Study of Factors Influencing the Bioaccessibility of Triazolone in Cherry Tomatoes Using a Static SHIME Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Samples
2.2. In-Vitro Digestion Model Based on Human SHIME
2.3. Chemical Analyses
2.4. Parameters of Bioaccessibility
2.5. Effect of Dietary Nutrition on Bioaccessibility
2.6. Statistical Analysis
3. Results and Discussion
3.1. Method Validation
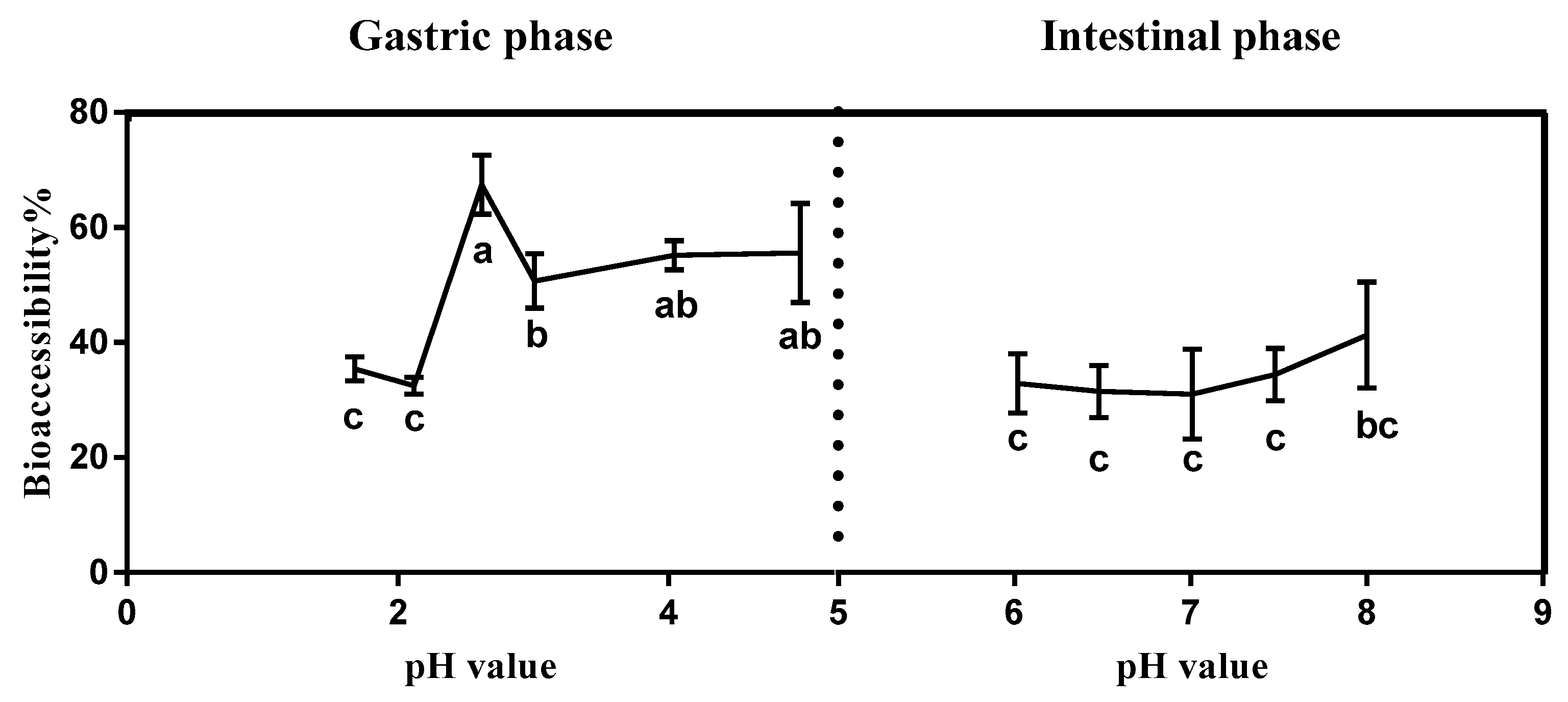
3.2. Effect of Gastrointestinal pH on Triazolone Bioaccessibility
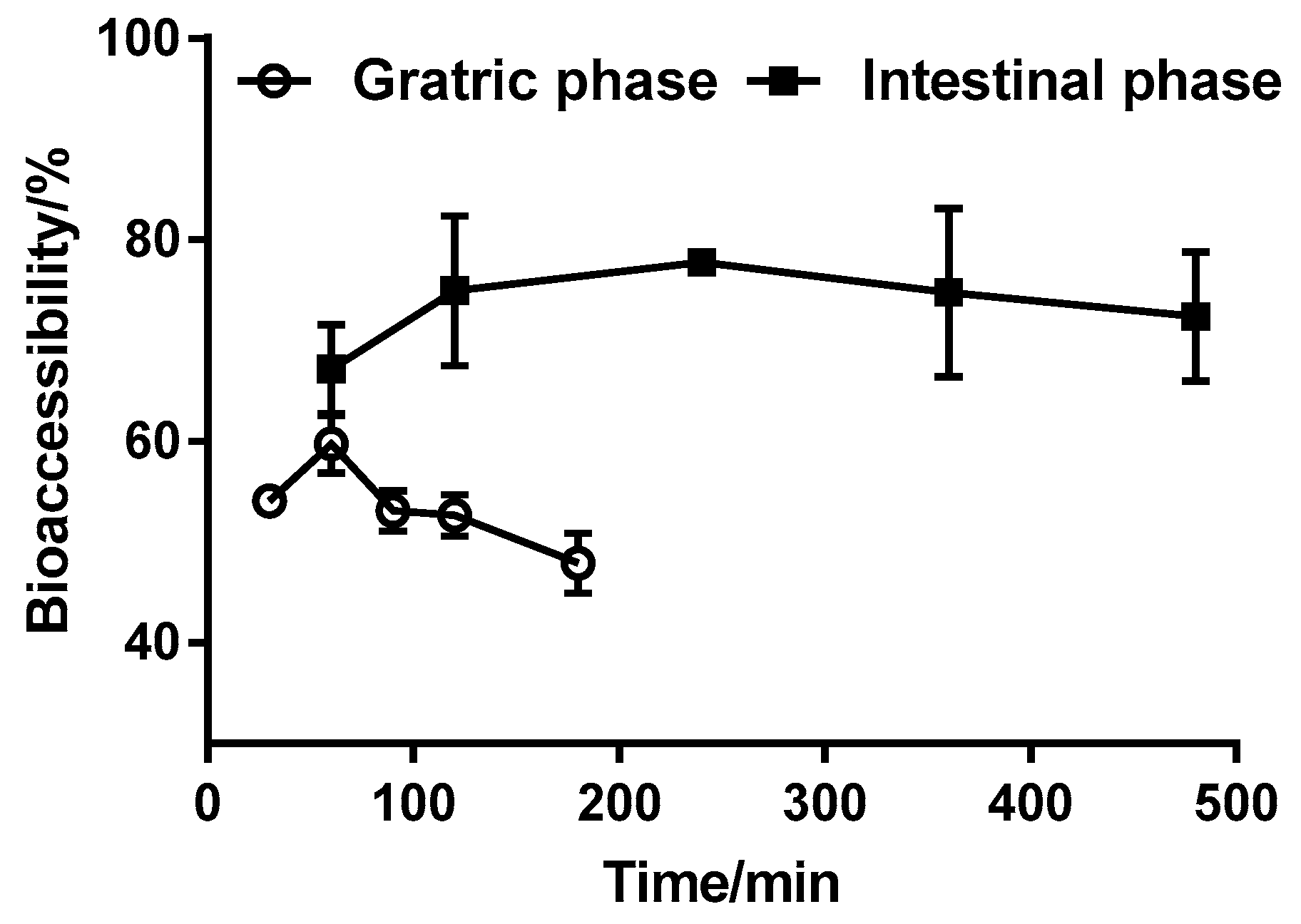
3.3. Effect of Digestion Time on Triazolone Bioaccessibility
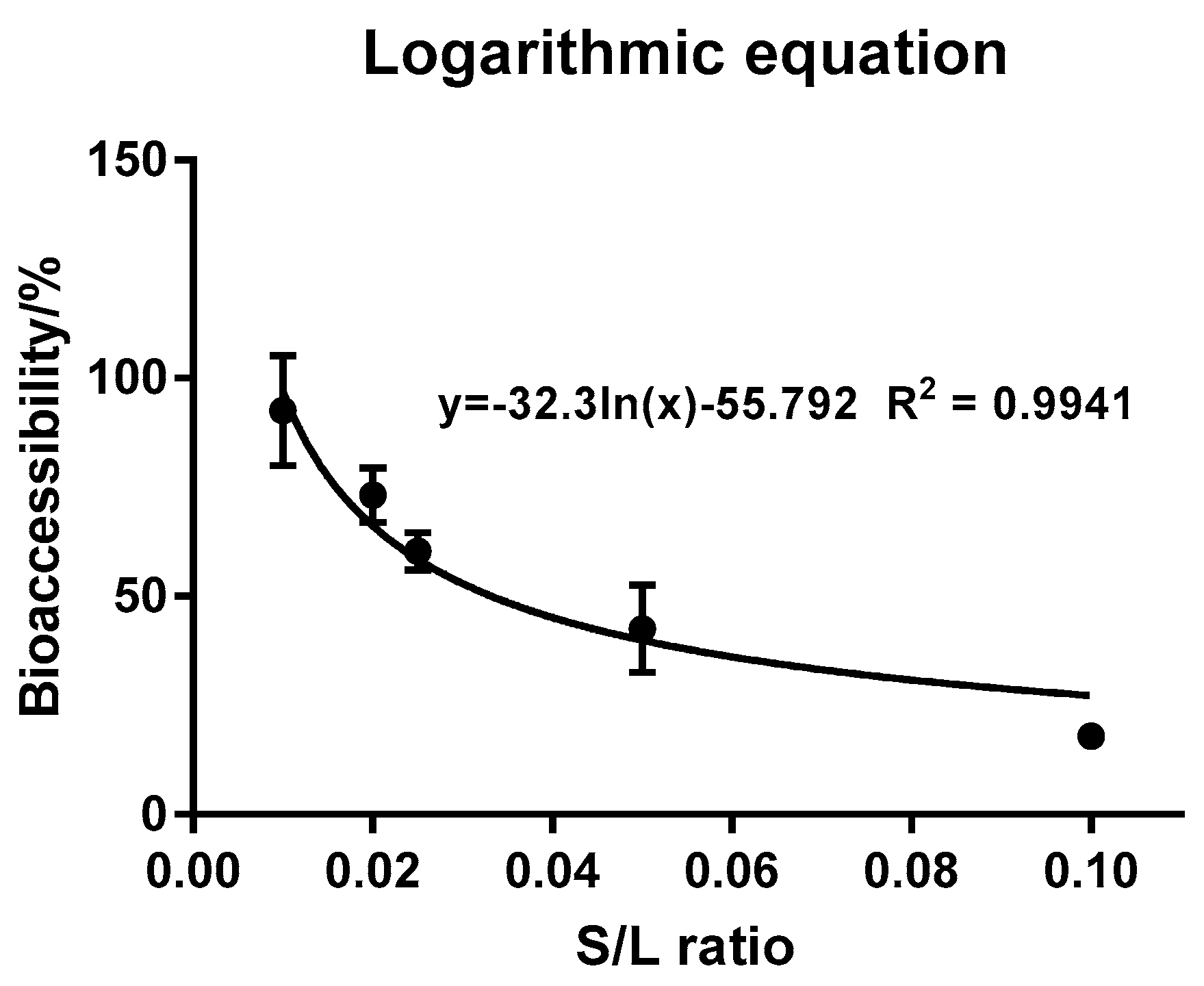
3.4. Effect of S/L Ratio on Triazolone Bioaccessibility
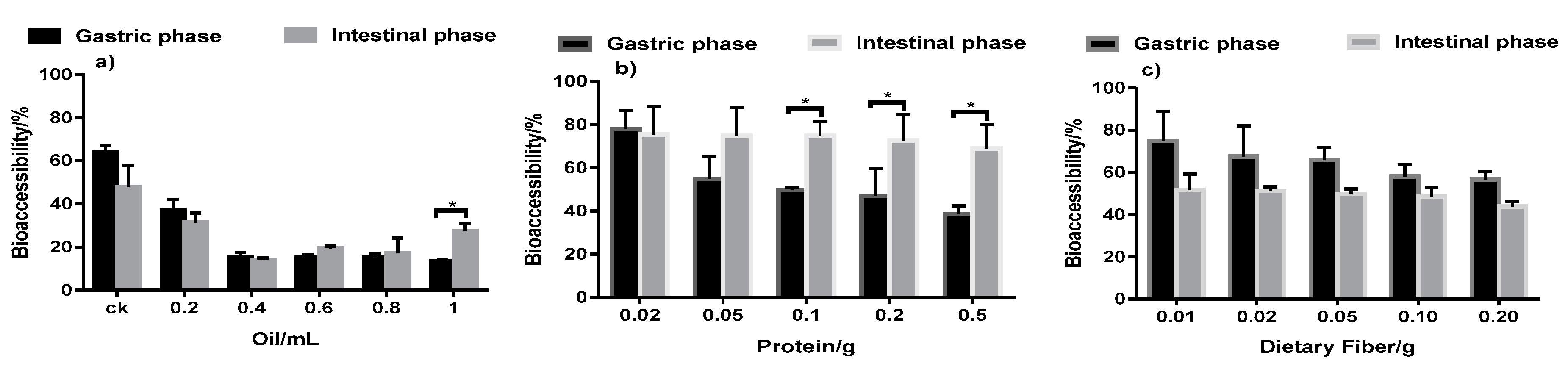
3.5. Effect of Dietary Nutrients on Triazolone Bioaccessibility
3.5.1. Effect of Oil on Triazolone Bioaccessibility
3.5.2. Effect of Protein and Dietary Fiber on Triazolone Bioaccessibility
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fosu, P.O.; Donkor, A.; Ziwu, C.; Dubey, B.; Kingsford-Adaboh, R.; Asante, I.; Nyarko, S.; Tawiah, R.; Nazzah, N. Surveillance of pesticide residues in fruits and vegetables from accra metropolis markets, ghana, 2010–2012: A case study in sub-saharan africa. Environ. Sci. Pollut. Res. 2017, 24, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, X.; Zhao, D.; Feng, T.; Zhou, J.; Sun, T.; Wang, J.; Wang, C. Seasonal variation and exposure risk assessment of pesticide residues in vegetables from xinjiang uygur autonomous region of china during 2010–2014. J. Food Compos. Anal. 2016, 58, 1–9. [Google Scholar] [CrossRef]
- Han, Y.; Liu, S.; Yang, J.; Zhong, Z.; Zou, N.; Song, L.; Zhang, X.; Li, X.; Pan, C. Residue behavior and processing factors of eight pesticides during the production of sorghum distilled spirits. Food Control 2016, 69, 250–255. [Google Scholar] [CrossRef]
