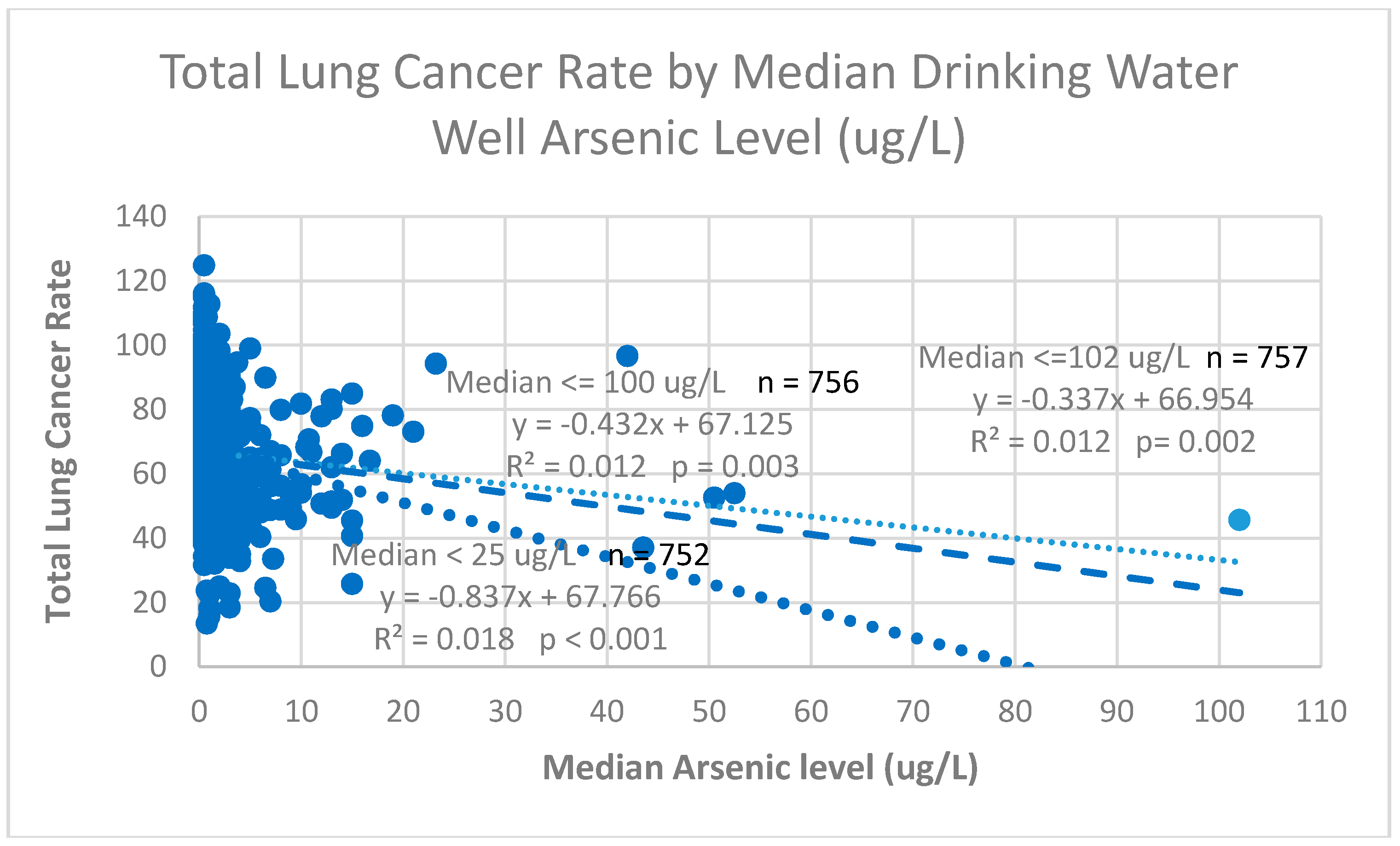
3.1.1. Outcome
3.1.2. Exposure
3.1.3. Co-Variates
3.1.4. Populations
3.2. Linear Regression Model
3.3. Poisson Log-Linear Regression Model
3.4. Restricted Poisson Log-Linear Model
3.5. Sensitivity Analysis
3.6. Stratified Risk Analysis
4. Discussion
4.1. Literature Review
4.2. Analogous Studies
5. Limitations and Strengths
5.1. Context of Exposure
5.2. Lung Cancer Risk at Low Arsenic Exposure
5.3. Toxicological Considerations
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Restrictions (Ln Mean Arsenic; GW Dependency ≥10%; ND 1/(Sqrt 2)) | |||||
|---|---|---|---|---|---|
| Population | n | Coef | SE | z | p |
| Total | 735 | −0.009 | 0.002 | −4.40 | <0.001 |
| Male | 682 | −0.021 | 0.003 | −7.33 | <0.001 |
| Female | 657 | −0.002 | 0.003 | −0.63 | 0.528 |
| Restrictions (Ln Mean As; NWIS; GW Dependency ≥10%; ND 1/(Sqrt 2)) | |||||
|---|---|---|---|---|---|
| Population | n | Coef | SE | z | p |
| Total | 639 | −0.012 | 0.003 | −5.04 | <0.001 |
| Male | 595 | −0.026 | 0.003 | −8.09 | <0.001 |
| Female | 571 | −0.003 | 0.004 | −0.67 | 0.500 |
| Restrictions (Daily Dosage; 1.1 L/day; GW Dependency ≥ 80%; As(Max) ≤ 50) | |||||
|---|---|---|---|---|---|
| Population | n | Coef | SE | z | p |
| Total | 393 | −0.004 | 0.001 | −3.67 | 0.000 |
| Male | 350 | −0.005 | 0.002 | −3.06 | 0.002 |
| Female | 333 | −0.003 | 0.002 | −1.80 | 0.071 |
| Restrictions (Dosage [mkd]; 70 kg; GW Dependency > 80%; As(Max) ≤ 50) | |||||
|---|---|---|---|---|---|
| Population | n | Coef | SE | z | p |
| Total | 393 | −304.4 | 82.9 | −3.67 | 0.000 |
| Male | 350 | −329.1 | 107.5 | −3.06 | 0.002 |
| Female | 333 | −226.3 | 125.6 | −1.80 | 0.071 |
References
- Chen, C.J.; Kuo, T.L.; Wu, M.M. Arsenic and cancers. Lancet 1988, 1, 414–415. [Google Scholar] [CrossRef]
- Wu, M.M.; Kuo, T.L.; Hwang, Y.-H.; Chen, C.J. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am. J. Epidemiol. 1989, 130, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Ferreccio, C.; Yuan, Y.; Calle, J.; Benitez, H.; Parra, R.L.; Acevedo, J.; Smith, A.H.; Liaw, J.; Steinmaus, C. Arsenic, tobacco smoke, and occupation: Associations of multiple agents with lung and bladder cancer. Epidemiology 2013, 24, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Ercumen, A.; Yuan, Y.; Steinmaus, C.M. Increased lung cancer risks are similar whether arsenic is ingested or inhaled. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Steinmaus, C.M.; Ferreccio, C.; Acevedo-Romo, J.; Yuan, Y.; Cortes, S.; Marshall, G.; Moore, L.E.; Blmes, J.R.; Liaw, J.; Golden, T.; Smith, A.H. Drinking water arsenic in Northern Chile: High cancer risks 40 years after exposure cessation. Cancer Epidemiol. Biomark. Prev. 2013, 22, 623–630. [Google Scholar] [CrossRef] [PubMed]
- D’Ippoliti, D.; Santelli, E.; De Sarlo, M.; Scortichini, M.; Davoli, M.; Michelozzi, P. Arsenic in Drinking Water and Mortality for Cancer and Chronic Diseases in Central Italy, 1990–2010. PLoS ONE 2015, 10, e0138182. [Google Scholar] [CrossRef] [PubMed]
- Mendez, W.M., Jr.; Eftim, S.; Cohen, J.; Warren, I.; Cowden, J.; Lee, J.S.; Sams, R. Relationships between arsenic concentrations in drinking water and lung and bladder cancer incidence in U.S. counties. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Ferdosi, H.; Dissen, E.K.; Afari-Dwamena, N.A.; Li, J.; Chen, R.; Feinleib, M.; Lamm, S.H. Arsenic in Drinking Water and Lung Cancer Mortality in the United States: An Analysis Based on US Counties and 30 Years of Observation (1950–1979). J. Environ. Public Health 2016, 2016, 1602929. [Google Scholar] [CrossRef] [PubMed]
- Dauphine, D.C.; Smith, A.H.; Yuan, Y.; Balmes, J.R.; Bates, M.N.; Steinmaus, C. Case-control study of arsenic in drinking water and lung cancer in California and Nevada. Int. J. Environ. Res. Public Health 2013, 10, 3310–3324. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S. Drinking Water Guidelines and Standards, Chapter 5.4 Drinking Water Quality Guideline on Arsenic; WHO International Water Standards; WHO Publications: Geneva, Switzerland, 2003; pp. 8–13. [Google Scholar]
- Tseng, W.P. Effects and dose–response relationships of skin cancer and Blackfoot disease with arsenic. Environ. Health Perspect. 1977, 19, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.P.; Chu, H.M.; How, SW.; Fong, J.M.; Lin, C.S.; Yeh, S. Prevalence of skin cancer in an endemic area of chronic arsenicism in Taiwan. J. Natl. Cancer Inst. 1968, 40, 453–463. [Google Scholar] [PubMed]