- Yadav, I.C.; Devi, N.L.; Syed, J.H.; Cheng, Z.; Li, J.; Zhang, G.; Jones, K.C. Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Sci. Total Environ. 2015, 511, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, S.G.; Kwon, Y.S.; Hong, S.M.; Seo, J.S. Development of cabbage reference material for multi-residue pesticide analysis. Appl. Biol. Chem. 2018, 61, 15–23. [Google Scholar] [CrossRef]
- Chitindingu, K.; Benhura, M.A.N.; Muchuweti, M. Invitro bioaccessibility assessment of phenolic compounds from selected cereal grains: A prediction tool of nutritional efficiency. LWT Food Sci. Technol. 2015, 63, 575–581. [Google Scholar] [CrossRef]
- Li, R.; Li, D.W.; Yan, A.L.; Hong, C.L.; Liu, H.P.; Pan, L.H.; Song, M.Y.; Dai, Z.X.; Ye, M.L.; Weng, H.X. The bioaccessibility of iodine in the biofortified vegetables throughout cooking and simulated digestion. J. Food Sci. Technol. 2018, 55, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Starr, J.; Han, J.; Zhang, L.; Lu, D.; Guan, R.; Xu, X.; Wang, X.; Li, J.; Li, W.; et al. The bioaccessibility of polychlorinated biphenyls (pcbs) and polychlorinated dibenzo-p-dioxins/furans (pcdd/fs) in cooked plant and animal origin foods. Environ. Int. 2016, 94, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, B.; Riethorst, D.; Brouwers, J.; Tack, J.; Annaert, P.; Augustijns, P. Evaluation of fasted state human intestinal fluid as apical solvent system in the caco-2 absorption model and comparison with fassif. Eur. J. Pharm. Sci. 2015, 67, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Yang, F.; Wei, C.Y.; Liang, T. Bioaccessibility of heavy metals in soils cannot be predicted by a single model in two adjacent areas. Environ. Geochem. Health 2016, 38, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-G.; Gao, Y.-P.; Lin, Q. Contamination, bioaccessibility and human health risk of heavy metals in exposed-lawn soils from 28 urban parks in southern China’s largest city, guangzhou. Appl. Geochem. 2016, 67, 52–58. [Google Scholar] [CrossRef]
- Warth, B.; Sulyok, M.; Krska, R. Lc-ms/ms-based multibiomarker approaches for the assessment of human exposure to mycotoxins. Anal. Bioanal. Chem. 2013, 405, 5687–5695. [Google Scholar] [CrossRef] [PubMed]
- Roncevic, S.; Spasojevic, J.; Maletic, S.; Jazic, J.M.; Isakovski, M.K.; Agbaba, J.; Grgic, M.; Dalmacija, B. Assessment of the bioavailability and phytotoxicity of sediment spiked with polycyclic aromatic hydrocarbons. Environ. Sci. Pollut. Res. 2016, 23, 3239–3246. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Xiao, J.J.; Feng, R.P.; Liu, Y.Y.; Liao, M.; Wu, X.W.; Hua, R.M.; Cao, H.Q. In-vitro bioaccessibility of five pyrethroids after human ingestion and the corresponding gastrointestinal digestion parameters: A contribution for human exposure assessments. Chemosphere 2017, 182, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Xiao, J.J.; Feng, R.P.; Liu, Y.Y.; Liao, M.; Wue, X.W.; Hua, R.M.; Cao, H.Q. Factors affecting the bioaccessibility and intestinal transport of difenoconazole, hexaconazole, and spirodiclofen in human caco-2 cells following in vitro digestion. J. Agric. Food Chem. 2017, 65, 9139–9146. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, H.J.; Juhasz, A.L.; Cui, X.; Ma, L.Q. Predicting the relative bioavailability of ddt and its metabolites in historically-contaminated soils using a tenax-improved physiologically-based extraction test (ti-pbet). Environ. Sci. Technol. 2016, 50, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, R.R.A.; Devesa, V.; Vélez, D.; Cervera, M.L.; Cadore, S. Study of the factors influencing the bioaccessibility of 10 elements from chocolate drink powder. J. Food Compos. Anal. 2016, 48, 41–47. [Google Scholar] [CrossRef]