- Chen, C.J.; Chen, C.W.; Wu, M.M.; Kuo, T.L. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br. J. Cancer 1992, 66, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Wu, M.M.; Lee, S.-S.; Wang, J.-D.; Cheng, S.-H.; Wu, H.-S. Atherogenicity and carcinogenicity of high-arsenic artesian well water. Multiple risk factors and related malignant neoplasms of Blackfoot disease. Arteriosclerosis 1988, 8, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Goycolea, M.; Haque, R.; Biggs, M.L. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am. J. Epidemiol. 1998, 147, 660–669. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (US EPA). National Primary Drinking Water Regulations; Arsenic and Clarifications to Compliance and New Source Contaminants Monitoring: Final Rule; Federal Register, 40 CFR parts 9, 141, and 142; United States Environmental Protection Agency (US EPA): Washington, DC, USA, 2001; pp. 6976–7066.
- National Cancer Institute (NCI). Available online: https://www.statecancerprofiles.cancer.gov/incidencerates/ (accessed on 26 September 2017).
- United States Geological Survey (USGS). Available online: http://water.usgs.gov/nawqa/trace/data/arsenic_nov2001 (accessed on 11 February 2013).
- United States Geological Survey (USGS). Available online: http://www.USGS/Water Use/Data (accessed on 15 December 2016).
- National Cancer Institute (NCI). Model-Based Small Area Estimates of Cancer Risk Factors and Screening Behaviors. 2016. Available online: http://sae.cancer.gov/nhis-brfss/methodology.html (accessed on 11 October 2016).
- Field, R.W. Environmental factors in cancer: Radon. Rev. Environ. Health 2010, 25, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Carmona, R.H. Surgeon General Releases National Health Advisory on Radon; U.S. Department of Health and Human Services: Washington, DC, USA, 2005. Available online: https://www.epa.gov/radon/health-risk-radon (accessed on 6 March 2018).
- United States Environmental Protection Agency (US EPA). Available online: https://www.epa.gov/radon/epa-map-radon-zones (accessed on 6 March 2018).
- US Census Bureau. American Fact Finder. Available online: https://factfinder.census.gov (accessed on 7 August 2017).
- Centers for Disease Control and Prevention (CDC). Diabetes Data and Trends. 2016. Available online: https://www.cdc.gov/diabetes/data/countydata/countydataindicators.html (accessed on 27 March 2018).