- Krishnan, R.; Meera, M.S. Assessment of inhibitory factors on bioaccessibility of iron and zinc in pearl millet (Pennisetum glaucum (L.) R. Br.) cultivars. J. Food Sci. Technol. 2017, 54, 4378–4386. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Ojeda, A.M.; Moreno-Rojas, R.; Sevillano-Morales, J.; Cámara-Martos, F. Influence of dietary components on minerals and trace elements bioaccessible fraction in organic weaning food: A probabilistic assessment. Eur. Food Res. Technol. 2017, 243, 639–650. [Google Scholar] [CrossRef]
- Kang, Y.; Pan, W.; Liang, S.; Li, N.; Zeng, L.; Zhang, Q.; Luo, J. Assessment of relative bioavailability of heavy metals in soil using in vivo mouse model and its implication for risk assessment compared with bioaccessibility using in vitro assay. Environ. Geochem. Health 2016, 38, 1183–11919. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Sun, H.J.; Li, H.B.; Luo, J.; Ma, L.Q. Assessment of cadmium bioaccessibility to predict its bioavailability in contaminated soils. Environ. Int. 2016, 94, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.R.; Basta, N.T.; Casteel, S.W.; Pace, L.W. An in vitro gastrointestinal method to estimate bioavailable arsenic in contaminated soils and solid media. Environ. Sci. Technol. 1999, 33, 642–649. [Google Scholar] [CrossRef]
- Yin, N.; Du, H.; Zhang, Z.; Cai, X.; Li, Z.; Sun, G.; Cui, Y. Variability of arsenic bioaccessibility and metabolism in soils by human gut microbiota using different in vitro methods combined with shime. Sci. Total Environ. 2016, 566–567, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Han, S.; Li, J.; Zhang, D.; Wu, M.; Sheng, G.; Fu, J. Factors Affecting the Bioaccessibility of Polychlorinated Biphenyls Using In vitro Test. In Proceedings of the International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009; pp. 1–4. [Google Scholar]
- Sun, G.X.; de Wiele, T.V.; Alava, P.; Tack, F.M.; Laing, G.D. Bioaccessibility of selenium from cooked rice as determined in a simulator of the human intestinal tract (Shime). J. Sci. Food Agric. 2017, 97, 3540–3545. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Mei, W.; JinSheng, D.; MingNa, S.; Yong, Z.; TongChun, G. Analysis of carbendazim and triadimefon in mixture by HPLC. J. Anhui Agric. Sci. 2007, 8767, 8786. [Google Scholar]
- Smith, E.; Scheckel, K.; Miller, B.W.; Weber, J.; Juhasz, A.L. Influence of in vitro assay pH and extractant composition on as bioaccessibility in contaminated soils. Sci. Total Environ. 2014, 473–474, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.X.; Li, J.L.; Zhang, X.Y.; Yu, Z.Q.; Wiele, T.V.D.; Han, S.Y.; Wu, M.H.; Sheng, G.Y.; Fu, J.M. Assessment of the bioaccessibility of polybrominated diphenyl ethers in foods and the correlations of the bioaccessibility with nutrient contents. J. Agric. Food Chem. 2010, 58, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, M.; Black, W.D.; Waisberg, C.M.; Hale, B. The effect of pH, time and dietary source of cadmium on the bioaccessibility and adsorption of cadmium to/from lettuce (Lactuca sativa L. Cv. Ostinata). Food Chem. Toxicol. 2004, 42, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Steinhart, H.; Beyer, M.G.; Kirchgessner, M. On the complex formation of proteins with cu Ions under Acidic conditions. Z. Lebensm. Unters. Forsch. 1975, 159, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Ravi, P. Dft study on the effect of relative positions of methyl-, nitro- and N → oxide groups on the molecular structure, thermal/kinetic stability, crystal density, heat of decomposition and performance characteristics of triazolones. Mol. Phys. 2017, 115, 1657–1666. [Google Scholar] [CrossRef]