- Lamm, S.H.; Ferdosi, H.; Dissen, E.K.; Li, J.; Ahn, J. A Systematic Review and Meta-Regression Analysis of Lung Cancer Risk and Inorganic Arsenic in Drinking Water. Int. J. Environ. Res. Public Health 2015, 12, 15498–15515. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.N.; Zu, R.; Kennedy, E.M.; Lam, T.; Liu, X.; Pizzurro, D.M.; Loftus, C.T.; Rhomberg, L.R. Quantitative assessment of lung and bladder cancer risk and oral exposure to inorganic arsenic: Meta-regression analyses of epidemiological data. Environ. Int. 2017, 106, 178–206. [Google Scholar] [CrossRef] [PubMed]
- Steinmaus, C.; Ferreccio, C.; Yuan, Y.; Acevedo, J.; Gonzalez, F.; Perez, L.; Cortes, S.; Balmes, J.R.; Liaw, J.; Smith, A.H. Elevated lung cancer in younger adults and low concentrations of arsenic in water. Am. J. Epidemiol. 2014, 180, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Bogen, K.T.; Chen, C.L.; Tsuji, J.S. Significant nonlinearity of lung cancer risk in relation to arsenic in drinking water in North West Taiwan. Toxicol. Sci. 2014, 138, 62. [Google Scholar]
- Chen, C.L.; Chiou, H.-Y.; Hsu, L.-I.; Hseueh, Y.-M.; Wu, M.M.; Chen, C.J. Ingested arsenic, characteristics of well water consumption and risk of different histological types of lung cancer in northeast Taiwan. Environ. Res. 2010, 110, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.G.; McDonald, J.C.; Cherry, N.M. Lung cancer and exposure to arsenic in rural Bangladesh. Occup. Environ. Med. 2008, 65, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Lamm, S.H.; Engel, A.; Kruse, M.B.; Feinleib, M.; Lai, S.-H.; Wilson, R. Arsenic in drinking water and bladder cancer mortality in the United States: An analysis based on 133 U.S. counties and 30 years of observation. J. Occup. Environ. Med. 2004, 46, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Lo, Y.S.; Guo, H.R. Lung Cancer Associated with Arsenic Ingestion: Cell-type Specificity and Dose Response. Epidemiology 2017, 28 (Suppl. 1), S106–S112. [Google Scholar] [CrossRef] [PubMed]
- Meacher, D.M.; Menzel, D.B.; Dillencourt, M.D.; Bic, L.F.; Schoof, R.A.; Yost, L.J.; Eickhoff, J.C.; Farr, C.H. Estimation of Multimedia Inorganic Arsenic Intake in the U.S. Population. Hum. Ecol. Risk Assess. 2002, 8, 1697–1721. [Google Scholar] [CrossRef]
- Aylward, L.L.; Ramasamy, S.; Hays, S.M.; Schoeny, R.; Kirman, C.R. Evaluation of urinary speciated arsenic in NHANES: Issues in interpretation in the context of potential inorganic arsenic exposure. Regul. Toxicol. Pharmacol. 2014, 69, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Lamm, S.H.; Robbins, S.; Chen, R.; Lu, J.; Goodrich, B.; Feinleib, M. Discontinuity in the cancer slope factor as it passes from high to low exposure levels--arsenic in the BFD-endemic area. Toxicology 2014, 326, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Abernathy, C.O.; Chappell, W.R.; Meek, M.E.; Gibb, H.; Guo, H.-R. Is ingested inorganic arsenic a “threshold” carcinogen? Fundam. Appl. Toxicol. 1996, 29, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Goering, P.L.; Aposhian, H.V.; Mass, M.J.; Cebrian, M.; Beck, B.D.; Waalkes, M.P. The enigma of arsenic carcinogenesis: Role of metabolism. Toxicol. Sci. 1999, 49, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chuang, Y.-C.; Lin, T.-M.; Wu, H.-Y. Malignant neoplasms among residents of a Blackfoot disease-endemic area in Taiwan: High-arsenic artesian well water and cancers. Cancer Res. 1985, 45, 5895–5899. [Google Scholar] [PubMed]