- Laird, B.D.; Tr, V.D.W.; Corriveau, M.C.; Jamieson, H.E.; Parsons, M.B.; Verstraete, W.; Siciliano, S.D. Gastrointestinal microbes increase arsenic bioaccessibility of ingested mine tailings using the simulator of the human intestinal microbial ecosystem. Environ. Sci. Technol. 2007, 41, 5542–5547. [Google Scholar] [CrossRef] [PubMed]
- Kania-Korwel, I.; Lehmler, H.J. Chiral polychlorinated biphenyls: Absorption, metabolism and excretion—A review. Environ. Sci. Pollut. Res. 2016, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Valderrama, J.; Wilde, P.; Macierzanka, A.; Mackie, A. The role of bile salts in digestion. Adv. Colloid Interface 2011, 165, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Ruby, M.V.; Davis, A.; Schoof, R.; Eberle, S.; Sellstone, C.M. Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environ. Sci. Technol. 1996, 30, 422–430. [Google Scholar] [CrossRef]
- Smith, B.A.; Kirk, J.L.; Stephenson, G.L. The influence of liquid to soil ratios on arsenic and lead bioaccessibility in reference and field soil. Hum. Ecol. Risk Assess. 2010, 16, 149–162. [Google Scholar] [CrossRef]
- Barreto, A.D.; Gutierrez, É.M.R.; Silva, M.R.; Silva, F.O.; Silva, N.O.C.; Lacerda, I.C.A.; Labanca, R.A.; Araújo, R.L.B. Characterization and bioaccessibility of minerals in seeds of Salvia hispanica L. Am. J. Plant Sci. 2016, 7, 2323–2337. [Google Scholar] [CrossRef]
- Min, L.U.; Zhang, F.; Han, S.Y.; Chen, L.I.; Ying-Xin, Y.U.; Zhang, D.P.; Ming-Hong, W.U.; Sheng, G.Y.; Jia-Mo, F.U. Effects of food components on bioavailability of ddts in carrots. Food Sci. 2009, 30, 44–47. [Google Scholar]