- Lamm, S.H.; Byrd, D.M.; Kruse, M.B.; Feinleib, M.; Lai, S.-H. Bladder cancer and arsenic exposure: Differences in the two populations enrolled in a study in southwest Taiwan. Biomed. Environ. Sci. 2003, 16, 355–368. [Google Scholar] [PubMed]
- Tsuji, J.S.; Alexander, D.D.; Perez, V.; Mink, P.J. Arsenic exposure and bladder cancer: Quantitative assessment of studies in human populations to detect risks at low doses. Toxicology 2014, 317, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. Inorganics and Hormesis. Crit. Rev. Toxicol. 2003, 33, 215–304. [Google Scholar] [CrossRef] [PubMed]
- Snow, E.T.; Sykora, P.; Durham, T.R.; Klein, C.B. Arsenic, mode of action at biologically plausible low doses: What are the implications for low dose cancer risk? Toxicol. Appl. Pharmacol. 2005, 207 (Suppl. 2), 557–564. [Google Scholar] [CrossRef] [PubMed]
- Gentry, P.R.; Yager, J.W.; Clewell, R.A.; Clewell, H.J., III. Use of mode of action data to inform a dose-response assessment for bladder cancer following exposure to inorganic arsenic. Toxicol. In Vitro 2014, 28, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Arnold, L.L.; Beck, B.D.; Eldan, M. Evaluation of the carcinogenicity of inorganic arsenic. Crit. Rev. Toxicol. 2013, 43, 711–752. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Chowdhury, A.; Arnold, L.L. Inorganic arsenic: A non-genotoxic carcinogen. J. Environ. Sci. 2016, 49, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Siliker, W.; Andersen, M.E.; Bogdanffy, M.S.; Bus, J.S.; Cohen, S.D.; Conolly, R.B.; David, R.M.; Doerrer, N.G.; Dorman, D.C.; Gaylor, D.W.; et al. Dose-dependent transitions in mechanisms of toxicity. Toxicol. Appl. Pharmacol. 2004, 201, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Slikker, W.; Andersen, M.E.; Bogdanffy, M.S.; Bus, J.S.; Cohen, S.D.; Conolly, R.B.; David, R.M.; Doerrer, N.G.; Dorman, D.C.; Gaylor, D.W.; et al. Dose-dependent transitions in mechanisms of toxicity: Case studies. Toxicol. Appl. Pharmacol. 2004, 201, 226–294. [Google Scholar] [PubMed]
- Andersen, M.E.; Clewell, R.J, III; Bermudex, E.; Dodd, D.E.; Willson, G.A.; Campbell, J.L.; Thomas, R.S. Formaldehyde: Integrating dosimetry, cytotoxicity, and genomics to understand dose-dependent transitions for an endogenous compound. Toxicol. Sci. 2010, 118, 716–731. [Google Scholar] [CrossRef] [PubMed]


| Variable | Mean | Median | Minimum | Maximum |
|---|---|---|---|---|
| Outcome | ||||
| Lung Cancer Rate | ||||
| (per 100,000) | 66.3 | 66.3 | 13.5 | 124.8 |
| Count (5-year estimate) | 463 | 136 | 8 | 11,459 |
| Exposure | ||||
| Dependency | 74% | 87% | 0% | 100% |
| Well Count | 8.7 | 2 | 1 | 274 |
| As Median (μg/L) | 2.1 | 0.8 | 0.5 | 102 |
| As Minimum (μg/L) | 1.2 | 0.5 | 0.5 | 42 |
| As Maximum (μg/L) | 11.1 | 1 | 0.5 | 950 |
| Variables | ||||
| Current Smoker (%) | 24.3 | 24.7 | 6.2 | 40.7 |
| Ex-Smoker (%) | 23.1 | 23.4 | 10.6 | 37.4 |
| Obesity (%) | 30.3 | 30.9 | 13.9 | 44.6 |
| Education (<HS) | 0.15 | 0.14 | 0.03 | 0.34 |
| Residency (Same Cnty) | 0.94 | 0.94 | 0.81 | 0.99 |
| Poverty | 0.16 | 0.16 | 0.04 | 0.53 |
| Income ($ K) | 47.4 | 44.8 | 23.9 | 106.1 |
| Rural | 0.49 | 0.48 | 0.00 | 1.00 |
| Population | ||||
| Male | 0.50 | 0.50 | 0.45 | 0.64 |
| Hispanic | 0.09 | 0.04 | 0.00 | 0.82 |
| White | 0.87 | 0.92 | 0.03 | 0.99 |
| Black | 0.07 | 0.02 | 0.00 | 0.69 |
| Asian | 0.02 | 0.01 | 0.00 | 0.22 |
| Other | 0.05 | 0.02 | 0.01 | 0.97 |
| Population | 154,960 | 38,966 | 2389 | 4,092,459 |
| Variable | Total | Male | Female |
|---|---|---|---|
| Unadjusted Median Model | |||
| N | 757 | 704 | 678 |
| As(Median) | −0.007 *** | −0.009 *** | −0.006 *** |
| Intercept | −5.801 *** | −5.637 *** | −5.937 *** |
| Total | Male | Female | |
| Adjusted Median Model | |||
| N | 748 | 695 | 669 |
| As (Median) | −0.001 * | −0.003 *** | −<0.001 |
| GW Dependency | −0.025 *** | −0.030 *** | 0.012 |
| Current Smoker Prevalence | 0.016 *** | 0.017 *** | 0.013 *** |
| Ex-smoker Prevalence | 0.010 *** | 0.008 *** | 0.011 *** |
| Radon (>4 pCi/L) | −0.004 | −0.003 | −0.006 |
| Obesity | 0.004 *** | 0.004 *** | −0.002 ** |
| Education (≥high school) | −0.022 *** | −0.017 *** | −0.021 *** |
| Residency (same county, prior year) | −0.084 | 0.477 *** | 0.165 |
| Poverty (<poverty live) | −0.340 ** | −0.689 *** | 0.021 |
| Median Household Income ($ K) | −0.001 * | −0.003 *** | 0.0006 |
| Rural | −0.288 *** | −0.214 ** | −0.297 *** |
| Male (%) | −1.421 *** | ||
| Hispanic | −0.896 *** | −0.831 *** | −1.029 *** |
| Black | −0.021 | 0.137 *** | −0.068 * |
| Asian | 0.220 *** | 0.311 *** | 0.129 |
| Other | −0.705 *** | −0.919 *** | −0.568 *** |
| Intercept | −3.488 *** | −4.823 *** | −4.408 *** |
| Variable | Total | Male | Female |
|---|---|---|---|
| Unadjusted Median Model | |||
| N | 394 | 351 | 334 |
| As(Median) | −0.008 *** | −0.010 *** | −0.007 *** |
| Intercept | −5.803 *** | −5.625 *** | −5.951 *** |
| Total | Male | Female | |
| Adjusted Median Model | |||
| N | 393 | 350 | 333 |
| As(Median) | −0.005 *** | −0.006 ** | −0.004 * |
| GW Dependency | 0.492 *** | 0.659 *** | 0.389 *** |
| Current Smoker Prevalence | 0.021 *** | 0.023 *** | 0.020 *** |
| Ex-smoker Prevalence | 0.015 *** | 0.011 *** | 0.016 *** |
| Radon (>4 pCi/L) | −0.003 | −0.015 | −0.008 |
| Obesity | 0.002 | −0.001 | −0.006 *** |
| Education (≥high school) | −0.022 *** | −0.016 *** | −0.022 *** |
| Residency (same county, prior year) | −0.069 | 0.052 | 0.337 |
| Poverty (<poverty live) | −0.495 ** | −0.749 *** | −0.123 |
| Median Household Income ($ K) | <0.001 | - <0.001 | 0.001 |
| Rural | −0.286 *** | −0.198 *** | −0.280 *** |
| Male (%) | −1.224 *** | ||
| Hispanic | −0.749 *** | −0.657 *** | −0.893 *** |
| Black | 0.017 | 0.230 *** | −0.061 |
| Asian | 1.207 *** | 0.822 *** | 1.682 *** |
| Other | −0.962 *** | −1.207 *** | −1.016 *** |
| Intercept | −4.352 *** | −5.473 *** | −5.067 *** |
| Restrictions (GW Dependency ≥ 80%; Max ≤ 100 μg/L) | |||||