- Anese, M.; Bot, F.; Panozzo, A.; Mirolo, G.; Lippe, G. Effect of ultrasound treatment, oil addition and storage time on lycopene stability and in vitro bioaccessibility of tomato pulp. Food Chem. 2015, 172, 685–691. [Google Scholar] [CrossRef] [PubMed]
| Pesticides | Fortified Level (mg kg−1) | Intestinal Juice | Gastric Juice | R2 | LOQ | ||
|---|---|---|---|---|---|---|---|
| Average Recovery (%) | RSD (%) | Average Recovery (%) | RSD (%) | (mg kg−1) | |||
| Triazolone | 0.5 | 82.21 | 2.71 | 109.74 | 8.43 | 0.9996 | 0.01 |
| 1 | 90.55 | 4.08 | 113.03 | 5.82 | |||
| 5 | 99.70 | 1.74 | 107.23 | 0.46 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-Y.; Xiao, J.-J.; Fu, Y.-Y.; Liao, M.; Cao, H.-Q.; Shi, Y.-H. Study of Factors Influencing the Bioaccessibility of Triazolone in Cherry Tomatoes Using a Static SHIME Model. Int. J. Environ. Res. Public Health 2018, 15, 993. https://doi.org/10.3390/ijerph15050993
Liu Y-Y, Xiao J-J, Fu Y-Y, Liao M, Cao H-Q, Shi Y-H. Study of Factors Influencing the Bioaccessibility of Triazolone in Cherry Tomatoes Using a Static SHIME Model. International Journal of Environmental Research and Public Health. 2018; 15(5):993. https://doi.org/10.3390/ijerph15050993
Chicago/Turabian StyleLiu, Yu-Ying, Jin-Jing Xiao, Yun-Yao Fu, Min Liao, Hai-Qun Cao, and Yan-Hong Shi. 2018. "Study of Factors Influencing the Bioaccessibility of Triazolone in Cherry Tomatoes Using a Static SHIME Model" International Journal of Environmental Research and Public Health 15, no. 5: 993. https://doi.org/10.3390/ijerph15050993
APA StyleLiu, Y.-Y., Xiao, J.-J., Fu, Y.-Y., Liao, M., Cao, H.-Q., & Shi, Y.-H. (2018). Study of Factors Influencing the Bioaccessibility of Triazolone in Cherry Tomatoes Using a Static SHIME Model. International Journal of Environmental Research and Public Health, 15(5), 993. https://doi.org/10.3390/ijerph15050993