|---|---|---|---|---|---|
| Population | n | Coef | SE | z | p |
| Total | 399 | −0.004 | 0.001 | −3.10 | 0.002 |
| Male | 359 | −0.005 | 0.002 | −3.54 | <0.001 |
| Female | 338 | −0.001 | 0.002 | −0.63 | 0.531 |
| Restrictions (GW Dependency ≥ 50%; Max ≤ 50 μg/L) | |||||
|---|---|---|---|---|---|
| Population | n | Coef | SE | z | p |
| Total | 513 | −0.006 | 0.001 | −5.05 | <0.001 |
| Male | 515 | −0.007 | 0.002 | −4.51 | <0.001 |
| Female | 496 | −0.008 | 0.002 | −4.563 | <0.001 |
| Restrictions (Ln Mean Arsenic; GW Dependency ≥ 80%; Max ≤ 50 μg/L) | |||||
|---|---|---|---|---|---|
| Population | n | Coef | SE | z | p |
| Total | 393 | −0.026 | 0.005 | −5.521 | <0.001 |
| Male | 350 | −0.036 | 0.006 | −5.81 | <0.001 |
| Female | 333 | −0.019 | 0.007 | −2.72 | 0.007 |
| Population | Concentration | Ceof. * | SE | z | p-Value |
|---|---|---|---|---|---|
| Total | >10–50 μg/L | −0.088 | 0.020 | −4.19 | 0.000 |
| Male | >10–50 μg/L | −0.093 | 0.027 | −3.46 | 0.001 |
| Female | >10–50 μg/L | −0.066 | 0.032 | −2.04 | 0.041 |
| Total | 1–10 μg/L | −0.045 | 0.008 | −5.71 | 0.000 |
| Male | 1–10 μg/L | −0.093 | 0.010 | −9.35 | 0.000 |
| Female | 1–10 μg/L | −0.017 | 0.012 | −1.45 | 0.146 |
| Reference (Year) | Location | μg/L | RR * |
|---|---|---|---|
| Dauphine et al. (2013) [6] | California/Nevada | 42.5 | 0.75 |
| Steinmaus et al. (2014) [28] | Chile | 35 | 1.24 |
| 80 a | 0.89 | ||
| Steinmaus et al. (2013) [5] | Chile | 52.5 b | 0.98 |
| Ferreccio et al. (2013) [3] | Chile | 60 | 0.77 |
| Smith et al. (2009) [4] | Chile | 12.8 | 0.7 |
| 35 c | 0.7 | ||
| Bogen et al. (2014) [29] | NE Taiwan | 3.26 | 0.57 |
| 25.9 | 0.73 | ||
| 74.3 | 0.68 | ||
| Chen et al. (2010b) d [30] | NE Taiwan | 30 | 1.1 |
| 75 | 0.99 | ||
| Mostafa et al. (2008) [31] | Bangladesh males | ||
| Non-Smokers | 30 | 0.9 | |
| 75 | 1.1 | ||
| Smokers | 30 | 1.25 | |
| 75 | 1.37 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamm, S.H.; Boroje, I.J.; Ferdosi, H.; Ahn, J. Lung Cancer Risk and Low (≤50 μg/L) Drinking Water Arsenic Levels for US Counties (2009–2013)—A Negative Association. Int. J. Environ. Res. Public Health 2018, 15, 1200. https://doi.org/10.3390/ijerph15061200
Lamm SH, Boroje IJ, Ferdosi H, Ahn J. Lung Cancer Risk and Low (≤50 μg/L) Drinking Water Arsenic Levels for US Counties (2009–2013)—A Negative Association. International Journal of Environmental Research and Public Health. 2018; 15(6):1200. https://doi.org/10.3390/ijerph15061200
Chicago/Turabian StyleLamm, Steven H., Isabella J. Boroje, Hamid Ferdosi, and Jaeil Ahn. 2018. "Lung Cancer Risk and Low (≤50 μg/L) Drinking Water Arsenic Levels for US Counties (2009–2013)—A Negative Association" International Journal of Environmental Research and Public Health 15, no. 6: 1200. https://doi.org/10.3390/ijerph15061200
APA StyleLamm, S. H., Boroje, I. J., Ferdosi, H., & Ahn, J. (2018). Lung Cancer Risk and Low (≤50 μg/L) Drinking Water Arsenic Levels for US Counties (2009–2013)—A Negative Association. International Journal of Environmental Research and Public Health, 15(6), 1200. https://doi.org/10.3390/ijerph15061200





